
Dissection of the Role of Multidrug Resistance Protein in Cancer
Lihan Xu
1,
†
a
, Zhouchang Yao
2,
†
b
and Enqian Zhu
3,
†
c
1
School of Life and Pharmaceutical Sciences, Dalian University of Technology, Panjin Campus, Panjin,124221, China
2
College of life science and technology, China Pharmaceutical University, Nanjing, 211198, China
3
School of Pharmacy, Nanjing University of Chinese Medicine, Nanjing, 210023, China
†
These authors contributed equally
Keywords: Multidrug Resistance, Cancer.
Abstract: Cancer is a major public health problem worldwide, with the mortality estimated to grow for years, especially
with the belated access to care due to the pandemic of coronavirus disease 2019. Despite the emergence of
various oncology therapies during the past several decades, multi-drug resistance remains an important
challenge in cancer healing, especially in chemotherapies. This review includes the classification of
chemotherapeutic agents. As over 90% of mortalities in chemotherapies are caused by multi-drug resistance,
this study also elaborates the mechanisms of multi-drug resistance, with a deep insight into distinct types of
multidrug resistance proteins and the inhibitors related to them. The mechanisms of multidrug resistance
proteins are yet to be fully understood, and a few novel inhibitors of multidrug resistance proteins are still
under development. Thus, there are challenges for effective chemotherapies overcoming multi-drug
resistance. However, a few oncology treatments, such as immunotherapy and targeted drug delivery via DNA
nanostructure have weakened the impact of multi-drug resistance. The aim of this review is not only to
demonstrate the latest data on the studies of multi-drug resistance but also to offer information to those who
are searching for novel oncology therapies with higher cure rates, and plan to contribute to future multidrug
resistance studies as well as the emerging discoveries on mechanisms of MRPs.
1 INTRODUCTION
As a major public health problem worldwide, cancer
is a group of diseases. Several cells in the body grow
rapidly, spread beyond their boundaries, and invade
other parts of the body (National Cancer Institute
2007, WHO 2021). The mortality rate of cancer
increased in most of the 20th century until it peaked
in 1991. Then with the boom in the detection and
treatment of cancer, the mortality by 31% between
1991 and 2018, which means 3.2 million deaths
caused by cancer were prevented. Various cancer
therapies, including chemotherapy, surgery,
radiotherapy, immunotherapy, and biologically
targeted therapy, contribute to the effectiveness of
chemotherapy as the most common oncology
treatment (Bugde et al. 2017).
a
https://orcid.org/0000-0002-7445-7092
b
https://orcid.org/0000-0002-8561-6685
c
https://orcid.org/0000-0003-1769-2958
Either extracted from plants or synthetic
compounds, chemotherapeutics can be classified into
alkylating agents, topoisomerase inhibitors,
antimetabolites, mitotic spindle inhibitors, and others
according to the mechanism of action (Bukowski,
Kciuk, Kontek 2020). Alkylating agents can cause the
transfer of alkyl groups to the DNA guanine residues
or intra or inter-strand cross links, leading to DNA
base mispairing and the inhibition of strand
separation in the process of DNA synthesis
(Nussbaumer, Bonnabry, Veuthey, Fleury-Souverain
2011). Topoisomerase inhibitors include
topoisomerase I inhibitors (topotecan, irinotecan) and
topoisomerase II inhibitors(teniposide, etoposide,
doxorubicin, anthracyclines), both of which can lead
to DNA strand breakthrough manipulating
topoisomerases in DNA replication (Bax,
Murshudov, Maxwell, Germe 2019). Antimetabolites
Xu, L., Yao, Z. and Zhu, E.
Dissection of the Role of Multidrug Resistance Protein in Cancer.
DOI: 10.5220/0011254400003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 671-679
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
671
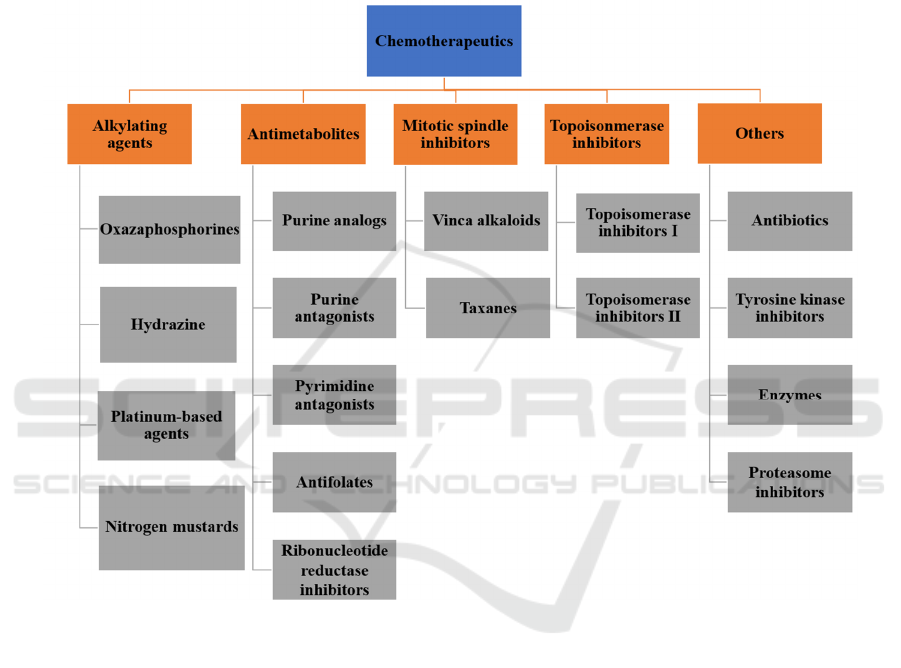
have interference in pathways of fundamental
biosynthesis, perturb the synthesis of DNA or RNA,
incorporate analogs of purine/pyrimidine with
different structures into DNA, or result in DNA
strand breaks by restraining a group of enzymes, for
instance, DNA polymerase, dihydrofolate reductase
(Marchi, O’Connor 2012). Mitotic spindle inhibitors
sustain activation of the spindle assembly checkpoint
(SAC), lengthening mitotic arrest, eventually
resulting in the cell's death. The entire inhibition
procedure is conducted in mitosis or in the G1 phase
of the following cell cycle (Sinha, Duijf, Khanna
2019). Besides the chemotherapeutic agents above,
other chemotherapeutic agents with distinct
mechanisms exist, for instance, tyrosine kinase
inhibitors, antibiotics, proteasome inhibitors, some
particular enzymes, including l-asparagincase, are
non-homogenous (Bukowski, Kciuk, Kontek 2020).
Figure 1: Classification of chemotherapeutics depending on their mechanism of action
However, the major challenge faced in cancer
chemotherapy is the less effective treatment due to
multidrug resistance (MDR), which remains the
second cause of death in developed countries (Garcia-
Mayea, Mir, Masson, Paciucci, LLeonart 2020).
MDR in oncology is defined as the ability of
resilience against drugs that are different in function
and structure. It was first discovered in experiments
with cultivated cells done by J.L. Biedler in 1970
(Biedler, Riehm 1970). Previous studies indicate that
multidrug resistance can appear before or during the
treatment, and it can be either acquired in the process
of chemotherapy or just inherent (Harris, Hochhauser
1992).
Mechanisms of MDR can be divided into several
categories: (1) promoted efflux of drugs by
transporters on the membrane of cancer cells, of
which ATP-binding cassette (ABC) transporters
function as main transporters (Chun, Kwon, Nam,
Kim 2015); (2) resistance to chemical substances
caused by microenvironment changes, for example,
cancer stem cell regulation (Li, Lei, Yao, et al. 2017);
(3) mutation in drug targets or feedback activation of
other targets and signaling pathways (Li, Lei, Yao, et
al. 2017); (4) decrease in drug uptake by influx
transporters; (5) promoted adaptability of cancer cells
enhanced by regulation of miRNA as well as
epigenetic regulation; (6) pathways of. apoptotic
signaling blocked as a result of altered level of B cell
lymphoma (BCL) family proteins expression or
mutant p53 pathway; (7) prompted metabolism of
xenobiotics (Bukowski, Kciuk, Kontek 2020).
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
672

MDR is most commonly led by the
overexpression of ABC, with a family of 49 human
membrane transporters involved in diverse
physiologic processes, in which P-glycoprotein (P-
gp), multidrug resistance-associated proteins
(MRPs), breast cancer resistance protein (BCRP) are
included (Liu 2019). P-glycoprotein (P-gp,
MDR1/ABCB1), as the first member of the ABC
transporter family, was found in drug-resistant
Chinese hamster ovary cells in 1976 by Victor Ling
functioning as an ATP binding cassette (ABC)
transporter (Juliano, Ling 1976). Experiments
indicate that the gene of P-gp, which was later
renamed ABCB1, can lead to drug resistance when
transfected into sensitive cells. Multidrug resistance-
associated protein 1 (MRP1/ABCC1), as the second
member of the ABC transporter family, discovered
by Cole et al. (Cole, Bhardwaj, Gerlach, et al. 1992),
can form resistance to vincristine etoposide,
doxorubicin (Mirski, Gerlach, Cole 1987). Different
from P-gp, which mainly transports hydrophobic
chemicals, the substrates of MRP1 and are
amphipathic organic acids with large hydrophobic
groups with a wide range of diversity in structure.
MRP1 also transports endobiotics, for instance,
hormones, pro-inflammatory molecules,
antioxidants, which P-gp doesn’t export (Kumar,
Jaitak 2019). Breast cancer resistance protein
(BCRP/ABCG2), as the third member of the ABC
transporter family, was first reported by Doyle et al.
in 1998, named from the drug selected breast cancer
cell line, i.e., MCF-7. Unlike P-gp or MRP1, both of
which are full transporters, BCRP is considered as the
shortest ABC transporter with one TMD and one
NBD. Through flocking by homodimerization or
heterodimerization with a disulfide bridge at Cys 603,
BCRP gains its full function (Kumar, Jaitak 2019).
2 MULTIDRUG RESISTANCE
PROTEINS (MRPS)
The ATP-binding cassette (ABC) transporters are
participants of a protein superfamily that are
recognized to transport intracellular and extracellular
materials, including metabolic products, lipids and
sterols, and xenobiotic drugs, and multidrug
resistance proteins (MRPs) belong to the largest
subfamily C in the ABC transporter superfamily
(Zhang, Wang, Gupta, Chen 2015). MRPs include
MRP1, MRP2 or cMOAT (the canalicular
multispecific organic anion transporter), MRP3,
MRP4 (cMOAT-B), MRP5 (cMOAT-C), MRP6,
MRP7, MRP8, and MRP9. The extended expression
of MRP is one of the integral motives of multidrug
resistance. MRPs have been implicated in mediating
multidrug resistance in tumor cells to many degrees
as the efflux extrude chemotherapeutic compounds
(or their metabolites) from malignant cells, so MRPs
are the groundwork for chemotherapeutic resistance
of many malignant tumors (Chen, Tiwari 2011).
2.1 Multidrug Resistance Protein 1
(MRP1)
MRP1(ABCC1) was once discovered in 1992, the
predominant member of the MRP subfamily-
associated MDR (Ma, Hu, Wang, et al. 2014). The
MRP1 gene is positioned in the lengthy arm 13.1
bands of human chromosome 16 with a molecular
weight of 190 kDa. The structure of MRP1 consists
of two transmembrane domains (TMD) and two
nucleotide-binding domains (NBD), and an extra N-
terminal TMD-TMD0, in which TMD is accountable
for substrate recognition, binding, and transport. At
the same time, NBD is concerned with ATP binding
and hydrolysis to grant strength for transport
(Johnson, Chen 2017). MRP1 transports a range of
therapeutic agents as properly as a variety of
physiological substrates. It may also play a role in
improving drug resistance in a range of cancers,
including lung cancer, breast cancer, persistent
lymphocytic leukemia, acute lymphocytic leukemia,
prostate cancer, and pediatric neuroblastoma (Munoz,
Henderson, Haber, Norris 2007). MRP1 is expressed
in the liver, kidney, intestine, brain, and other tissues,
transporting structurally numerous necessary
endogenous substances (e.g., leukotriene and
conjugated estrogen) and heterogeneous biological
and their metabolites, such as a number conjugates,
anti-cancer drugs, heavy metals, organic anion, and
lipids, with features concerned in inflammation,
detoxification, and oxidative stress (Nasr, Lorendeau,
Khonkarn, et al. 2020, He, Li, Kanwar, Zhou 2011).
2.2 Multidrug Resistance Protein 2
(MRP2)
ABCC2 encodes MRP2, which was once first
identified and cloned from rat liver cells. MRP2 not
only exists in tumor cells but also in the small
intestine, liver, kidney, placental barrier, and blood-
brain barrier (Zhang, Wang, Gupta, Chen 2015,
ostendorp, Beijnen, Schellens 2009). Similar to
MRP1, MRP2 has two MSDs characteristic of ABC
transporters, in addition to a third NH2-terminal MSD
(MSD0) and a COOH-terminal region. Sequences
Dissection of the Role of Multidrug Resistance Protein in Cancer
673

inside MSD0 of MRP2 are required for its activity and
plasma membrane trafficking, as are sequences within
its COOH terminal region (Chen, Tiwari 2011).
MRP2 promotes the excretion of drugs and chemicals,
especially MRP2 mediates ATP-dependent outflow of
various drugs and chemical compounds (including
glucosidic acid, sulfate, and glutathione complexes),
so drugs and chemical substances can be eliminated
from cells (Wen, Joy, Aleksunes 2017).
2.3 Multidrug Resistance Protein 3
(MRP3)
The amino acid sequence homology of MRP3 and
MRP1 is the highest, about 58%. MRP3 used to be
often positioned in the basement membrane of
hepatocytes and more often than expressed in the
adrenal gland, kidney, small intestine, colon,
pancreas, and gallbladder. However, the expression
stage of MRP3 was once low in the lung, spleen,
stomach, and tonsil (Zhang, Wang, Gupta, Chen
2015, Chen, Tiwari 2011). One of the features of
MRP3 is to mediate the transport of anionic
complexes and optimize the endogenous lipophilic
substances, exogenous resources, and glycosides of
bile sulfate. At the same time, it can also preserve the
stability of bile acid metabolism and adjust the
transport of soluble compounds in bile (Pérez-Pineda,
Baylón-Pacheco, Espíritu-Gordillo, Tsutsumi,
Rosales-Encina 2021).
2.4 Multidrug Resistance Protein 4
(MRP4)
MRP4 (ABCC4) was first discovered in human T
lymphoid cell lines in 1999. MRP4 is a vast substrate-
specific carrier, dispensed in nearly all tissues and
cells, inclusive of lung, ovary, testis, kidney,
intestine, liver, brain, pancreas, prostate, and more
than a few blood cells, and expressed in a range of
human tissues (examples are the basolateral and
apical plasma membranes from the liver and kidneys)
(Pérez-Pineda, Baylón-Pacheco, Espíritu-Gordillo,
Tsutsumi, Rosales-Encina 2021). MRP4 (MOAT-B)
is a lipophilic anion efflux pump capable of
conferring resistance to huge varying from substrates,
including nucleotide analogs, MTX, and glutathione
(GSH). Compared with other transgenic egg whites,
ABCC4 has a typical ABC transporter core structure,
specifically two transmembrane domains and two
nucleotide-binding domains (Zhang, Wang, Gupta,
Chen 2015). MRP4 is a plausible therapeutic target
for MDR. MRP4 was once noticeably expressed in
myeloid progenitors, and various endogenous
molecules are transported out of cells. MRP4 protects
cell function through 6-mercaptopurine (6-MP)
efflux. However, it can make most cancer cells
resistant to anticancer drugs and limit the sensitivity
of the tumor to radiotherapy and chemotherapy (Ma,
Hu, Wang, et al. 2014).
3 INHIBITORS
3.1 MRP1 Inhibitor - Sulindac
Sulindac appears to have favourable characteristics as
a potential MRP-1 inhibitor since in vitro inhibition
is evident at concentrations achievable in serum with
standard doses of the agent. Also, sulindac is
relatively nontoxic and well-tolerated because of the
small number of NSAIDs that might be used,
especially when used in an acute setting. The primary
biological metabolites and certain analogues of it
have been demonstrated to have pro-apoptotic
actions. Sulindac potentially inhibition of MRP-1-
mediated doxorubicin resistance coupled with other
activities such as anti-angiogenesis, which has been
described for sulindac and led to a potentiation of the
toxicity of doxorubicin without using toxic
concentrations. Also, sulindac is able to potentiate the
anti-tumour activity of doxorubicin in some animal
models (Anticancer Research 2004).
3.2 MRP1 and MRP2
Inhibitor - Nonsymmetrical 1,
4-Dihydropyridines
It is non-symmetrical compounds that have been
investigated to inhibit MRP1 and MRP2, both with a
non-symmetric framework. It is developed by novel
non-symmetrically substituted 1,4-dihydropyridines
in a different approach than the known so-called one-
pot reaction. The reaction mixture consists of the
afore-described three compounds to result in the
molecular 1,4-dihydropyridine scaffold.
Nonsymmetrical 1,4-Dihydropyridines express
MRP1 and MRP2 using the fluorescent
carboxyfluorescein diacetate (CFDA) as MRP
substrate. The respective cells were pre-incubated
with the potential inhibitors, and then the fluorescent
substrate was added. The substrate uptake was
measured by flow cytometry detecting the
corresponding fluorescence of the respective cells.
The fluorescence was related to the untreated control
cells measured to give a fluorescence activity ratio
(FAR) value (Pharmaceuticals 2020).
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
674
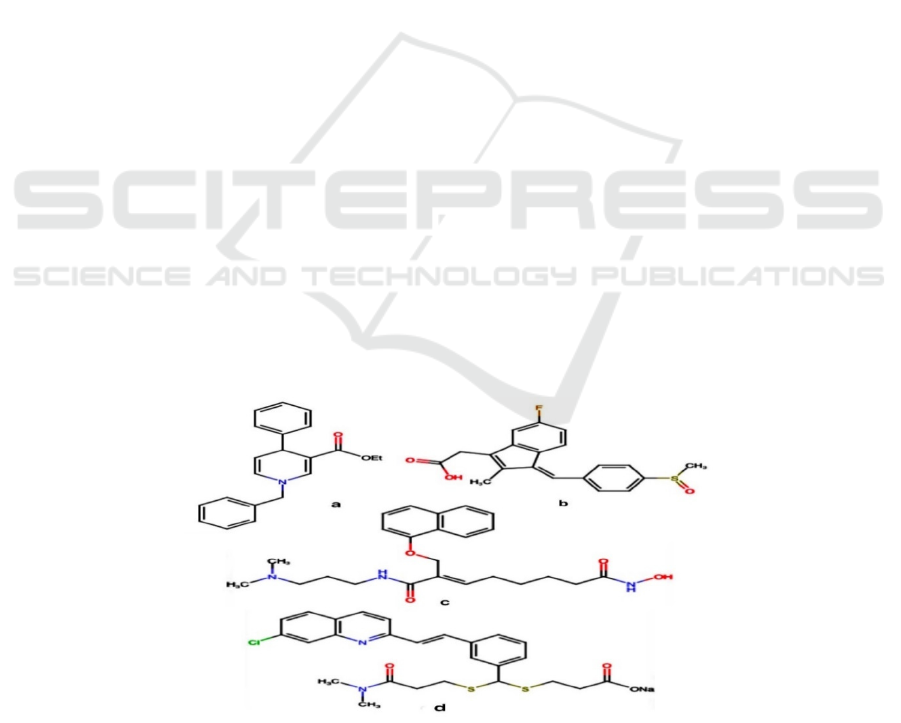
3.3 MRP3 and MRP4
Inhibitor - CG200745
CG200745, (E)- N(1)-(3-(dimethylamino)propyl)-
N(8)-hydroxy-2-((naphthalene-1-loxy) methyl) oct-2-
enediamide, is a recently developed HDAC inhibitor.
As a novel, HDAC inhibitor, CG200745, is an
intravenous hydroxamate-based pan-HDAC inhibitor.
Its inhibitory effect on cell growth has been
demonstrated in several types of cancer cells,
including prostate cancer, renal cell carcinoma, and
colon cancer in mono- and combinational-therapy
with other anticancer drugs. CG200745 was well
tolerated at the tested doses with no dose-limiting
toxicities in the first human study. The effect of
CG200745 on pancreatic cancer cell apoptosis was
tested by Western blot analysis, which indicated that
CG200745 increased the expression of pro-apoptotic
proteins, BAX, and p21. CG200745 induced the
expression of apoptotic proteins (PARP and caspase-
3) and increased the levels of acetylated histone H3.
CG200745 with gemcitabine/erlotinib showed
significant growth inhibition and synergistic
antitumor effects in vitro. In vivo,
gemcitabine/erlotinib and CG200745 reduced tumor
size up to 50%. CG200745 enhanced the sensitivity of
gemcitabine-resistant pancreatic cancer cells to
gemcitabine and decreased the level of ATP-binding
cassette-transporter genes, especially MRP3 and
MRP4. The novel HDAC inhibitor, CG200745, with
gemcitabine/erlotinib, had a synergistic anti-tumor
effect on pancreatic cancer cells. CG200745
significantly improved pancreatic cancer sensitivity to
gemcitabine, with a prominent antitumor effect on
gemcitabine-resistant pancreatic cancer cells
(Scientific Reports 2017).
3.4 MRP4 Inhibitor - MK-571
MK571 is a multidrug resistance protein-1, multidrug
resistance protein-2, and multidrug resistance
protein-4 (MRP1, MRP2, and MRP4) inhibitor. It has
been widely used to demonstrate the role of Mrp2 in
the cellular efflux of drugs, xenobiotics, and their
conjugates. Increasing the dosing concentration of
MK-571 in the in vivo study is restricted by its
solubility. Higher exposure of MK-571 in blood cells
and tissues may increase the intracellular
concentration of Methotrexate caused by MRP4
inhibition. MK-571 was selected as the concomitant
drug possessing inhibitory potency for MRP
transporters, demonstrating a typical bile-excretion
pharmacokinetic property. It is efficient in inhibiting
MRP1, MRP2, and MRP4 in cancer therapy (etm
2012).
Suppressing drug efflux is an important aim in
many drug development programs, so as in cancer
therapy. Chemically modifying or redesigning an
anticancer drug to completely bypass MRPs is
challenging. But no specific rules have been found,
as they can recognize diverse structures that permeate
cellular membranes. At the same time, modifications
of an anticancer drug without diminution of drug
potency are much harder. In comparison, drug
delivery systems offer seemingly innumerable
possibilities and provide the potential for safer and
high-dose delivery of anticancer drugs while using
noninvasive tracking techniques that are target-
specific. In these cases, controlling drug release
inside the cells could be significant, and the
exploration of inhibitors is of great importance for
our future.
a: MRP1 and MRP2 inhibitor - Nonsymmetrical 1,4-Dihydropyridines
b: MRP1 inhibitor -Sulindac
c: MRP3 and MRP4 inhibitor -CG200745
d: MRP4 inhibitor - MK-571
Figure 2. The structure of inhibitors.
Dissection of the Role of Multidrug Resistance Protein in Cancer
675

Table 1: Structure and function of MRPs and related inhibitors (NR: not report).
MRPs Chromosomal
localization
Exon
coding
Amino
acids
Physiological
function
major drugs substrate
inhibitors
Reference
MRP1 6p13.11-13 31 1531 Maintain the
dynamic
balance of GSH
in vivo and
protect cells
from the toxic
damage of
bilirubin
amycin,
vincristine,
etoposicIe,
Methotrexate,
camptothec,
Irinotecan, and
its active
metabolites SN-
38,
cyclophosphami
de
diphenylsulfami
de, benzazolone,
Indometacin,
Verapamil,
quercetin,
Genistein,
cyclosporin A,
steroids,
glibenpiride,
Glucovance
(Bakos,
Homolya 2007,
Wei, Sun, Liu
2010)
MRP2 10q23-24 32 1541 Transport of
hydrophobic,
uncharged
molecules or
water-soluble
anionic
compounds
cisplatin,
etoposicIe,
Vinblastine,
camptothec,
Methotrexate,
Olmesartan,
lopinovi
r
furosemide (Kruh,
Belinsky, Gallo,
Lee 2007)
MRP3 17q21.3 31 1527 Transport bile
salts and various
organic acids,
E217βG can be
transported
efficiently
etoposicIe,
acetaminophen,
glucuronic acid
glycosides,
vincristine,
Methotrexate
etoposicIe,
Methotrexate
(Chu, Huskey,
Braun, Sarkadi,
Evans, Evers
2004)
MRP4 13q32.1 31 1325 It plays a key
role in the
protection of
cells and cell
signaling
pathways by
regulating the
redistribution
and excretion of
various
inhibitory cell
growth drugs,
antiviral drugs,
antibiotics, and
cardiovascular
drugs in vivo
and in the
kidney
Methotrexate, 6-
mercaptopurine
,6-thioguanine,
Adefovir,
topotecan
celecoxib,
rofecoxib,
diclofenac
(Russel,
Koenderink,
Masereeuw
2008)
MRP5 3q27 NR 1437 It mediates the
signal
transduction
process of
biological cells
and binds GSH
and its
compounds and
even heavy
metals
6-
mercaptopurine,
6-thioguanine,
Adefovir, heavy
metals, S-GSH
Diprophenyl
sulfamide,
sulphinpyrazone
,
benzbromarone,
(Homolya,
Váradi, Sarkadi
2003)
MRP6 16p13.1 31 1503 S-conjugated
GSH transport
is associated
with pulmonary
elastic fibrosis
leukotriene
C4(LTC4), N-
hexyl cis-butene
diimide, S-GSH,
dinitrophenol,
podophylloside,
doxorubicin,
cisplatin,
Daunorubicin
Indometacin,
disulfonamide,
Benzbromarone
(Hendig,
Langmann,
Kocken, et al.
2008)
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
676

MRP7 6p12-21 22 1492 Modulate the
transport effects
of E217βG
17-β-D-
glucuronside,
paclitaxel,
Vinblastine,
SN-38, amycin,
carboplatin
imatinib
(Chen, Hopper-
Borge, Belinsky,
Shchavelev,
Kotova, Kruh
2003)
MRP8 16q12.1 28 1382 Transport of
nucleotides,
transport of
glycocholate
and taurocholate
5-FU, Adefovir,
Methotrexate,
Chile Acid
NR (Che, Guo,
Belinsky,
Kotova, Kruh
2005)
MRP9 16q12.1 29 1359 Transport
nucleotides,
immune
markers of
b
reast cance
r
NR NR (Zhou, Wang,
Di, et al. 2008)
4 CONCLUSIONS
This paper reviews the status and trend of research of
the last five years based on the papers published in
major educational technology journals. In summary,
this paper shows that:
the current status of cancer epidemiology with
the pandemic of COVID-19;Bottom: 4,2 cm;
the oncology treatments correlated to
multidrug resistance; Right: 2,6 cm.
molecular mechanism of multidrug resistance
the classification and insight of multidrug
resistance protein; Bottom: 4,2 cm;
the definition, physicochemical property, and
function of MRPs inhibitors. Right: 2,6 cm.
Due to the coronavirus disease 2019 (COVID-19)
pandemic, the situation of oncology treatment in
2020 was relatively distinct. A short-term decrement
in cancer incidence is followed by the growth in
advanced-stage disease, causing increased mortality,
resulting from delays in diagnosis and treatment
caused by unavailable or belated access to care.
Unfortunately, the consequence of COVID-19 is
estimated to last for years to qualify, which burdens
cancer therapy, even more, making effective tumor
treatments of significance. The findings included in
this paper could be good references for those
searching for novel oncology therapies with higher
cure rates, and plan to contribute to future multidrug
resistance studies and the emerging discoveries on
mechanisms of MRPs. In addition, the insight could
be helpful to future researches on inhibitors of MRPs
as well.
However, provided with cancer treatments in
existence, multidrug resistance can be avoided in
immunotherapy, or weakened by targeted drug
delivery via DNA nanostructure.
REFERENCES
A novel HDAC inhibitor, CG200745, inhibits pancreatic
cancer cell growth and overcomes gemcitabine
resistance January 2017 Scientific Reports 7(1):41615
DOI:10.1038/srep41615
Bukowski K, Kciuk M, Kontek R. Mechanisms of
Multidrug Resistance in Cancer Chemotherapy. Int J
Mol Sci. 2020;21(9). doi:10.3390/ijms21093233
Bax BD, Murshudov G, Maxwell A, Germe T. DNA
Topoisomerase Inhibitors: Trapping a DNA-Cleaving
Machine in Motion. J Mol Biol. 2019;431(18):3427-
3449. doi:10.1016/j.jmb.2019.07.008
Biedler JL, Riehm H. Cellular Resistance to Actinomycin
D in Chinese Hamster Cells in Vitro: Cross-Resistance,
Radioautographic, and Cytogenetic Studies. Cancer
Res. 1970;30(4):1174-1184.
Bakos E, Homolya L. Portrait of multifaceted transporter,
the multidrug resistance-associated protein 1
(MRP1/ABCC1). Pflugers Arch. 2007;453(5):621-641.
doi:10.1007/s00424-006-0160-8
Cancer. Accessed May 17, 2021.
https://www.who.int/news-room/fact-
sheets/detail/cancer
Chun S-Y, Kwon Y-S, Nam K-S, Kim S. Lapatinib
enhances the cytotoxic effects of doxorubicin in MCF-
7 tumorspheres by inhibiting the drug efflux function
of ABC transporters. Biomedicine & Pharmacotherapy.
2015;72:37-43. doi:10.1016/j.biopha.2015.03.009
Cole SP, Bhardwaj G, Gerlach JH, et al. Overexpression of
a transporter gene in a multidrug-resistant human lung
cancer cell line. Science. 1992;258(5088):1650-1654.
doi:10.1126/science.1360704
Chen Z-S, Tiwari AK. Multidrug Resistance Proteins
(MRPs/ABCCs) in Cancer Chemotherapy and Genetic
Diseases. FEBS J. 2011;278(18):3226-3245.
doi:10.1111/j.1742-4658.2011.08235.x
Chu X-Y, Huskey S-EW, Braun MP, Sarkadi B, Evans DC,
Evers R. Transport of ethinylestradiol glucuronide and
ethinylestradiol sulfate by the multidrug resistance
proteins MRP1, MRP2, and MRP3. J Pharmacol Exp
Ther. 2004;309(1):156-164.
doi:10.1124/jpet.103.062091
Dissection of the Role of Multidrug Resistance Protein in Cancer
677

Chen Z-S, Hopper-Borge E, Belinsky MG, Shchaveleva I,
Kotova E, Kruh GD. Characterization of the transport
properties of human multidrug resistance protein 7
(MRP7, ABCC10). Mol Pharmacol. 2003;63(2):351-
358. doi:10.1124/mol.63.2.351
Chen Z-S, Guo Y, Belinsky MG, Kotova E, Kruh GD.
Transport of bile acids, sulfated steroids, estradiol 17-
beta-D-glucuronide, and leukotriene C4 by human
multidrug resistance protein 8 (ABCC11). Mol
Pharmacol. 2005;67(2):545-557.
doi:10.1124/mol.104.007138
Expression levels of multidrug resistance-associated
protein 4 (MRP4) in human leukemia and lymphoma
cell lines, and the inhibitory effects of the MRP-specific
inhibitor MK-571 on methotrexate distribution in rats
10.3892/etm.2012.627
Garcia-Mayea Y, Mir C, Masson F, Paciucci R, LLeonart
ME. Insights into new mechanisms and models of
cancer stem cell multidrug resistance. Seminars in
Cancer Biology. 2020;60:166-180.
doi:10.1016/j.semcancer.2019.07.022
Harris AL, Hochhauser D. Mechanisms of Multidrug
Resistance in Cancer Treatment. Acta Oncologica.
1992;31(2):205-213.
doi:10.3109/02841869209088904
He S-M, Li R, Kanwar JR, Zhou S-F. Structural and
functional properties of human multidrug resistance
protein 1 (MRP1/ABCC1). Curr Med Chem.
2011;18(3):439-481.
doi:10.2174/092986711794839197
Homolya L, Váradi A, Sarkadi B. Multidrug resistance-
associated proteins: Export pumps for conjugates with
glutathione, glucuronate or sulfate. Biofactors.
2003;17(1-4):103-114. doi:10.1002/biof.5520170111
Hendig D, Langmann T, Kocken S, et al. Gene expression
profiling of ABC transporters in dermal fibroblasts of
pseudoxanthoma elasticum patients identifies new
candidates involved in PXE pathogenesis. Lab Invest.
2008;88(12):1303-1315.
doi:10.1038/labinvest.2008.96
Increased Anti-tumour Efficacy of Doxorubicin when
Combined with Sulindac in a Xenograft Model of an
MRP-1-positive Human Lung Cancer March 2004
Anticancer Research 24(2A):457-64
Johnson ZL, Chen J. Structural Basis of Substrate
Recognition by the Multidrug Resistance Protein
MRP1. Cell. 2017;168(6):1075-1085.e9.
doi:10.1016/j.cell.2017.01.041
Juliano RL, Ling V. A surface glycoprotein modulating
drug permeability in Chinese hamster ovary cell
mutants. Biochimica et Biophysica Acta (BBA) -
Biomembranes. 1976;455(1):152-162.
doi:10.1016/0005-2736(76)90160-7
Kumar A, Jaitak V. Natural products as multidrug
resistance modulators in cancer. European Journal of
Medicinal Chemistry. 2019;176:268-291.
doi:10.1016/j.ejmech.2019.05.027
Kruh GD, Belinsky MG, Gallo JM, Lee K. Physiological
and pharmacological functions of Mrp2, Mrp3 and
Mrp4 as determined from recent studies on gene-
disrupted mice. Cancer Metastasis Rev. 2007;26(1):5-
14. doi:10.1007/s10555-007-9039-1
Li Y-J, Lei Y-H, Yao N, et al. Autophagy and multidrug
resistance in cancer. Chin J Cancer. 2017;36.
doi:10.1186/s40880-017-0219-2
Liu X. ABC Family Transporters. In: Liu X, Pan G, eds.
Drug Transporters in Drug Disposition, Effects and
Toxicity. Advances in Experimental Medicine and
Biology. Springer; 2019:13-100. doi:10.1007/978-981-
13-7647-4_2
Marchi E, O’Connor OA. Safety and efficacy of
pralatrexate in the treatment of patients with relapsed or
refractory peripheral T-cell lymphoma. Ther Adv
Hematol. 2012;3(4):227-235.
doi:10.1177/2040620712445330
Mirski SE, Gerlach JH, Cole SP. Multidrug resistance in a
human small cell lung cancer cell line selected in
adriamycin. Cancer Res. 1987;47(10):2594-2598.
Ma S, Hu Y, Wang F, et al. Lapatinib Antagonizes
Multidrug Resistance-Associated Protein 1-Mediated
Multidrug Resistance by Inhibiting Its Transport
Function. Mol Med. 2014;20(1):390-399.
doi:10.2119/molmed.2014.00059
Munoz M, Henderson M, Haber M, Norris M. Role of the
MRP1/ABCC1 multidrug transporter protein in cancer.
IUBMB Life. 2007;59(12):752-757.
doi:10.1080/15216540701736285
Nasr R, Lorendeau D, Khonkarn R, et al. Molecular
analysis of the massive GSH transport mechanism
mediated by the human Multidrug Resistant Protein
1/ABCC1. Scientific Reports. 2020;10(1):7616.
doi:10.1038/s41598-020-64400-x
Novel Nonsymmetrical 1,4-Dihydropyridines as Inhibitors
of Nonsymmetrical MRP-Efflux Pumps for Anticancer
Therapy July 2020 Pharmaceuticals 13(7):146
DOI:10.3390/ph13070146
Nussbaumer S, Bonnabry P, Veuthey J-L, Fleury-
Souverain S. Analysis of anticancer drugs: A review.
Talanta. 2011;85(5):2265-2289.
doi:10.1016/j.talanta.2011.08.034
Oostendorp RL, Beijnen JH, Schellens JHM. The
biological and clinical role of drug transporters at the
intestinal barrier. Cancer Treatment Reviews.
2009;35(2):137-147. doi:10.1016/j.ctrv.2008.09.004
Pérez-Pineda SI, Baylón-Pacheco L, Espíritu-Gordillo P,
Tsutsumi V, Rosales-Encina JL. Effect of bile acids on
the expression of MRP3 and MRP4: An In vitro study
in HepG2 cell line. Annals of Hepatology.
2021;24:100325. doi:10.1016/j.aohep.2021.100325
Russel FGM, Koenderink JB, Masereeuw R. Multidrug
resistance protein 4 (MRP4/ABCC4): a versatile efflux
transporter for drugs and signalling molecules. Trends
Pharmacol Sci. 2008;29(4):200-207.
doi:10.1016/j.tips.2008.01.006
Sinha D, Duijf PHG, Khanna KK. Mitotic slippage: an old
tale with a new twist. Cell Cycle. 2019;18(1):7-15.
doi:10.1080/15384101.2018.1559557
The therapeutic potential of targeting ABC transporters to
combat multi-drug resistance: Expert Opinion on
Therapeutic Targets: Vol 21, No 5. Accessed May 19,
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
678

2021.
https://www.tandfonline.com/doi/full/10.1080/147282
22.2017.1310841?scroll=top&needAccess=true
What Is Cancer? - National Cancer Institute. Published
September 17, 2007. Accessed May 17, 2021.
https://www.cancer.gov/about-
cancer/understanding/what-is-cancer
Wen X, Joy MS, Aleksunes LM. In Vitro Transport
Activity and Trafficking of MRP2/ABCC2
Polymorphic Variants. Pharm Res. 2017;34(8):1637-
1647. doi:10.1007/s11095-017-2160-0
Wei N, Sun H, Liu G. [Advances in the targeting ATP-
binding cassette transporters to overcome tumor multi-
drug resistance]. Yao Xue Xue Bao. 2010;45(10):1205-
1211.
Zhang Y-K, Wang Y-J, Gupta P, Chen Z-S. Multidrug
Resistance Proteins (MRPs) and Cancer Therapy.
AAPS J. 2015;17(4):802-812. doi:10.1208/s12248-
015-9757-1
Zhou S-F, Wang L-L, Di YM, et al. Substrates and
inhibitors of human multidrug resistance associated
proteins and the implications in drug development.
Curr Med Chem. 2008;15(20):1981-2039.
doi:10.2174/092986708785132870
Dissection of the Role of Multidrug Resistance Protein in Cancer
679
