A Proposed Total Synthesis of Sesquiterpenoids from
Chrysanthemum indicum
Weng Xiujie
1,*
, Tsz Laam Kiang
2
and Wufei Ji
3
1
Guangdong Technion-Israel Institute of Technology, Shantou Guangdong, China
2
Shanghai American School Puxi, Shanghai, 201107, China
3
Shenzhen College of International Education, Shenzhen Guangdong, 518043, China
Keywords: Retrosynthesis, Sesquiterpenes.
Abstract: The proposed retrosynthesis of a guaianolide-type sesquiterpenoid. The first route begins with the
construction of a 5,7,5-ring sesquiterpenoid through the connection α, β-cyclopentenone derivative from
Pauson-Khand reaction and trans lactone by a special Barbier reagent. Firstly, angelic acid is used to complete
the synthesis via esterification with enclosing central ring sesquiterpenoid. The second route starts with the
fabrication of a modified trans configured lactone with methylene. A following Pauson-Khand reaction
appends α, β-cyclopentenone on the lactone. Finally, angelic acid completes the synthesis by esterification
with exterior hydroxy of sesquiterpenoid.
1 INTRODUCTION
Chrysanthemum indicum is a genus of flowers that
belongs to the Asteraceae family. The dried flower
heads of Chrysanthemum indicum have been used for
tea preparations and have also been used in traditional
Chinese and Korean medicine for the treatment of
fever, migraine, eye irritation, hypertension, vertigo,
and respiratory diseases (Youssef et al. 2020, Kim et
al 2021). Over 190 isolated chemical constituents
have been identified from the Chrysanthemum
indicum plant to date, including phenylpropanoids,
terpenoids, flavonoids, and phenolic acids. Various
extracts and monomeric compounds from
Chrysanthemum indicum have different
pharmacological characteristics, such as having anti-
inflammatory, anti-oxidation, antipathogenic,
anticancer, immune regulation, and hepatoprotective
effects (Shao, Sun, Li, Chen 2020).
New compounds recently isolated from
Chrysanthemum indicum include three guaianolide
lactones and four 9-oxonerolidol glucosides. The
target molecule, compound 1, is a guaianolide-type
sesquiterpenoid isolated from Chrysanthemum
indicum flowers. (Kim et al 2021) Compound 1 may
provide some pharmacological value, as its molecular
structure is similar to the tumor inhibitors eupatorin
acetate and eupachlorin acetate, which are found in
Eupatorium rotundifolium. (Kupchan, Kelsey,
Cassady 1968)
In this paper, two detailed retrosynthetic
strategies and proposed synthetic routes of producing
the target molecule are presented
2 THE ANALYSIS OF
SESQUITERPENOIDS FROM
CHRYSANTHEMUM INDICUM
2.1 Sesquiterpenoids from
Chrysanthemum Indicum
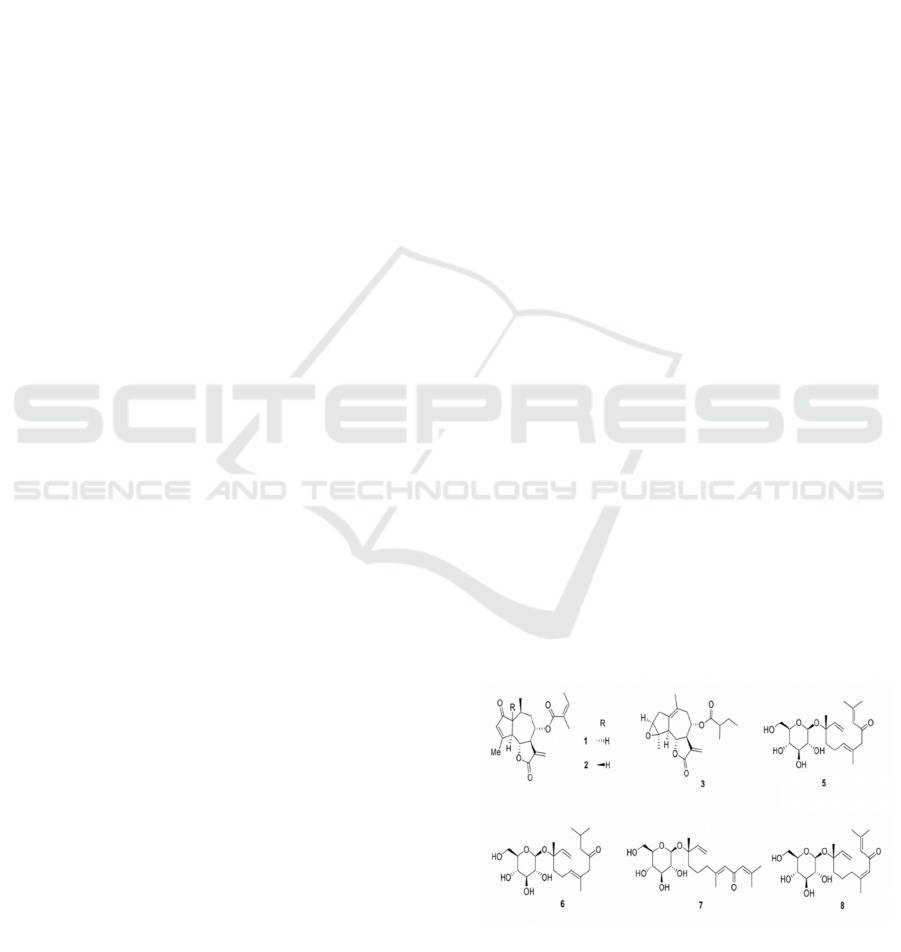
Scheme 1: Three new guaianolide lactones (1−3) and four
new 9-oxonerolidol glucosides (5−8) isolated from the
flowers of Chrysanthemum indicum.
666
Xiujie, W., Kiang, T. and Ji, W.
A Proposed Total Synthesis of Sesquiterpenoids from Chrysanthemum indicum.
DOI: 10.5220/0011253200003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 666-670
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
Compound 1 features six contiguous stereocenters,
four of which are situated on common atoms
connecting two rings. A feature of critical importance
is the stereochemistry surrounding the lactone ring in
the synthetic design. Specifically, the 5,7-fused
lactone being trans-configured poses difficulties.
Furthermore, the α, β-cyclopentenone structure is
also synthetically challenging.
2.2 Synthetic Difficulties of Compound
1
Scheme 2: Molecular formula of Compound 1.
3 SYNTHETIC ROUTE OF
COMPOUND 1
3.1 Synthetic Route 1 of Compound 1
3.1.1 Retrosynthetic Strategy for Synthetic
Route 1.
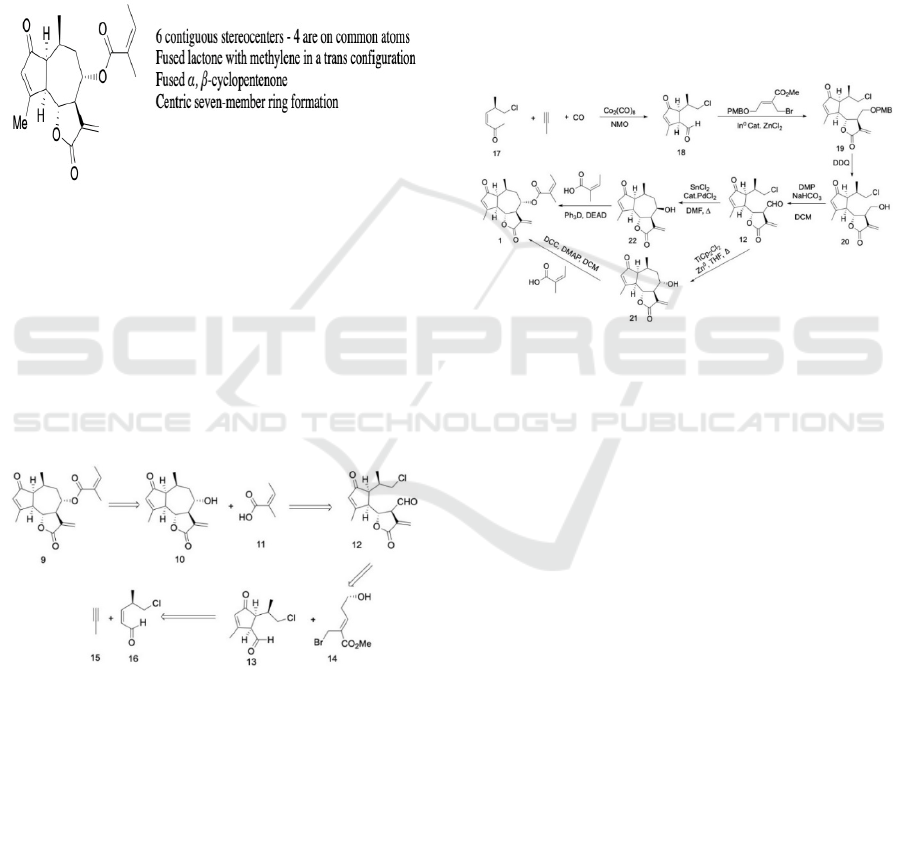
Scheme 3: Retrosynthetic Strategy for Synthetic route 1.
After evaluating the structure of compound 1, the first
retrosynthetic analysis was proposed. The target
molecule can be divided into three major fragments,
the eastern angelic acid, the western α, β-
cyclopentenone structure, and the southern trans
configured lactone. Disconnecting the 5,7,5-ring
system and the angelic acid eliminates the possibility
of the eastern angelic acid side structure interfering
with other synthesis reactions. The hydroxyl group on
the seven-membered ring is a good cleavage point
from a retrosynthetic perspective as it provides a
convenient separation of the α, β-cyclopentenone
structure and the southern trans configured lactone.
The construction of southern trans configured lactone
relies on the nucleophilic attack of the Barbier
reagent 14 to the aldehyde of the α, β-
cyclopentenone, leading to transesterification. The
stereostructure of the lactone is also a synthetic
challenge. The construction of the α, β-
cyclopentenone 13 with exterior 2-chloro-isopropyl
chain could be achieved through a PK reaction of
designed alkynes and alkenes. However, since the
reaction does not possess chiral selectivity, it may
lead to the formation of impurities.
3.1.2 Synthetic Route 1
Scheme 4: Synthetic route 1.
The first proposed synthetic route begins at
compound 17, an α, β-cyclopentenone 18 decorated
with various groups that is derived from the Pauson-
Khand (PK) reaction between 17 and propyne.
Theoretically, compound 14 is one of the derived
isomers formed from the reaction. Since the PK
reaction does not possess chiral selectivity, the
formation of 18 may be in a low yield. Noteworthily,
the Nazarov Cyclization reaction of specific modified
divinyl ketones constructs 18, which reduces the
byproducts resulting from the PK reaction.
Compound 19 can be merged with allylic bromide
under In
0
-mediated allylation conditions to produce
lactone 20 in good yield and with the correct trans
configuration in the major diastereomer. (Hu,
Musacchio, Shen, Tao, Maimone, 2019)
The following steps focus on correcting the
different attached functional groups and closing the
centric seven-member ring. DDQ (2,3-dichloro-5,6-
dicyanobenzoquinone) hydrolyzes the PMB (4-
methoxybenzyl ester) protecting group in compound
19, and compound 20 is formed with a free alcohol
group. A Dess-Martin periodinane oxidation is
applied for aldehyde generation in compound 12. To
close the centric seven-member ring and form
compound 21, zinc and titanocene (III) complexes are
A Proposed Total Synthesis of Sesquiterpenoids from Chrysanthemum indicum
667
used as catalysts, which allows the allylic chloride
group in compound 12 to act as a nucleophilic zinc
reagent that adds to the aldehyde in a Barbier
reaction. (Estévez et al. 2009) This reaction mitigates
potential compatibility issues that may have been
present if a Grignard reagent had been used instead,
as a Barbier reaction can work with a reactive group.
The resulting compound, compound 21, is the core
framework of the target molecule. Finally, Steglich
esterification between angelic acid and compound 21
completes the formation of the target molecule.
An alternate proposed sub-route for forming the
target molecule after compound 12 is created
employs exogenous transition-metals in combination
with SnCl
2
as catalysts, and the addition of catalytic
quantities of PdCl
2
, which can close the centric ring.
This produces 22 in high yield, but gives the incorrect
stereochemical outcome at the hydroxyl group, which
contrasts the correct outcome in 21. (Hu, Musacchio,
Shen, Tao, Maimone, 2019)
However, the incorrect stereo configuration on
the hydroxyl group of 22 can be amended by the
Mitsunobu reaction to give the target molecule, 1.
3.2 Synthetic Route 1 of Compound 1
3.2.1 Retrosynthetic Strategy for Synthetic
Route 2
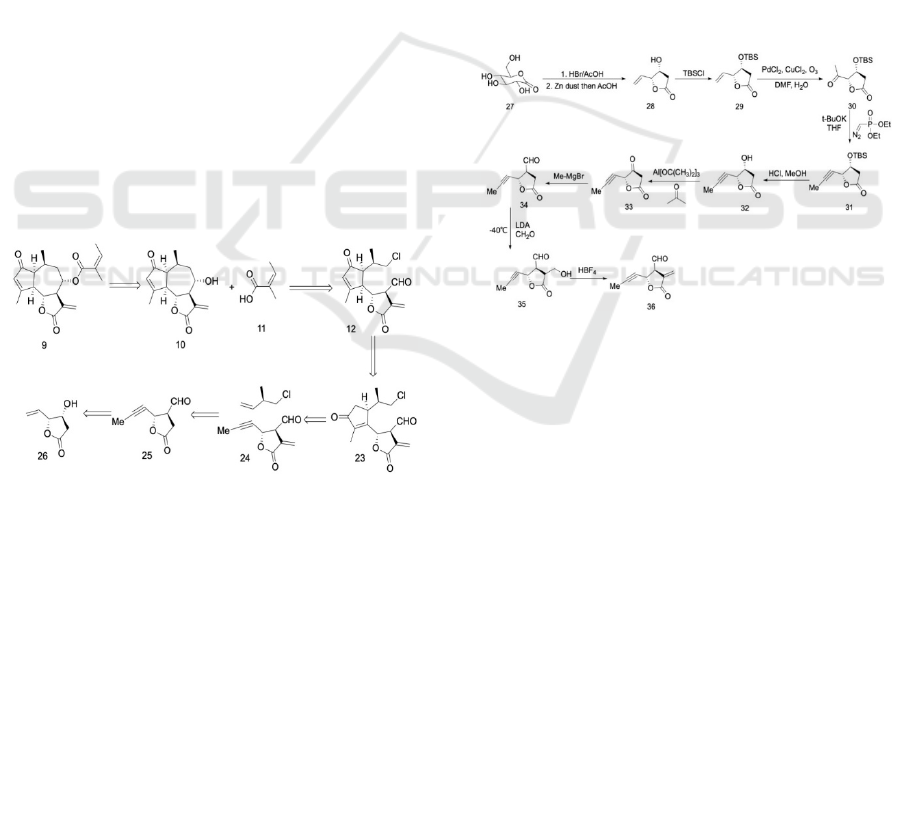
Scheme 5: Retrosynthetic Strategy for Synthetic route 2.
A second viable retrosynthetic analysis was also
proposed. In this retrosynthetic route, the target
molecule is disconnected into two major fragments,
the eastern angelic acid, and the 5,7,5-ring system.
The fused centric seven-member ring is cleaved due
to the presence of a hydroxyl group. The fabrication
of the α, β-cyclopentenone is a challenge in the
synthetic design. This could be resolved by a PK
reaction of alkyne 24 and an alkene with chlorine,
which then modifies the α, β-cyclopentenone.
Consequently, the structure of the core framework,
the 5,7,5-ring system, is formed. The unusual
structure of compound 24 requires further synthetic
consideration. The structure of compound 24 is
similar to the β-hydroxy-γ-vinyl-γ-lactone 26, which
is a common building block in natural products.
Hence, a retrosynthetic path connecting compound 24
and the β-hydroxy-γ-vinyl-γ-lactone 31 can be
formed. Due to the high reactivity of the exterior
vinyl bond at the α- position on the lactone, the
modification of α-position will be at a later stage. To
obtain 25, the vinyl bond in β-hydroxy-γ-vinyl-γ-
lactone 26 requires a chain extension and
transformation of the vinyl to an alkyl. Furthermore,
its hydroxyl also requires chain prolongation and
further functionalization. Compound 26 has a
structure similar to many natural products, which
results in multiple potential synthetic strategies. The
synthetic strategy for creating D-glucono-δ-lactone
was chosen. (Song, Hollingsworth 2001)
3.2.2 Synthetic Route 2. Preparation of the
Modified Stereo Lactone 36
Scheme 6: Synthetic route 2: Preparation of the modified
stereo lactone 36.
The second synthetic proposal begins with the
construction of compound 27, which undergoes a
Pauson-Khand reaction. Starting from D-glucono-δ-
lactone 27, chiral pool material is used to complete
the synthesis of the β-hydroxy-γ-vinyl-γ-lactone
enantiomers 28, which is further used to synthesize
compound 36. The synthetic route then treats 27 with
30% HBr in AcOH (acetic acid) at 60 ℃. This is
followed by a reaction with Zn dust and 50% aq.
AcOH (acetic acid) at room temperature and then at
reflux, producing compound 28 in 58% over yield.
(Song, Hollingsworth 2001) The free alcohol is then
protected with the TBS (tert-butyldimethylsilyl)
group. The homologation of lactone 29 is carried out
through the Wacker Oxidation of the terminal vinyl
bond to aldehyde, to produce compound 30.
Following this, the Seyferth-Gilbert alkyne formation
is used to generate molecule 31. (Fernandes 2020)
Removal of the TBS (tert-butyldimethylsilyl)
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
668
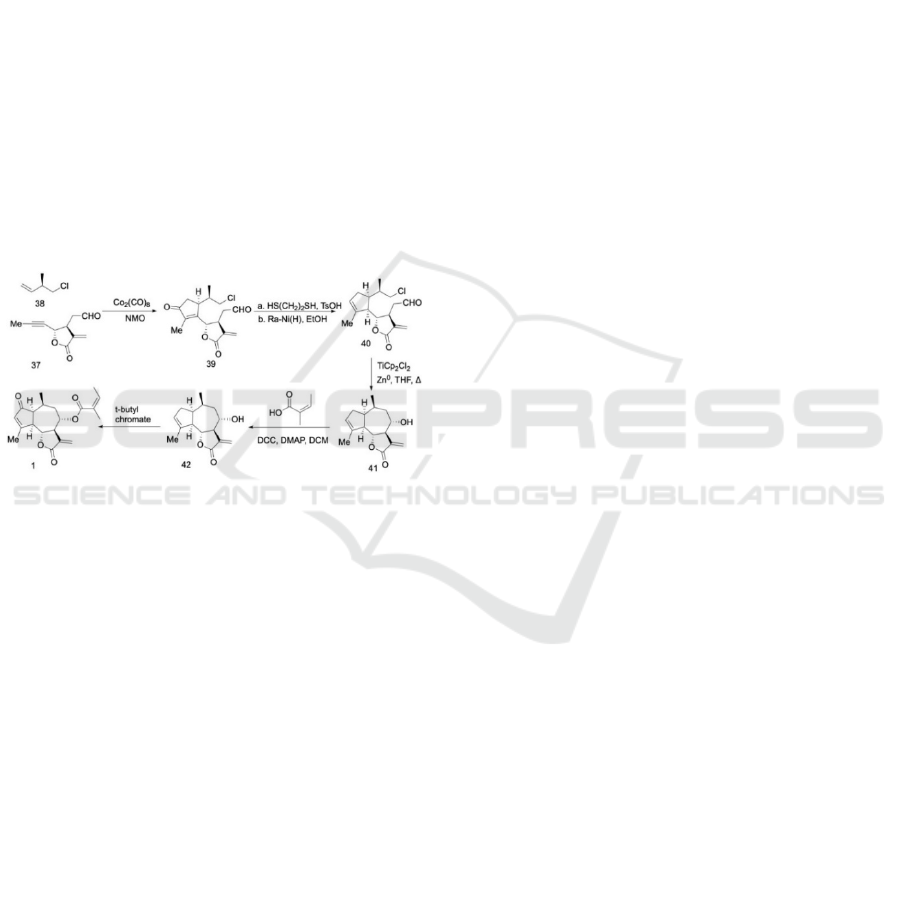
protection group is driven by hydrochloric acid in
MeOH. Due to the presence of an unsaturated bond,
Oppenauer oxidation is utilized to convert the alcohol
into ketone 33, in preparation for the elongation of the
side chain on the lactone in the next step. The ketone
responds to the Grignard reagent and participates in
C-C coupling reactions, which lengthens the size of
the chains surrounding the lactone, forming
compound 34. The active α-position of lactone is
available for hydroxylation by LDA (Lithium
diisopropylamide) in methanal under a controlled -40
℃ temperature, resulting in 35 in 92% yield.
(Baitinger, Mayer, Trauner 2010) In the presence of
fluoroboric acid, vinyl group formation becomes
possible via the dehydration of the hydroxyl group.
The carbon radical reactivity is inhibited by the non-
coordinating anion, thus producing 36.
3.2.3 Synthetic Route 2. Completion of the
Synthesis of Compound 1
Scheme 7: Synthetic route 2: Completion of the synthesis
of compound 1.
With compound 37 in place, the α, β-cyclopentenone
39 is derived from alkyne 37 and alkene 38, and
undergoes a PK reaction using dicobalt octacarbonyl.
Theoretically, there could be four products of the
reaction, 39 is one of them. To eliminate the ketone
on cyclopentene, treatment of 39 with dithiol in
TsOH (p-Toluenesulfonic acid) is followed by Raney
Nickel in EtOH, which leads to alkenyl shift due to
the conjugation effect, yielding compound 40. A Ti-
catalyzed Barbier-Type allylation generates centric
seven-member ring closure, thus producing the 5,7,5
fused ring system 41 with a hydroxyl group of the
desirable configuration for further esterification.
(Estévez et al. 2009) Angelic acid attaches to the 5,7,5
fused ring system via straightforward DCC-coupling
(DDC: N, N′-Dicyclohexylcarbodiimide) and gives
42. Compound 1, the target molecule, is then formed
via carbonylation on cyclopentenone by t-butyl
chromate. (Dodson 1955)
4 CONCLUSIONS
The compound 1 which isolated from the
Chrysanthemum indicum may exists some
pharmacological value, for its molecular structure is
similar to the tumor inhibitors eupatorin acetate and
eupachlorin acetate, which are found in Eupatorium
rotundifolium. In this paper, two retrosynthetic
strategies and proposed synthetic routes are proposed.
The target molecule, compound 1, is comprised of
three parts: an α, β-cyclopentenone structure, a trans
configured lactone, and an angelic acid structure. By
synthesizing each component and assembling them,
the target molecule can be obtained. Future efforts to
supplement the theoretical synthesis of
Sesquiterpenoids from Chrysanthemum Indicum
could focus on further researching the relevant
stereochemistry. An emphasis could be placed on
fine-tuning the stereochemical challenges of the
proposed synthetic routes.
AUTHOR
CONTRIBUTIONS
X.W., T. K., and W.J. evaluated the construction of
the lactone, researched details regarding the
characteristics of the target molecule, and
participated in the discussion. T. K and W.J.
researched the background of the target molecule.
X.W. designed the major parts of route 1. T. K. and
W.J. helped in the completion of route 1. X.W.
designed route 2 and undertook the writing work of
route 2. All authors worked on the retrosynthesis
strategy and wrote the paper. All authors read and
agreed on the content of the paper.
REFERENCES
Baitinger, I., Mayer, P. & Trauner, D. Toward the total
synthesis of maoecrystal V: Establishment of
contiguous quaternary stereocenters. Org. Lett. 12,
5656–5659 (2010).
Dodson, R. M. The Synthesis and Stereochemistry of. 29,
1142–1148 (1955).
Estévez, R. E. et al. Ti-catalyzed Barbier-type allylations
and related reactions. Chem. - A Eur. J. 15, 2774–2791
(2009).
Fernandes, R. A. The Potential of β-Hydroxy-γ-vinyl-γ-
lactone in the Synthesis of Natural Products and
Beyond. European J. Org. Chem. 2020, 634–645
(2020).
Hu, X., Musacchio, A. J., Shen, X., Tao, Y. & Maimone, T.
J. Allylative approaches to the synthesis of complex
guaianolide sesquiterpenes from apiaceae and
A Proposed Total Synthesis of Sesquiterpenoids from Chrysanthemum indicum
669

asteraceae. J. Am. Chem. Soc. 141, 14904–14915
(2019).
Kim, J. G. et al. Sesquiterpenoids from Chrysanthemum
indicum with Inhibitory Effects on NO Production . J.
Nat. Prod. (2021) doi:10.1021/acs.jnatprod.0c01121.
Kupchan, S. M., Kelsey, J. E. & Cassady, J. M. (Received
in USA 11 July 1968; reoeived in UK for publication
24. 2, 5913–5918 (1968).
Shao, Y., Sun, Y., Li, D. & Chen, Y. Chrysanthemum
indicum L.: A Comprehensive Review of its Botany,
Phytochemistry and Pharmacology. Am. J. Chin. Med.
48, 871–897 (2020).
Song, J. & Hollingsworth, R. I. Homochiral 4-hydroxy-5-
hexenoic acids and their derivatives and homologues
from carbohydrates. Tetrahedron Asymmetry 12, 387–
391 (2001).
Youssef, F. S. et al. Chrysanthemum indicum and
chrysanthemum morifolium: Chemical composition of
their essential oils and their potential use as natural
preservatives with antimicrobial and antioxidant
activities. Foods 9, (2020).
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
670
