
The Investigation of P7 Peptide Delivery Targeting Cdc24 in
Ras-driven Pancreatic Cancer by L-fucose-bound Liposome
Yurui Yang
1,+
, Hongbin Lan
2,+
, Huawei Zhu
3,*
and Yiran Zhu
4
1
Department of Pharmacology and Toxicology, University of Toronto, Toronto, Ontario M5S 2E8, Canada
2
School of Pharmacy, University College London, London, WC1E 6BT, U.K.
3
College of life science, South China Agricultural University, Guangzhou, Guangdong, 510642, China
4
RDFZ Xishan High School, Beijing, 100193, China
+
These authors should be regarded as co-first authors because they contributed to the work equally
Keywords: Cell Division Control Protein 42, Ras-driven Pancreatic Cancer, Liposome, Reduced Cytotoxicity.
Abstract: Pancreatic adenocarcinoma is one of the most common leading causes of cancer deaths with an increasing
incidence in the developed world. Most pancreatic cancers are induced by Ras protein abnormality. Since Ras
is involved in many important cellular functions, targeting Ras alone is difficult with little progression.
Currently, the only surgical resection offers the potential cure to this disease. However, among those who
have the chance to receive surgery, most of them suffer from recurrence within a year. The previous study
has developed novel peptides targeting Cell division control protein 42 (Cdc42), which is a type of Rho family
small GTPase activated by Ras and achieved great success in inhibition of tumor cell growth. Since Cdc42 is
also expressed in normal cells, a suitable drug delivery system is required for targeted therapy. This study
investigates the cytotoxic and cell penetration effects of L-fucose-bound liposome-P7 targeting pancreatic
cancer cells, in both in vitro and in vivo conditions. The experiments will use know human pancreatic cancer
cell lines, human pancreatic epithelial cell lines, and Xenograft Murine Models. The concentration of P7 in
each L-fucose-bound liposome will be assessed through half-log dilution. Cell proliferation and cytotoxicity
are measured through colony formation assay, MTT assay, and Annexin V/ propidium iodide (PI) assay. The
cell penetration effect will be reflected by fluorescence microscopy. There are three most possible results: (1)
L-fucose-bound liposome-P7 inhibits the pancreatic cancer cell proliferation in both in vitro and in vivo cell
lines without causing significant cytotoxicity to normal cells; (2) L-fucose-bound liposome-P7 only inhibits
the cancer cell proliferation in vitro cell cultures without causing significant damage to normal cells; (3) L-
fucose-bound liposome-P7 inhibits tumor cell proliferation in both normal pancreatic epithelial cells and
cancer cells. The result of our study will provide important information for deciding whether to continue P7
peptide development in clinical trials. Future studies should focus on improving the drug delivery system and
investigating P7 effects on transformed tumor cells.
1 INTRODUCTION
Pancreatic adenocarcinoma, ranked as the fourth
leading cause of cancer deaths in the United States,
has poor outcomes and an increasing incidence in the
developed world (McGuigan, et al, 2018, Patra, et al,
2010). The incidence rates vary between countries.
Generally, the highest incidence rates are detected in
Europe and North America, while the lowest
incidence rates are measured in Africa and South-
central Asia (McGuigan, et al, 2018). This type of
cancer often has a late detection and ineffective
treatment at the advanced stage, which contributes to
a poor 5-year survival rate of 2-9% (McGuigan, et al,
2018). Nowadays, even with advancements in
detection and management, the 5-year survival rate
remains relatively unchanged with negligible
improvements. Currently, the only treatment that
offers a potential cure for pancreatic cancer is
surgical resection. However, due to the late detection
of the disease, only 20% of patients have the chance
to receive surgery, and most of them may suffer from
disease recurrence within a year (Zeng, et al, 2019).
The addition of adjuvant chemotherapy has been
shown to improve long-term prognosis in some
patients, while other patients have developed chemo-
Yang, Y., Lan, H., Zhu, H. and Zhu, Y.
The Investigation of P7 Peptide Delivery Targeting Cdc24 in Ras-driven Pancreatic Cancer by L-fucose-bound Liposome.
DOI: 10.5220/0011251700003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 651-660
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
651

resistance with unclear mechanisms, which
significantly limits the effect of these therapeutic
drugs. Therefore, novel drug developments are
necessary (Zeng, et al, 2019).
About 95% of Pancreatic cancer are driven by the
mutations in Ras family genes (McGuigan, et al,
2018, Patra, et al, 2010). However, targeting Ras gene
alone is a hard task since it is involved in many
essential signaling pathways promoting cell
proliferation and differentiation (Arias-Romero,
Chernoff, 2013). Alternatively, targeting the
downstream small GTPase that is activated by Ras is
favorable. Cdc24 is an important downstream
effector of Ras and plays an important role in Ras-
induced transformation, and previous studies have
developed a novel cyclic peptide, P7, targeting Cdc24
with nanomolar affinity. P7 is tested for binding to
the binding surface of Cdc24, preventing it from
interacting with its downstream effectors and thus
inhibits the transformation pathway in Ras-induced
tumorigenesis (Tetley, Murphy, Bonetto et al, 2020).
Although the study showed a promising effect of P7
peptide as a Cdc24 inhibitor in Ras-driven cancers,
the peptide entry strategy has become another
challenge. Cell-penetrating peptide (CPP) was tagged
to the P7 and helped the delivery in the previous
study. However, the results showed a significant
cytotoxic effect with reduced target engagement
(Tetley, Murphy, Bonetto et al, 2020). Therefore, an
alternative drug delivery system with minimal
cytotoxicity and greater efficacy is necessary to be
developed.
Nanoscale drug delivery system using liposomes
is an emerging technology in cancer treatment.
Liposomes are composed of a lipid bilayer that is
enclosed as a hollow sphere with an aqueous phase
inside. Accordingly, it can encapsulate and stabilize
drugs in either aqueous compartments or lipid
bilayers, depending on the properties of drugs. Also,
the similarity of liposomes to the biological
membrane reduces their toxicity and enables the
enhanced permeability and retention (EPR) effects to
tumor tissues (Malam, Loizidou, Seifalian, 2009).
Specifically, most solid tumors have the nature of
vascular abnormalities, like hypervascularization,
aberrant vascular architecture, and a lack of
lymphatic drainage (Malam, Loizidou, Seifalian,
2009). Taking advantage of the adjustability of
nanoparticles' size, nanoscale anticancer drugs
designed ideally in a moderate size are unable to
penetrate through tight endothelial junctions of
normal blood vessels. However, they can selectively
extravasate in the tumor tissue relying on the tumor's
abnormal vascular characteristics, thereby reaching
several fold drug concentrations in the tumor than
that in the normal tissue (Malam, Loizidou, Seifalian,
2009). Therefore, liposome is a good candidate for P7
peptide delivery.
The surface of liposomes can be modified by
taking advantage of characteristics of pancreatic
adenocarcinoma. Since 80% of pancreatic cancer
cells overexpress carbohydrate antigen-19-9 (CA19-
9) and thus recruit large amounts of fucose as an
energy source, the fucose-bound liposome can be
generated for targeted delivery (Papahadjopoulos,
Heath, Bragman, Matthay, 1985, Yoshida, Takimoto,
Murase, et al, 2012). A previous study has applied a
14C-labeled L-fucose binding assay in the pancreatic
cell lines, and the result indicated the presence of
high-affinity L-fucose specific receptors (Yoshida,
Takimoto, Murase, et al, 2012). Furthermore, the
inhibition of endocytosis by chroloquine resulted in a
suppression of drug delivery (Yoshida, Takimoto,
Murase, et al, 2012). These results together supported
that L-fucose-bound liposome enters the pancreatic
cancer cells via receptor-mediated endocytosis
(Yoshida, Takimoto, Murase, et al, 2012).
In order to investigate the drug delivery system
with minimal side effects and better efficacy, a
comparative study should be designed. In the present
study, we asked whether targeting the delivery of P7
with L-fucose-bound liposome can increase the cell
penetration, enhance cytotoxicity to tumor cells and
reduce cytotoxicity to normal cells compared with
CPP-tagged P7 both in in vitro and in vivo conditions.
We chose BxPC-3 and AsPC-1 pancreatic cancer cell
lines, which secreted substantial amounts of CA-19-
9 molecules (Yoshida, Takimoto, Murase, et al,
2012). We hypothesized that treatment of P7 peptide
delivered in L-fucose liposomes to Ras-driven
pancreatic cancer can increase cell penetration,
peptide stability and reduce cytotoxicity to normal
cells. In the present study, we will treat AsPc-1,
BxPC-3, and normal pancreatic epithelial cells
(hTRET-HPNE) in culture or as a mouse xenograft
model with increasing amounts of P7-liposome
assessed by half log dilution series with various
liposome and/or P7 peptide concentrations and
measure cytotoxicity by cell counts, colony formation
assay, MTT assay, Annexin V/PI, and cell
penetration by fluorescently labeled liposomes.
2 METHOD & MATERIAL
This experiment will use two human pancreatic
cancer cell lines (AsPC-1 and BxPC-3), and one well
studied non-cancerous pancreatic cell line (hTRET-
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
652
HPNE) for in vitro studies and mouse xenograft
models for in vivo studies. Mouse xenograft models
include new subcutaneous and orthotopic models
which are generated from previous research and will
be used for the in vivo study (Yoshida, Takimoto,
Murase, et al, 2012). The subcutaneous model will
establish with mice bearing AsPC-1 cell lines, which
the orthotopic model will use mice bearing BxPC-3
cell lines. The mice will be housed under specific
pathogen-free conditions. Animal studies will be
carried out according to the Guide for the Care and
Use of Laboratory Animals of the National Institutes
of Health. All surgery will be performed under
sodium pentobarbital anesthesia, and all efforts will
be made to minimize suffering. P7 peptide and L-
fucose bound liposomes will be made according to
the method used by Tetley et al (Tetley, Murphy,
Bonetto et al, 2020). and Yoshida et al (Yoshida,
Takimoto, Murase, et al, 2012).
2.1 Material
PBS (pH 7.4), Purified P7 peptide, L-fucose-bound
liposome-P7, Crystal violet 0.5% (wt/vol) in H2O,
Glutaraldehyde 6.0% (vol/vol), Trypsin-EDTA
(0.05%), Appropriate culture medium containing
serum, RPMI 1640 (Gibco) plus 10% FBS, L-
glutamine, 1% penicillin-streptomycin, and binding
buffer 10X: 0.1 M HEPES/NaOH, pH 7.4; 1.4 M
NaCl; 2.5 nM CaCl, Class 2B biocabinet, 500 mg
MTT powder, methanol, ethanol, DMS and
acidified isopropanol, Plateshaker, Pipettes 0.001–1
mL, single channel and 0.01–0.3, multichannel, Class
2B hood, Benchtop centrifuge, Microplate reader, O2
incubator, L-fucose-bound liposome-P7, L-fucose-
bound liposome without P7, liposome alone, CPP-P7,
P7 alone and PBS.
2.2 Method
a) Half log dilution
To find out the optimal drug concentration of P7
and L-fucose-P7 liposomes, half-log dilution will be
applied in both in vitro and in vivo studies. The stock
solution of pure P7 without liposome will be prepared
by dissolving P7 peptide in PBS (pH=7.4) in a
concentration of 1mg/ml. Varying concentrations of
P7 solutions will be constructed by diluting the stock
solution with PBS to seven final concentrations at
0.1mg/ml, 0.0316mg/ml, 10 ug/ml, 3.16 ug/ml, 1
ug/ml, 0.316 ug/ml, 0.1 ug/ml. These solutions will
be used to test their cytotoxicity and proliferation
inhibition effect on cell lines. Similarly, the stock
solution of L-fucose-bound liposome-P7, keep the
amount of P7 inside the liposome the same as the pure
P7 group.
Then, L-fucose-bound liposome-P7 stock
solution will be parallelly diluted with P7 group,
resulting in varying concentrations with the inside p7
concentrations of 0.1mg/ml, 0.0316mg/ml, 10 ug/ml,
3.16 ug/ml, 1 ug/ml, 0.316 ug/ml, 0.1 ug/ml, which
will be used to test cytotoxicity and proliferation
inhibition effect on cell lines. The concentration
range of P7 and L-fucose-bound liposome-P7
concentration that achieve the significant inhibitory
effect will be obtained from the in vitro MTT assay.
Subsequently, three doses will be selected to test in
vivo studies for testing treatment effects, determining
the optimal in vivo drug concentration, which will be
used in subsequent experiments.
b) Peptide synthesis and purification
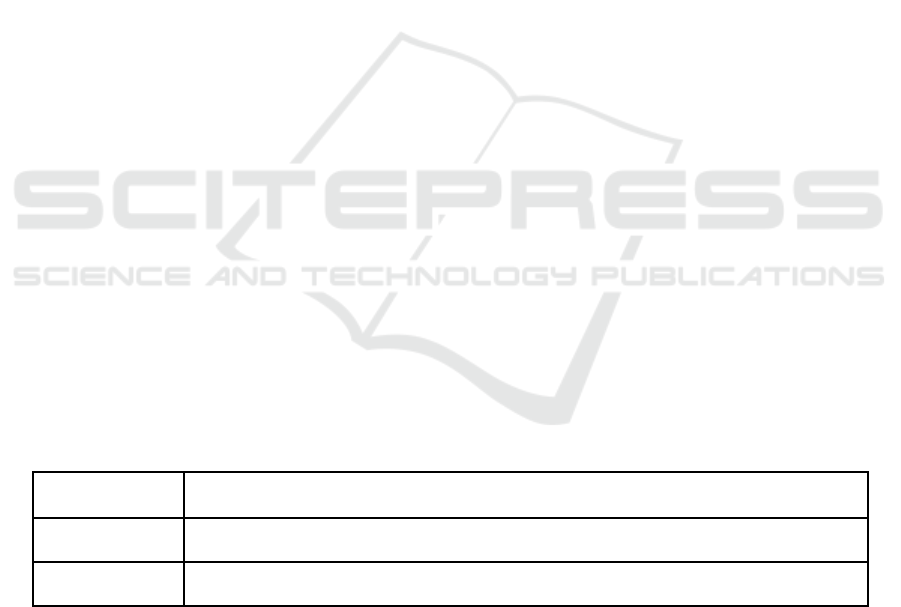
Table 1: The peptide sequence of P7 (Tetley, Murphy, Bonetto et al. 2020).
Peptide Sequence
P7 P S I C H V H R P D W P C W Y R
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16
Table 1 shows the sequence of P7 peptide.
Synthesis and purification of the target peptide use
the method from Tetley et al (Tetley, Murphy,
Bonetto et al, 2020). Standard Fmoc solid-phase
chemistry is used to synthesize a 10μmol scale
peptide with an amidated C terminus on the
automated peptide synthesizer. After the deprotection
of Fmoc, the N terminus will be acetylated and
biotinylated as described in Tetley et al (Tetley,
Murphy, Bonetto et al, 2020). Subsequently, the resin
is washed with methanol and dichloromethane, then
dried overnight. 5% water, 5% phenol, 5%
thioanisole, 2.5% ethanedithiol in TFA is used to
deprotect and cleave the peptide from the resin for 3
h. Then, the peptide will precipitate in diethyl ether at
-20 ℃. A C18 column in reverse-phase HPLC is used
The Investigation of P7 Peptide Delivery Targeting Cdc24 in Ras-driven Pancreatic Cancer by L-fucose-bound Liposome
653

to purify the peptide with a linear gradient elution of
20 to 50% acetonitrile in 0.1% TFA. Then, the
determination of molecular masses uses a mass
spectrometry.
c) Preparation of CPP-tagged P7
The addition of CPP, which is a C-terminal Nona-
arginine motif (9R), is the same as the motif used in
Tetley et al (Tetley, Murphy, Bonetto et al. 2020).
Carboxyfluorescein (FAM) will be linked to the N-
terminal of the P7 peptide. Named the product
without FAM and with FAM as CPP-tagged-P7 and
CPP-tagged-P7-FAM, respectively.
d) Preparation of P7 encapsulated in liposomes
Preparation of L-fucose bound liposomes has
been described previously (Tetley, Murphy, Bonetto
et al. 2020). Generally, DPPC, Chol, ganglioside,
DCP, and DPPE will be mixed at different molar
ratios, and cholic acid will be added for micelle
formation. The mixture will be dissolved in
methanol/chloroform (1:1, v/v), and the solvent will
be evaporated at 37°C to produce a lipid film, which
will be dried under vacuum. For the P7 peptide
preparation, P7 peptide containing solution will be
added to the lipid film and sonicated to obtain
uniform micelles in the buffer, which will then be
ultrafiltered. Hydrophilization treatment and L-
fucose conjugation on the surface of liposomes will
be carried out by methods modified from Yamazaki
et al (Yamazaki, Kodama, Gabius, 1994). Aminated
L-fucose will be conjugated to the liposome surface
using DTSSP, which is a type of cross-linking agent.
e) In vitro cell culture
The pancreatic cell lines AsPC-1 and BxPC-3 will
be cultured in RPMI 1640 (Gibco) plus 10% FBS, L-
glutamine, and 1% penicillin-streptomycin
(Yamazaki, Kodama, Gabius 1994). Non-cancerous
pancreatic cell line (hTRET-HPNE) will be cultured
in DMEM (Gibco) with 10% FBS, 5% L-glutamine,
and 1% penicillin-streptomycin. All cell lines will be
kept in a 5% CO2 humidified atmosphere at 37 °C.
f) Colony formation assay
In our study, we use cell colony formation assay
to measure the ability of a cell to divide and form a
colony after delivering P7 peptide delivered in l-
fucose liposomes to Ras-driven pancreatic cancer.
We will first prepare 6-well plates and harvest
exponentially growing cells. Then, we replate 50 cells
per dish, waiting for 2 h at 37 Degree Celsius.
Followed by incubation in the incubator until cells in
control dishes have formed sufficiently large clones.
Harvest cells from a donor culture using
trypsinization, which makes the cells completely
detach from the flask and float in the medium,
allowing us to see the cells more clearly. When the
cells start to detach from the culture dishes, resuspend
the cells in medium to inhibit trypsinization. Then,
neutralize the trypsin solution and count the cells.
Dilute the cell suspension and seed into flasks.
Remove the medium and rinse the cell by PBS. After
PBS wash, 2-3 ml of a mixture of 6.0%
glutaraldehyde and 0.5% crystal violet will be added.
Leave this for at least 30 min. Finally, remove the
glutaraldehyde crystal violet mixture and dry at room
temperature. After this, we count the colony
formation of cells in different plates that with the
different treatments. We will use Equal. 1 to calculate
the surviving fraction of cells.
𝐏𝐄 =
𝐍𝐮𝐦𝐛𝐞𝐫 𝐨𝐟 𝐜𝐨𝐥𝐨𝐧𝐢𝐞𝐬 𝐜𝐨𝐮𝐧𝐭𝐞𝐝
𝐍𝐮𝐦𝐛𝐞𝐫 𝐨𝐟 𝐜𝐞𝐥𝐥𝐬 𝐩𝐥𝐚𝐭𝐞𝐝
× 𝟏𝟎𝟎 Equal.
1(Franken, Rodermond, Stap, Haveman, Van-Bree
2006)
g) MTT assay
In this study, we will use MTT assay to
investigate the killing effect of targeted drugs (L-
fucose-bound liposome-P7) on pancreatic cancer
cells and drug sensitivity in established cell lines.
Subsequently, MTT assay is also used to determine
drug concentration that is required to achieve 50%
growth inhibition as compared to the growth of the
untreated control (50% inhibitory concentration,
IC50). Briefly, set up bottom plates (120 mL) to be
filled with PBS. If the drug is unstable, the drug
diluent will be freshly prepared before adding cells,
and 20 ml of total drug and 30 ml of drug solution
must be added to the plate. If testing a stable drug,
plates can be prepared with 30 mL drug
concentrations and can be stored at −20°C for later
use. Cultured pancreatic cell lines for 4 days to
determine the optimal effect for most standard drugs.
After the appropriate incubation time, add 1:10
volume of MTT solution (5 mg/mL), Shake plates for
5 min on a plate shaker by slowly increasing the
shaking speed to a maximum of 900 shakes/min, then
incubate the plate for another 4–6 h at 37°C in a CO
2
incubator. 150 mL of acidified isopropanol is added
to each well, mixing the rows with drugs. The cell
lines were divided into six groups and mixed with
drugs according to the table. 150 mL of acidified
isopropanol is added to each well, mixing the rows
with drugs. The cell lines were divided into six
groups and mixed with drugs according to table 1. In
terms of measurement, the optimal density (OD) is
measured at 540 and 720 nm to get a more exact
measurement. The pancreatic cell survival is
calculated by: (OD treated well [−blank])/(mean OD
control well [−blank])×100. The LC50 (the drug
concentration which results in 50% pancreatic cell
survival) can be calculated.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
654
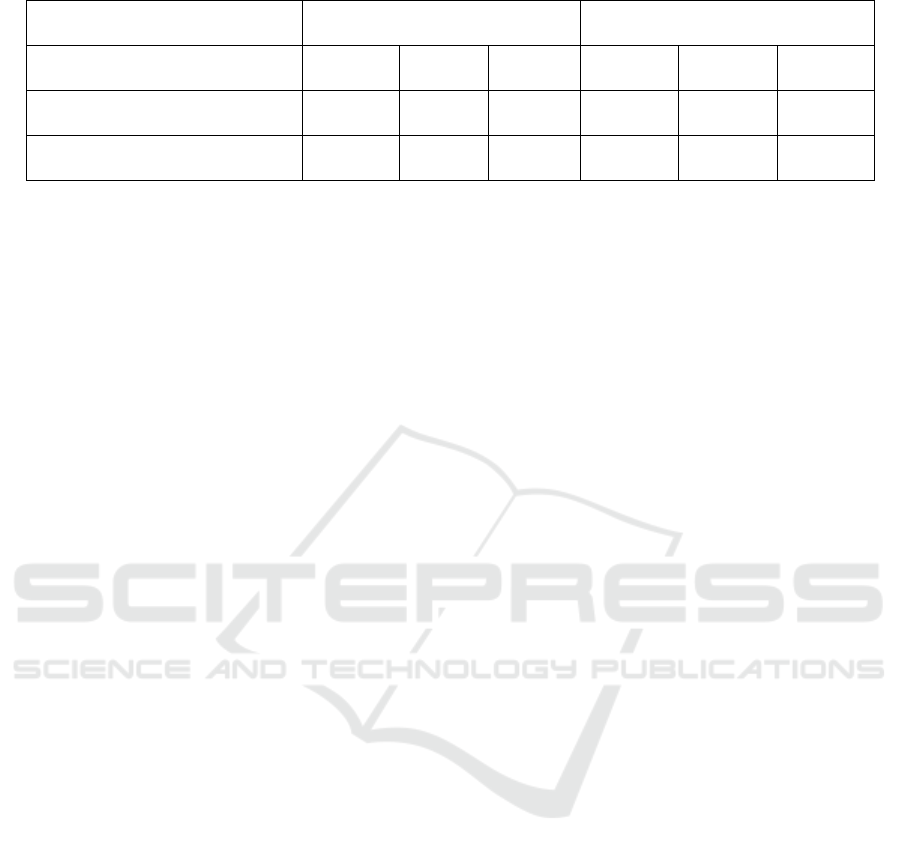
Table 2: Different drugs put in the cell lines (Van-Meerloo, Kaspers, Cloos 2011).
AsPC-1 cell line BxPC-3 cell line
Serial numbe
r
1 2 3 4 5 6
L-fucose-
b
ound liposome - + + - + +
P7 - - + - - +
Note. “+” represents a significant decrease in cell proliferation compared with negative controls
*
. “-” is not
significantly different from negative control.
Table 2 shows the possible results of cell
proliferation of L-fucose-liposome and P7 peptide in
two different cell lines (AsPC-1 and BxPC-3) in MTT
assay.
h) Fluorescence polarization
Cell penetration effect will be detected by
confocal laser microscopy and fluorescence
microscopy. As discussed in previous work (Tetley,
Murphy, Bonetto et al, 2020), cells will be plated in
Lab-Tek chambered coverglasses at 1 X 10
4
cells/chamber (liposome citation). L-fucose-
liposome-P7 or liposome-P7 will be added to cells at
an optimal P7 concentration determined by half log
dilution (predicted around 0.7μg/ml). There will be
six different groups. The experimental group includes
FAM-L-fucose-P7 liposome. The negative control
group includes FAM-P7, FAM-liposome, FAM-L-
fucose liposome, and FAM-L-fucose. Positive
control group consists of FAM-CPP-P7 alone. Each
treatment will be mixed with cancer cell lines and
normal cells. Then each group will receive a beam of
Fluorescence microscope which will be used to detect
the intensity of excited fluorescent probes (Moerke
2009).
If L-fucose-p7 liposome is not bound to the Cdc42
protein, when they are hit by polarized light, the light
is going to be deflected. Since the complex is small,
the angle of rotation will be big, and the light it emits
is unpolarized, which is more diffuse and less intense,
so the degree of deflection will not be strong (Moerke
2009). However, when the L-fucose-P7 liposome
binds to the Cdc42 protein, the complex becomes
more massive. The Cdc42 protein is too large to rotate
freely, so the large complex keeps the light in the
polarized state, hence producing very fluorescent
light. By comparing the fluorescence intensity,
whether the Cdc42 protein binds to the drug can be
known. If the drug binds to Cdc42 protein, very
strong fluorescence can be obtained. On the contrary,
if they are not combined, the fluorescence intensity
will be weak. By comparing the content of
fluorescent agents in cells, the cell penetration of
drugs can be known. For example, the higher the
intensity of fluorescent light in the cell, the higher the
amount of drug entering the cell, so the cell is more
penetrating. However, if the content of fluorescent
agents in cells is relatively low, it indicates that the
drug content of forbidden cells is relatively low, and
the cell penetration is poor.
i) Annexin V/PI assay
In order to assess the cytotoxicity of newly
synthesized L-fucose-liposome-P7 compared with
previously developed CPP-P7, we apply annexin
V/PI assay to determine the fraction of apoptotic and
necrotic cells (Kabakov, Gabai, 2018). We will first
harvest the AsPC-1, BxPC-3 and hTRET-HPNE cell
lines and wash twice in PBS at 4 degree celsius and
resuspend in 1 X buffer. Then aliquot 100 μl cells into
fluorescence-activated cell sorter (FACS) tubes and
add 5 μl FITC-Annexin V and/or 10 μl PI (50 ug/ml
Propidium Iodide 10x). The mixture will incubate for
15 min at room temperature in the dark. Finally, 400
μl binding buffer (Binding buffer 10x : 0.1 M
HEPES/NaOH, pH 7.4; 1.4 M NaCl; 2.5 nM CaCl)
will be added to each tube and the result will be
analyzed by flow cytometry on FACS.
j) Fluorescence microscopy
Cell penetration effect will be detected by
fluorescence microscopy. As discussed in previous
work, cells will be plated in Lab-Tek chambered
cover glasses at 1 X 10
4
cells/chamber (Tetley,
Murphy, Bonetto et al. 2020). L-fucose-liposome-P7-
GFP or liposome-P7-GFP will be added to cells at an
optimal P7 concentration determined by half log
dilution (predicted around 0.7μg/ml). According to
Tetley et al. (Tetley, Murphy, Bonetto et al, 2020),
cells will be cultured in complete medium for 30 min
and then replaced with fresh medium. Cells will be
washed twice with PBS and fixed with 4%
paraformaldehyde 30 min and 2 hours post-treatment
at room temperature for 15 min. Then, the cells will
be washed 3 times using PBS and exposed to DAPI
staining the nuclei. The distribution of P7 peptide can
The Investigation of P7 Peptide Delivery Targeting Cdc24 in Ras-driven Pancreatic Cancer by L-fucose-bound Liposome
655

be assessed by fluorescence microscopy by
comparing the fluorescent intensity.
k) Peptide stability study
Inject P7 and L-fucose-bound liposome-P7 into
mice from the subcutaneous model group and detect
the drug plasma concentration after injection of 1, 2,
4, 8, 12 and 48 hours. Make profiles of drug plasma
concentration with time by HPLC. Compare the drug
plasma concentration curve, the stability of peptide to
the serum protease can be analyzed.
l) Animal model development
The subcutaneous model and orthotopic model
have been previously described (Tetley, Murphy,
Bonetto et al. 2020). Briefly, in the subcutaneous
model, mice aged 4 to 6 weeks will be modified with
AsPC-1 cell line to allow the growth of tumor to 5
mm in diameter. AsPC-1-bearing mice will be treated
with CPP-tagged P7 (2 mg/kg), L-fucose-P7-
Liposome (2 mg/kg), via the tail vein twice a week.
At 4, 8, 11, 15, 18, and 22 days after transplantation,
tumor volumes will be measured by IVIS imaging.
Representative image of mice treated with P7. For D-
mannose pre-treatment in the in vivo experiment for
L-fucose bound liposome, 5 mg of D-mannose will
be injected through the tail vein 5 minutes before
administration of agents (Tetley, Murphy, Bonetto et
al, 2020).
In the orthotopic model, BxPC-3-Luc cells in 100
µl PBS will be orthotopically injected into the
pancreas of nude mice (ages 4 to 6 weeks).
Bioluminescence will be measured on day 0-4 post-
injection, and the mice will be randomly assigned into
different groups (placebo groups, CPP-tagged P7
group and L-fucose bound liposome-P7 group)
before the initiation of treatment. The mice received
injection twice in the first week, and then received
injection once in week 2 and once in week 3. All mice
will be sacrificed on the day after the last injection
but before a final bioluminescence measurement.
m) Statistical Analysis
Results will be presented as means (± SD) for
each sample. All statistical significance of all
numerical data will be analyzed using the student’s
T-Test on GraphPad Prism® at (p <0.05).
3 POSSIBLE RESULTS
Table 3: In Vitro Cell proliferation comparison of L-fucose-liposome-P7 with negative controls on cancer cells and normal
cells.
Result 1 Result 2 Result 3 Result 4 Result 5
C
N
C
N
C
N
C
N
C
N
Colony
formation assay
+ - + _ ++ + + + - -
MTT assay
+ - + - ++ + + + - -
Annexin /PI
assay
A L A L D D A A L L
Note. In table 3, “C” represents tests in cancer cells group and “N” means in normal cells. “+” represents a
significant decrease in cell proliferation compared with negative controls*. “-” is not significantly different from
negative controls. Negative controls include L-fucose-liposome, Liposome without L-fucose and L-fucose. “A”
means apoptotic cell population is significantly larger than live and necrotic cells, while “L” represents live cells
population is larger than apoptotic and necrotic cells and “D” represents population of necrosis dead cells is
higher than both apoptotic and necrotic cells.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
656
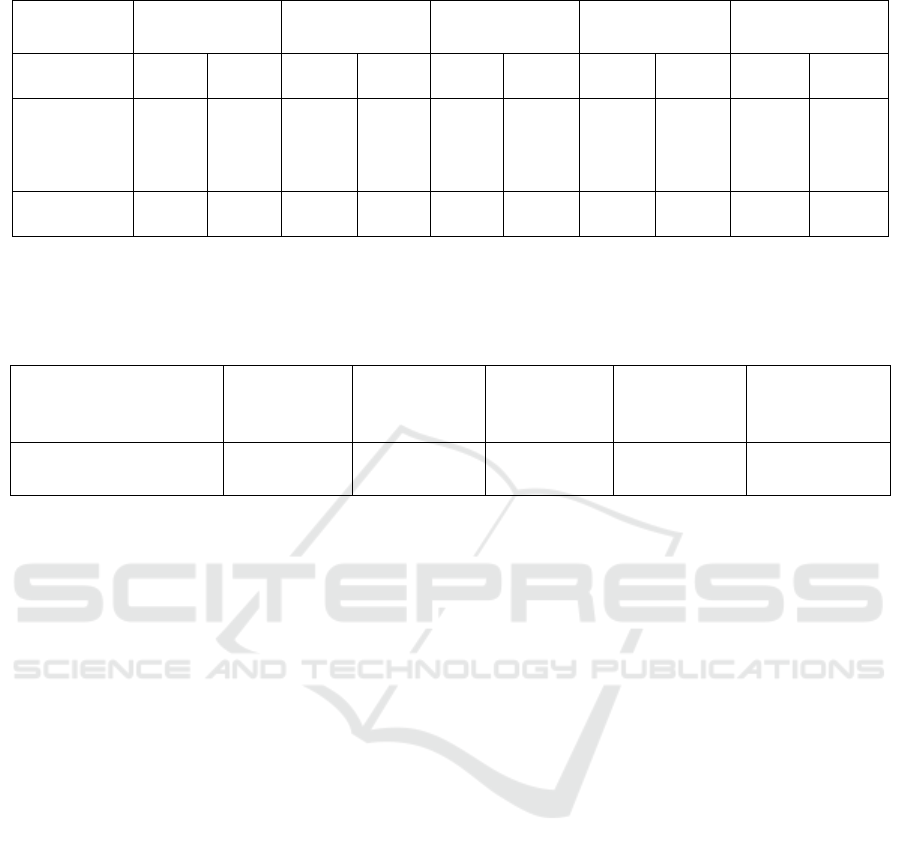
Table 4: Cell proliferation inhibition comparison of L-fucose-liposome-P7 with P7 and CPP-P7.
Result 1 Result 2 Result 3 Result 4 Result 5
P7 Cpp-P7 P7 Cpp-P7 P7 Cpp-P7 P7 Cpp-P7 P7 Cpp-P7
Colony
formation
assay
+ ++ - + ++ + - + - -
MTT assay + ++ - + ++ + - + - -
Note. In table 4, “+” represents a significant higher cell proliferation inhibition effect of L-fucose-liposome-P7
in cancer cell lines compared with that of p7 and cpp-p7, respectively. “-” indicates that no significant greater
proliferation inhibition effect of L-fucose-liposome-P7 compared with p7 or cpp-p7, respectively.
Table 5: Possible Results on Cell penetration of L-fucose-liposome-P7 in vitro.
Cell penetration by L-
fucose-liposome-P7 in vitro
Result 1 Result 2 Result 3 Result 4 Result 5
Fluorescence microscopy + + + - +
Note. In table 5, “+" represents the fluorescence intensity inside the cancer cells is significantly higher than that
outside. “-” indicates that fluorescence intensity inside cancer cells is not significantly higher than outside.
3.1 Possible Results
Possible result 1: As shown in table 3 and table 4, L-
fucose-bound liposome-P7 has significantly greater
cell anti-proliferative effect to pancreatic cancer cells
compared with all negative controls, P7 peptide and
CPP-P7. Also, fluorescence intensity indicates that L-
fucose-bound liposome-P7 has good penetrability to
cancer cells (Table 5). Moreover, the sizes of tumors
in animal models have significantly decreased by
treating with L-fucose-bound liposome-P7.
Additionally, L-fucose-bound liposome-P7 shows
significant higher inhibitory effects on the cell growth
of in vitro cancer cell samples and in vivo xenograft
mice models without affecting normal pancreatic
epithelial cells.
Possible Result 2: L-fucose-bound liposome-P7
exerts significant greater inhibitory effect on
proliferation of the pancreatic cancer cells in the
determined human pancreatic cancer cell lines,
compared with CPP-P7 and negative controls but not
P7 peptide. Also, it shows cytotoxicity to pancreatic
cancer cells but not non-cancerous cells, and it
controls/reduces mean tumor diameter in vivo animal
models without affecting normal pancreatic cells.
Specially, L-fucose-bound liposome-P7 show
greater cytotoxicity and inhibitory effect on
proliferation of pancreatic cancer cell lines but no
significant inhibitory effect and cytotoxicity on
normal pancreatic cell lines compared with all
negative control groups. Also, L-fucose-liposome,
Liposome without L-fucose and L-fucose alone do
not show inhibitory effect on growth of both
pancreatic cancer cell lines and normal pancreatic cell
lines, while P7 alone shows comparable cell
proliferation inhibition to L-fucose-bound liposome-
P7 on pancreatic cancer cell lines.
Possible Result 3: L-fucose-bound liposome-P7
shows cytotoxicity to cancer cells to a large extent
and revels cytotoxicity to normal cells (Table 3).
Also, L-fucose-bound liposome-P7 reduces mean
tumor diameter in vivo mice models but causes side
effects to mice.
L-fucose-bound liposome-P7 exerts greater
cytotoxicity to both pancreatic cells and normal
pancreatic epithelial cells in vitro and in vivo. Also,
all negative control groups do not show significant
cell cytotoxicity to the pancreatic cancer cells in vitro
and in vivo studies. As shown in table 3, results of
Annexin V/PI assay indicates that L-fucose-bound
liposome-P7 causes cell deaths by necrosis to
pancreatic cells and normal pancreatic epithelial
cells. In table 4, L-fucose-bound liposome-P7 shows
a significant higher cell proliferation inhibition effect
in cancer cell lines compared with P7 and CPP-P7.
Moreover, fluorescence microscopy results show that
The Investigation of P7 Peptide Delivery Targeting Cdc24 in Ras-driven Pancreatic Cancer by L-fucose-bound Liposome
657

L-fucose-bound liposome-P7 has poor penetration to
cancer cells (Table 5).
Possible Result 4: L-fucose-bound liposome-P7
inhibits cell growth of both pancreatic cancer and
normal cells (Table 3). Fluorescence imaging results
reflect poor cell penetration of L-fucose-bound
liposome-P7 to pancreatic cancer cells.
L-fucose-bound liposome-P7 exerts great
inhibitory effects on cell growth of cancer cells and
normal cells mainly by apoptosis (Table 3). In vivo
animal study, the sizes of tumors are decreased by L-
fucose-bound liposome-P7. As shown in table 4, L-
fucose-bound liposome-P7 shows a significant higher
cytotoxicity to cancer cell lines compared with CPP-
P7 but not P7. FAM-L-fucose-liposome and FAM-L-
fucose-bound liposome-P7 are observed to locate and
accumulate significantly in tumor sites, while FAM-
P7, FAM-liposome, FAM-L-fucose and FAM-CPP-
P7 do not show significant target delivery to the
pancreatic tumor site.
Possible Result 5: L-fucose-bound liposome-P7
does not exert any significant effect to either
pancreatic cancer cells, or normal pancreatic
epithelial cells (Table 3). Also, all negative control
groups show not significant effect on the growth of
both pancreatic cancer cells and normal pancreatic
cells. Colony formation assay, MTT assay and
Annexin assay all demonstrate insignificant effect on
pancreatic cancer cells and normal cell lines (Table
3). Although L-fucose-bound liposome-P7 shows
good cell penetration to cancer cells (Table 5), it has
no comparable cytotoxicity to P7 and CPP-P7 on in
vitro pancreatic cancer cells (Table 4). Also,
fluorescence imaging experiments of FAM-L-fucose-
bound liposome-P7 and all negative control groups
including FAM-P7, FAM-liposome, FAM-L-fucose
liposome, and FAM-L-fucose and positive control
(FAM-CPP-P7) do not show significant distribution
and accumulation in the targeted pancreatic tumor
site.
4 DISCUSSION
Previous studies report that P7 peptide binds with
Cdc42 protein and inhibits the downstream functions
exerted by Ras-mediated signaling, leading to
blockage of cancer cell transformation in Ras-driven
cancers (Malam, Loizidou, Seifalian, 2009).
However, the lack of a proper delivery system slows
down the drug development processes. To determine
a better delivery system that would enhance
therapeutic effects of P7 peptide and reduce OFF-
target cytotoxicity, this study uses L-fucose-modified
liposome in comparison with CPP-tagged P7 peptide
used in previous studies to deliver P7 peptide to two
well studied pancreatic cancer cell lines from humans
and to in vivo pancreatic cancer animal models.
Possible result 1 indicates a great cytotoxic effect and
proliferation inhibitory effect on pancreatic cancer
cells without affecting normal pancreatic epithelial
cells, indicating target delivery of L-fucose-bound
liposome-P7 to pancreatic cancer cells. Fluorescence
imaging indicates that L-fucose-bound liposome-P7
has good penetrability to cancer cells, which may
explain its higher cytotoxicity than P7. According to
Tetley et al. (Tetley, Murphy, Bonetto et al. 2020),
binding with CPP reduces the efficacy of P7, which
explain the much higher cytotoxicity of L-fucose-
bound liposome-P7 than CPP-P7 (Table 3). Overall,
possible result 1 fully supports our hypothesis that L-
fucose-modified liposome has a significant treatment
effect with enhanced cell penetration and targeted
delivery to pancreatic cancer cells. Further studies
assessing the pancreatic cancer cell transformation
should be done for a thorough understanding of the
blockage of Ras-driven transformation. More
complex and representative animal models should
also be done in preclinical trials before entering
clinical trials to prevent potential damages that could
not be found in mouse models.
Similar with possible result 1, possible result 2
indicates L-fucose-modified liposome P7 peptides
achieve the great efficacy with minimal side effects
and toxicity, which is consistent with previous
investigations in targeted delivery of L-fucose
liposome to pancreatic cancer cells. Differently, in
possible result 2, L-fucose-modified liposome P7
shows significant greater proliferation inhibition
effect than cpp-p7 but not p7, which indicates that the
efficacy of P7 is not enhanced by L-fucose liposome.
This could be also possibly because binding with L-
fucose liposome reduce the efficacy of P7 when
increasing drug distribution of L-fucose-bound
liposome-P7 to cancer cells. This result partially
supports our hypothesis that L-fucose-modified
liposome has targeted drug delivery to the tumor site
and increased cell penetration to cancer cells.
Compared with possible result 1 and 2, possible
result 3 shows that L-fucose-modified liposome P7
peptides has strong cytotoxicity and side effects at the
same time. It may be due to the existence of other
unknown molecular mechanisms induced by L-
fucose-modified liposome P7 peptides in the cell,
resulting in the apoptosis of normal cells and
pancreatic cancer cells induced. Simultaneously,in
result 3 both L-fucose-modified liposome P7 and
cpp-p7 shows significant proliferation inhibition and
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
658

L-fucose-modified liposome P7 is stronger, which
may because the 9R motif binds non-specifically to
other moieties or causes aggregation in the
lysate(Tetley, Murphy, Bonetto et al, 2020).This
result partially supports our hypothesis that L-fucose-
modified liposome has targeted drug delivery to the
tumor site but also has strong cytotoxicity to the
normal cell.
Possible result 4 shows that L-fucose-modified
liposome P7 peptide has cytotoxicity to both cancer
cells and normal cells by apoptosis (Table 4), while
L-fucose-modified liposome P7 has poor penetration
to the cancer cells, indicating the possibility that L-
fucose-modified liposome P7 induce an unknown
path on the cell surface which cause apoptosis of
cells. compared with cell. by cell apoptosis, cell
intracellular content is not released while by cell
necrosis, cell intracellular content is released which is
prone to cause inflammatory reaction (Helewski,
Kowalczyk-Ziomek, Konecki, 2006). Hence, drug
cytotoxicity induced by cell apoptosis is more
desirable than by cell necrosis. However, L-fucose-
modified liposome P7 causes significant harm to
normal pancreatic cells which would potentially
increase the suffering of patients. Hence, future work
should modify liposome carriers or find a new
strategy to increase the cell penetration of P7, which
can eventually achieve the efficacy of P7. Overall,
possible result 4 does not support our hypothesis.
There could be many reasons for the failure of
possible result 5. However, we have set up several
negative controls to eliminate the problem. If it does
not show any alteration compared with negative
controls, which include P7 alone, liposome alone, L-
fucose-liposome alone, it is likely that P7 is unable to
exert its inhibitory effect in pancreatic cancer cells. If
P7 alone shows a significant inhibitory effect in
pancreatic cancer cells compared with L-fucose-
liposome-P7, then probably the delivery system is the
problem that prevents P7 from exerting its effect.
Other failed results in animal models could be due to
enzymatic activity in the animal body, clearing
liposomes before they reach the pancreas. Therefore,
further study should investigate liposome
modifications that make them last longer in the
animal body is necessary.
5 CONCLUSION
In conclusion, the objective of our experiment was to
determine whether targeting P7 delivery with L-
focused binding liposomes can increase cell
penetration, enhance cytotoxicity to tumor cells, and
reduce cytotoxicity to normal cells compared to CPP-
tagged P7 under in vitro and in vivo conditions. This
study explores the therapeutic effect of L-fucose-P7
liposome in CA19-9 overexpressing pancreatic
cancer cell lines and Xenograft Murine Models. The
results of this study will examine whether L-fucose-
P7 liposome has a better therapeutic effect and a
weaker OFF-target effect compared with CPP-
tagged-P7 used in previous studies and preparing it
for entering clinical trials. These possible results and
the experiment itself provide the potential for future
improvement in the delivery system including
adjustment of liposome size to increase half-life and
adding monoclonal antibodies specific for pancreatic
cancer cells overexpressing EGFRs. The
modifications on liposome surface proteins will alter
pharmacokinetics of the P7 peptide to make it more
efficacious and the enhanced targeting skill of the
liposome will potentially minimize the OFF-tumor
effects.
ACKNOWLEDGEMENT
We would like to thank Hongbin Lan, Yurui Yang,
Huawei Zhu, and Yiran Zhu who
cooperatively contribute to this paper, especially
Hongbin Lan and Yurui Yang who did wonderful
work to this paper equally and they two should be
regarded as co-first authors. Moreover, we sincerely
thank Professor Arthur Salomon and teachers who
give useful guidance and helps.
REFERENCES
Arias-Romero, L.E., Chernoff, J. (2013) Targeting Cdc42
in cancer. Expert Opin Ther Targets., 17(11):1263-
1273.
Franken, N.A., Rodermond, H.M., Stap, J., Haveman, J.,
Van-Bree, C. (2006) Clonogenic assay of cells in vitro.
Nat Protoc., 1(5): 2315-9.
Helewski, K. J., Kowalczyk-Ziomek, G. I., & Konecki, J.
(2006). Apoptosis and necrosis-. Wiadomoci
Lekarskie, 59(9-10), 679-684.
Kabakov, A.E., Gabai, V.L. (2018) Cell Death and Survival
Assays. Methods Mol Biol. 1709: 107-127.
Malam, Y., Loizidou, M., Seifalian, A.M. (2009)
Liposomes and nanoparticles: nanosized vehicles for
drug delivery in cancer. Trends Pharmacol Sci., 30(11):
592-599.
McGuigan, A., Kelly, P., Turkington, R. C., Jones, C.,
Coleman, H. G., & McCain, R. S. (2018) Pancreatic
cancer: A review of clinical diagnosis, epidemiology,
treatment and outcomes. World J
The Investigation of P7 Peptide Delivery Targeting Cdc24 in Ras-driven Pancreatic Cancer by L-fucose-bound Liposome
659

Gastroenterol., 24(43), 4846–4861.
Moerke, N.J. (2009) Fluorescence Polarization (FP) Assays
for Monitoring Peptide-Protein or Nucleic Acid-Protein
Binding. Curr Protoc Chem Biol. 1(1): 1-15.
Papahadjopoulos, D., Heath, T., Bragman, K., Matthay, K.
(1985) New methodology for liposome targeting to
specific cells. Ann N Y Acad Sci., 446: 341-348.
Patra, C.R., Bhattacharya, R., Mukhopadhyay, D.,
Mukherjee, P. (2010) Fabrication of gold nanoparticles
for targeted therapy in pancreatic cancer. Adv Drug
Deliv Rev., 62(3): 346-361.
Tetley, G.J.N., Murphy, N.P., Bonetto S., et al. (2020) The
discovery and maturation of peptide biologics targeting
the small G-protein Cdc42: A bioblockade for Ras-
driven signaling. J Biol Chem., 295(9): 2866-2884.
Van-Meerloo, J., Kaspers, G.J., Cloos, J. (2011) Cell
sensitivity assays: the MTT assay. Methods Mol Biol.,
731: 237-45.
Yamazaki, N, Kodama, M., Gabius, H.J. (1994)
Neoglycoprotein-liposome and lectin-liposome
conjugates as tools for carbohydrate recognition
research. Methods Enzymol., 242: 56–65.
Yoshida, M., Takimoto, R., Murase, K., et al. (2012)
Targeting anticancer drug delivery to pancreatic cancer
cells using a fucose-bound nanoparticle approach.
PLoS One., 7(7): e39545.
Zeng, S., Pöttler, M., Lan, B., Grützmann, R., Pilarsky, C.,
Yang, H. (2019) Chemoresistance in Pancreatic
Cancer. Int J Mol Sci., 20(18): 4504.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
660
