
Based on Network Pharmacology and Molecular Docking Technology
to Explore the Mechanism of Coptis in the Treatment of Diabetic
Nephropathy
Yuexing Ma
1,2,3,+,*
, Zixuan Luo
2,3,+
, Simin Liu
2,3
, Haoyi Zheng
2,3
, Rongbin Pan
4,*
, Zhixin Zhu
2,3,*
,
Zirong Peng
2,3,*
, Mengyu Hou
1
, Xuening Huang
1
and Xin Qiao
1
1
Jiangxi University of Chinese Medicine, Nanchang, Jiangxi, 330004, China
2
Science and Technology College, Jiangxi University of Chinese Medicine, Nanchang, Jiangxi, 330004, China
3
Nanchang Medical College, Nanchang, Jiangxi, 330004, China
4
Jiangzhong Cancer Research Center, Jiangxi University of Chinese Medicine, Nanchang, Jiangxi, 330004, China
Rongbin Pan’s e-mail: PRB2019@jxutcm.edu.cn; Yuexing Ma ’s e-mail: ma-yuexing@qq.com;
+The same contribution to research
Keywords:
Huanglian, Diabetic Nephropathy, Network Pharmacology, Molecular Docking.
Abstract:
Objective Based on network pharmacology and molecular docking to explore the mechanism of Coptis in the
treatment of diabetic nephropathy. Methods Search and screen the main active ingredients in Coptis chinensis
through the TCM System Pharmacology Database and Platform (TCMSP) and obtain the corresponding
targets. Through the four databases of GeneCards, OMIM, PharmGkb, and TTD, the genes related to diabetic
nephropathy are searched and merged, and the target genes of the effective component target and the disease-
related gene intersection are obtained through the R language. Cytoscape 3.8.0 software was used to construct
a drug component-target-disease regulation network, and a protein interaction network was constructed
through the STRING online website. Using R language, GO enrichment analysis and KEGG enrichment
analysis were performed on the potential targets of Huanglian in the treatment of diabetic nephropathy. In
AutoDockTools-1.5.6, the molecular docking of key target proteins and main active ingredients is realized.
As a result, 10 active ingredients of Coptidis for treating diabetic nephropathy were obtained, including:
berberine, quercetin, etc.; corresponding to 104 target genes, including: PTSG2, CCL2, MAPK1, etc. Among
them, PTSG2 is the core of the PPI network Protein; KEGG pathway enriched to obtain 166 pathways,
including: IL-17 signaling pathway, TNF signaling pathway, NF-kappa B signaling pathway, VEGF signaling
pathway, etc. The results of molecular docking showed that berberine (berberine) has binding properties to
PTSG2. Conclusion Through network pharmacology, the target and mechanism of Coptidis in the treatment
of diabetic nephropathy are predicted.1 Introduction.
1 INTRODUCTION
1.1 Diabetes Pathogenesis and
Treatment Research
Diabetes (diabetes mellitus, DM) is a metabolic
disease caused by insufficient insulin secretion or the
inability of insulin to act. Continued maintenance of
high blood sugar and long-term metabolic disorders
may cause damage to the whole body tissues and
organs, especially the kidneys, and their dysfunction
and failure.
Diabetic kidney disease (DKD) is the most
common microvascular complication of diabetes. It is
a kidney disease caused by DM. (Neal, 2017); (Xing,
2021) It has become the leading cause of end-stage
kidney disease globally (Perkovic, 2019); (Verma,
2018); (Xing, 2021), and its prevalence is increasing
year by year (Liu, 2013). Clinical manifestations are
generally proteinuria, hypertension, edema, etc. (Ritz,
2010); (Xing, 2021), and in severe cases, it can even
cause renal failure and life-threatening. The
pathogenesis of DKD is complicated. Modern
research believes that the occurrence of DKD may be
related to oxidative stress, inflammation, metabolic
status, activation of NF-κB, and activation of the
renin-angiotensin-aldosterone system. (Haraguchi,
2020); (Xing, 2021) Among them, the inflammatory
638
Ma, Y., Luo, Z., Liu, S., Zheng, H., Pan, R., Zhu, Z., Peng, Z., Hou, M., Huang, X. and Qiao, X.
Based on Network Pharmacology and Molecular Docking Technology to Explore the Mechanism of Coptis in the Treatment of Diabetic Nephropathy.
DOI: 10.5220/0011250500003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 638-645
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

response plays an important role in the pathogenesis
of DKD. (Friedman, 2004); (Li, 2008) Inflammatory
factors include IL, NF-κB, TNF-α, TGF-β1, etc. (Lu,
2012); inflammatory factors not only cause kidney
Damage can also activate some signal channels to
aggravate the inflammatory response (Lu, 2010),
which further stimulates the development of DKD. At
present, Western medicine lacks effective treatment
for DKD, and studies have shown that Chinese and
Western medicine treatment will be the development
trend of the treatment of DKD.
1.2 Research Purpose and Introduction
From ancient times to the present, there have been
many discussions on Xiaoke in the literature and
classics, and the earliest relevant discussion appears
in the "Huangdi Neijing" (Wang, 2011); (Zhang,
2017). Diabetic nephropathy belongs to the category
of "diabetes" or "nephropathy" in traditional Chinese
medicine, and Huanglian is used to treat diabetic a
lot." Huanglian has a bitter taste and a cold nature. It
has the effects of clearing heat and dampness, purging
fire and detoxification. It is used for damp heat,
fullness of damp heat, vomiting and sourness, red
eyes, diminishing thirst, etc. Materia Medica Justice"
says: "Coptis rhizome has great bitterness and severe
cold, bitterness and dampness, cold overcomes heat,
and can vent all excess damp and fire, and the residual
heat of heart, spleen, liver, and kidney; the fire of
gallbladder, stomach, large intestine, and small
intestine, nothing will be incurable." (Peng, 2018)
Modern pharmacological studies (Fu, 2021);
(WANG, 2019) have shown that Coptidis has anti-
inflammatory, hypoglycemic, antitoxin, and anti-
tumor effects. Berberine, the main component of
Coptis, can effectively treat diabetic nephropathy.
(Li, 2016); (Yang, 2019)
Therefore, this article intends to use network
pharmacology methods to collect and analyze
relevant data from major databases. Through methods
such as drawing, tabulation, screening, and docking,
we will explore and verify the target of Coptidis on
DKD at the molecular level, and predict its
mechanism of action.
2 MATERIAL AND METHODS
2.1 Database and Software Preparation
Commonly used software: PERL (strawberry-perl-
5.32.1.1-64bit), R language (R-4.0.4-win), Cytoscape
(Cytoscape_v3.8.0), ChemOffice (Chem3D.exe),
PyMOL, AutoDockTools-1.5.6, vina and other
Databases: TCMSP, GeneCard, OMIM, PharmGKB,
TTD
2.2 Acquirement of the Effective
Components of Coptis and Its
Target
The TCSMP database was used to search for Coptidis
Rhizoma Coptidis, and the effective components of
Coptidis Rhizoma Coptidis and its corresponding
target data were screened based on the criteria of oral
bioavailability (OB) ≥ 30% and druglikeness (DL) ≥
0.18 in the TCMSP database. Obtain the target name
of the corresponding person in the UniProt database.
2.3 Acquisition and Screening of
Diabetic Nephropathy Related
Genes
Search with "Diabetic Kidney Disease" and "diabetic
nephropathy" in GeneCards, OMIM, TTD, and
PharmGKB databases to obtain DKD-related gene
sets and make their intersection Venn diagrams.
2.4 Obtain the Intersection of the
Target of the Effective Component
of Coptis and DKD-Related Genes
Use R language and corresponding R language scripts
to perform online analysis on the target of Coptidis
active ingredient and DKD disease-related genes,
draw the Venn diagram of the intersection of drug
ingredient targets and disease-related genes, and get
the intersection genes. This intersection gene is the
target of Coptidis for DKD.
2.5 Constructing the Mechanism
Network of Coptis Chinensis in
Regulating DKD
Upload the relevant files to the graphical display and
analysis software Cytoscape_v3.8.0, use Cytoscape
to construct a drug-component-target network
relationship diagram, and analyze the corresponding
relationship between the effective components and
Based on Network Pharmacology and Molecular Docking Technology to Explore the Mechanism of Coptis in the Treatment of Diabetic
Nephropathy
639
the target, and the results are usually displayed by
Degree. The higher the degree value of the target
gene, the greater the number of connected nodes,
which means that this node is more important in the
network relationship graph.
2.6
Construct (PPI) Protein Interaction
Network and Screen Core Genes
Upload the intersection gene file of the effective
component target of Coptis chinensis and DKD
disease-related genes to the STRING online website,
select the species as "Human", enter the website to
select all genes, set the minimum required interaction
score to lowest confidence (0.4000), and then
analyze, Get the PPI protein interaction network and
related documents. Finally, the file was imported into
Cytoscape to obtain the final core gene through three
screenings.
2.7
Enrichment Analysis of GO and
KEGG Pathways
Using R and its scripts, set qvalueFilter=0.05 (P
value≤0.05) and working directory of related files.
Then perform GO (gene ontology) enrichment
analysis and KEGG (Kyoto encyclopedia of genes
and genomes) pathway enrichment analysis in R-
4.0.4-win respectively, Obtain available enrichment
bubble chart and pathway enrichment histogram.
2.8
Molecular Docking Verifies the
Binding Relationship Between the
Active Ingredient and the Target
Download the 2D structure of the small molecule
ligand from the PubChem database
(http://pubchem.ncbi.nlm.nih.gov/), import it into
ChemOffice to convert the 2D structure of the small
molecule ligand into a 3D design, and optimize it to
the minor free energy structure, Get the 3D structure
of small molecule ligand. Download the protein
receptor structure of the selected active ingredient
from the PDB database (http://www.rcsb.org), import
the PyMOL software to remove water molecules and
small molecule ligands to obtain the protein receptor
file. Use AutoDockTools-1.5.6 software to
hydrogenate the protein receptor obtained in the
previous step and then convert the small molecule
ligand and protein receptor file format to determine
the functional pockets on the protein receptor
roughly. Use vina to perform molecular docking
between the target and the target protein to obtain the
score of the molecular docking result. The lower the
free energy, the better the binding.
3 RESULTS
3.1
Screening and Intersection of
Active Ingredient Targets and
Disease-Related Genes
Through the TCM System Pharmacology Database
and Platform (TCMSP), we searched and screened 14
main active ingredients in Coptis and 148
corresponding targets. In the GeneCards, OMIM,
TTD, and PharmGKB databases, 3506 DKD (DN)
disease-related genes (after deduplication) were
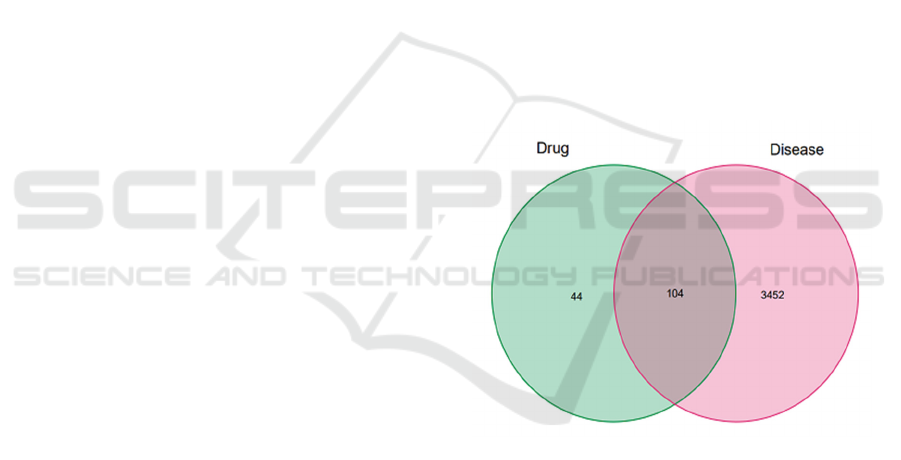
explored. The Venn diagram is drawn by the
intersection of the active ingredient targets and
disease-related genes (see Figure 1), and ten active
ingredients of Coptidis for treating diabetic
nephropathy include: berberine, etc., and 104
corresponding target genes include: PTSG2, MMP3,
MAPK1, etc
Figure 1: Venn diagram of the intersection of the
corresponding target of the practical components of
Coptidis and DKD-related genes.
3.2
The Mechanism Network of
Coptidis Regulating DKD
The analysis software Cytoscape_v3.8.0 was used to
construct and visualize the drug component-target
network of Coptidis regulated DKD (see Figures 2).
There are 277 nodes, including ten active ingredients,
104 target genes, and 163 edges. Among them,
PTGS2, ESR1, AR, and PTGS1 have slightly more
connections, and the Degree value is higher, which
may play an essential role in the treatment.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
640
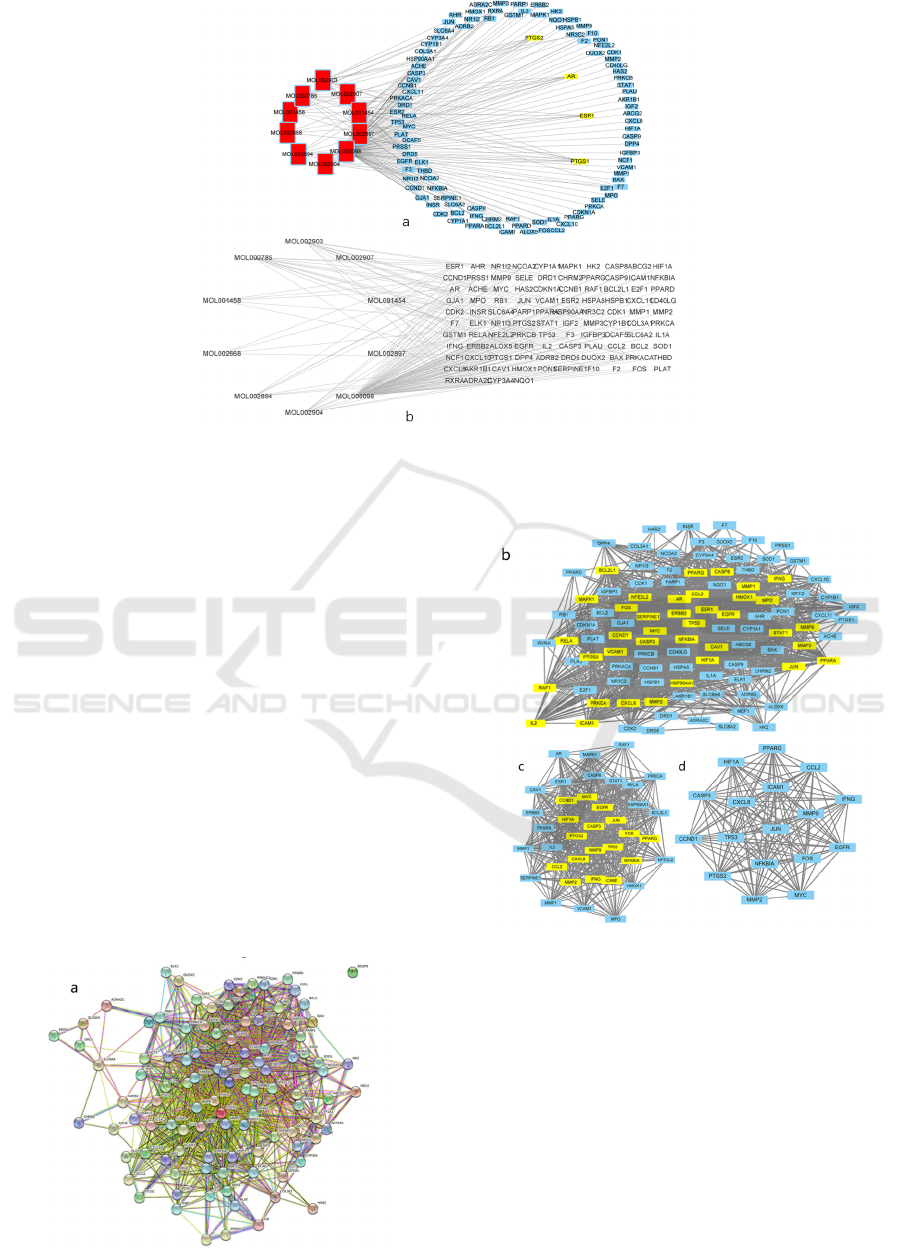
Figure 2: a: Rhizoma Coptidis regulates the DKD network, in which genes PTGS1, PTGS2, ESR1, AR, etc. are more
connected b: Coptis Rhizoma controls the DKD network, in which the active ingredients MOL000098, MOL000758,
MOL001454, etc. are more connected.
3.3 PPI Protein Interaction Network
The lowest confidence (0.4000) was screened through
the STRING online website to construct and visualize
the protein interaction network of the target genes for
the treatment of diabetic nephropathy (DKD) in
Coptis Chinensis (see Figure 3 a). There are 103
nodes and 1466 edges in the PPI network. The result
of importing the network into Cytoscape is visualized
as network 1. Select DC (Betweenness), CC
(Closeness), DC (Degree), EC (Eigenvector), and
LAC which are all greater than the median value.
Thirty-eight nodes generate network 2, and 17 nodes
generate network three by repeating the screening
criteria (see Figure 3 bcd). Network 3 is the core
network gene. Among them, the target genes may be
related to the treatment of diabetic nephropathy
(DKD) by Huanglian, from which genes can be
selected for molecular docking.
Figure 3: a: The PPI network of Coptis treatment of DKD
targets, where each sphere represents a protein or gene, and
the number of lines with different colors represents the
relationship b: the PPI network of a picture is imported into
the visualized network diagram of Cytoscape c: the gene
network is shown in b Diagram of the first-level core
network screened by specific criteria d: Diagram b:
Diagram of the core gene network filtered by specific
criteria
Based on Network Pharmacology and Molecular Docking Technology to Explore the Mechanism of Coptis in the Treatment of Diabetic
Nephropathy
641
3.4
GO and KEGG Enrichment
Analysis
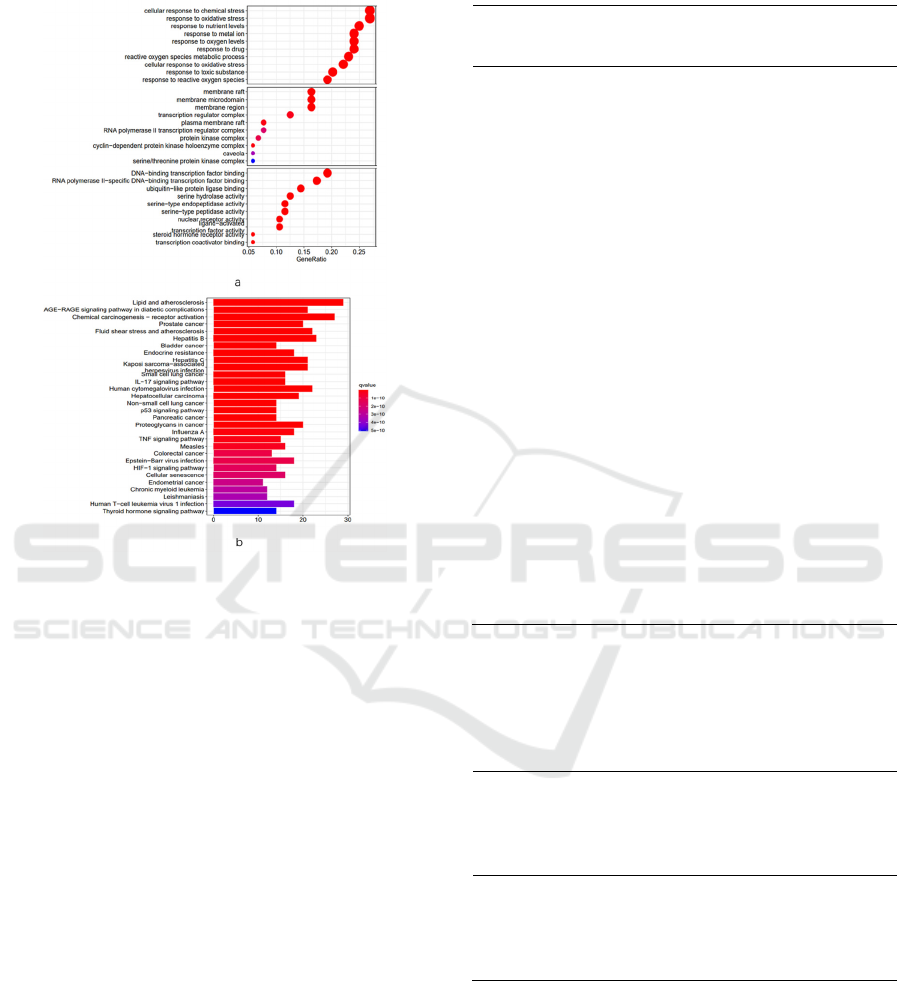
Figure 4 : a: GO enrichment analysis bubble chart, the
larger the value of generation, the more significant the
enrichment, the redder the bubble color, the more relevant
b: the histogram of KEGG pathway enrichment analysis,
the longer the column indicates that the gene is in this
pathway, The more significant the enrichment, the redder
the color suggests, the more relevant
The results of GO enrichment analysis (see Figure 4a)
show that BP mainly includes cellular Response to
chemical stress, Response to oxidative stress,
Response to nutrient levels, Response to the drug, etc.
KEGG pathway enrichment analysis has 165
pathways, and the top 30 pathways are plotted as a
histogram (see Figure4b). The results showed that
Lipids and atherosclerosis, AGE-RAGE signaling
pathway in diabetic complications, Prostate cancer,
IL-17 signaling pathway, TNF signaling pathway,
NF-kappa B signaling pathway, and other metabolic
pathways have significant gene enrichment. Screen
the IL-17 signaling pathway, TNF signaling pathway,
NF-kappa B signaling pathway, and VEGF signaling
based on the selected PTGS2 and refer to relevant
literature VEGF signaling pathway and draw a table
(see Table 1)
Table 1:According to the specific data of the four signal
pathways determined by PTGS2, including name, number
of genes, specific gene names, etc.
Serial
numbe
r
Path
way
Number
of genes
Gene
hsa046
57
IL-
17
signa
ling
path
way
16
PTGS2/HSP90AA1/MMP3/
RELA/FOS/
MMP9/MAPK1/JUN/CASP
3/NFKBIA/
CASP8/MMP1/CCL2/CXCL
8/IFNG/
CXCL10
hsa046
68
TNF
signa
ling
path
way
15
PTGS2/MMP3/RELA/FOS/
MMP9/MAPK1/
JUN/CASP3/NFKBIA/CAS
P8/ICAM1/CCL2/
SELE/VCAM1/CXCL10
hsa040
64
NF-
kapp
a B
signa
ling
path
way
12
PTGS2/RELA/BCL2/BCL2
L1/PLAU/NFKBIA
/ICAM1/VCAM1/CXCL8/P
RKCB/PARP1/
CD40LG
hsa043
70
VEG
F
signa
ling
path
way
7
PTGS2/CASP9/MAPK1/RA
F1/PRKCA/PRKCB
/HSPB1
3.5
Molecular Docking Results
Table 2: PTGS2 molecular docking result scoring table
(select the first five docking positions with the lowest free
energy)
mode affinity(kcal/mol) dist from
best
mode
(rmsd
l.b.
)
dist from
best
mode
(rmsd
u.b.
)
1 -9.2 0.000 0.000
2 -9.0 37.418 38.338
3 -8.9 47.593 48.959
4 -8.8 2.188 3.850
5 -8.7 1.948 7.680
The molecular docking technology was used to verify
the binding degree of the docking between the
corresponding receptor protein corresponding to the
receptor protein berberine and the related small-
molecule ligand of PTGS2, and the minimum free
energy was selected and visualized with PyMOL (see
Figure 5). The docking results show that the free
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
642
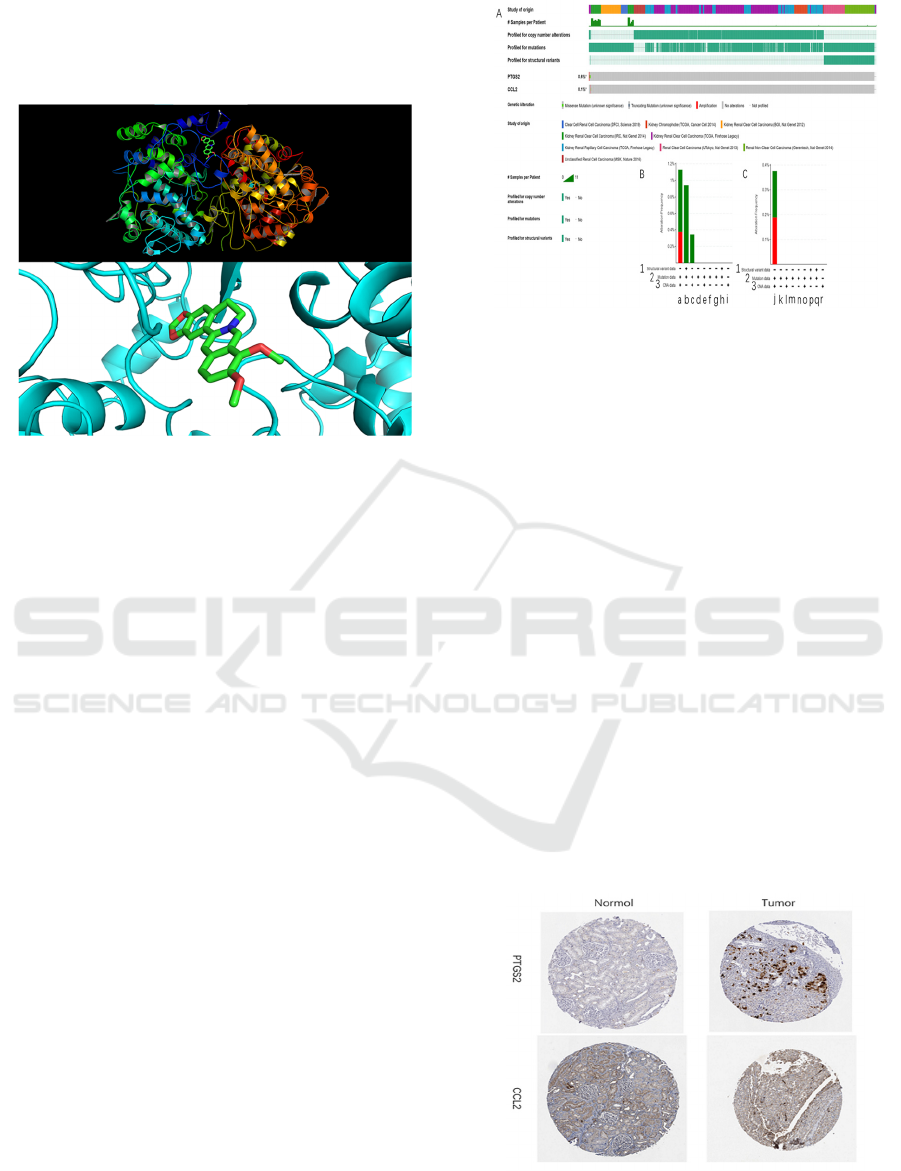
energy of the receptor protein and the corresponding
small molecule ligand is low, indicating that the target
protein has a better binding ability than the small
molecule (see Table 2)
Figure 5: Select the minimum free energy docking form,
and use PyMOL to visualize the panorama and details.
3.6
PTSG2, CCL2 Gene Mutation
Analysis
It is predicted that the genes related to DKD may be
PTGS2 and CCL2. Studies have shown that increased
expression of renal tubules MCP-1/CCL2 will
promote kidney damage, and the level of urine MCP-
1/CCL2 will gradually increase with the progress of
DKD, and the severity of it will deepen. (Morii,
2003); (Zhu, 2013). It shows that CCL2 and its
pathway are closely related to DKD. They are
analyzed through the online website
(http://www.cbioportal.org/). The analysis results are
shown in Figure 6: a: PTSG2 has Missense Mutation,
Truncating Mutation unknown gene mutation, and
CCLI2 has a strange gene mutation in Missense
Mutation. b: PTGS2 may have genetic mutations in
Kidney Renal Clear Cell Carcinoma, Renal Clear Cell
Carcinoma, Kidney Renal Papillary Cell Carcinoma,
and Kidney Renal Papillary Cell Carcinoma. Among
them, Kidney Renal Clear Cell Carcinoma and Renal
Clear Cell Carcinoma are more likely to occur. Nearly
40% of the genetic mutations in Renal Clear Cell
Carcinoma will be amplified. CCL2 is less likely to
be mutated in Kidney Renal Clear Cell Carcinoma,
but nearly 50% of the possible genetic mutations will
be strengthened after the genetic mutation.
Figure 6: A: Comparative analysis of gene mutations of
PTGS2 and CCL2 in kidney-related cancers. B: Analysis of
PTGS2 mutations [1:Structural variant data 2:Mutation
data 3:CAN data a:Kidney Renal Clear Cell Carcinoma
(TCGA, Firehose Legacy) b:Renal Clear Cell Carcinama
(UTOkyo, Nat Genet 2013) c:Kidney Renal Papillary Cell
Carcinama (TCGA, Firehose Legacy) d:Clear Cell Renal
Cell Caricinoma (DFCI, Science 2019) e:Kidney
Chromophobe(TCGA, Cancer Cell 2014) f:Kidney Renal
Clear Cell Carcinoma(BGI, Nat Genet 2012) g: Kidney
Renal Clear Cell Carcinoma (IRC, Nat Genet 2014) h:Renal
Non-Clear Cell Carcinoma (Genentech, Nat Genet 2014)
i:Unclassificied Renal Cell Cacinoma (MSK, Nature 2016)
C: Analysis of CCL2 mutations. [1:Structural variant data
2:Mutation data 3:CAN data j: Kidney Renal Clear Cell
Carcinoma(TCGA, Firehose Legacy) k: Clear Cell Renal
Cell Caricinoma(DFCI, Science 2019) l: Kidney
Chromophobe(TCGA, Cancer Cell 2014) m: Kidney
Renal Clear Cell Carcinoma(BGI, Nat Genet 2012) n:
Kidney Renal Clear Cell Carcinoma(IRC, Nat Genet 2014)
o: Kidney Renal Papillary Cell Carcinama(TCGA, Firehose
Legacy) p: Renal Clear Cell Carcinama(UTOkyo, Nat Genet
2013) q: Renal Non-Clear Cell Carcinoma(Genentech,
Nat Genet 2014) r: Unclassificied Renal Cell
Cacinoma(MSK, Nature 2016)] (In B and C, the green part
represents Mutation, and the red part represents
Amplification)
Figure 7: Comparison of the expression map of the two
genes in normal kidney tissue and tumor tissue. Image is
taken from Human Protein Atlas
(http://www.proteinatlas.org) online database
Based on Network Pharmacology and Molecular Docking Technology to Explore the Mechanism of Coptis in the Treatment of Diabetic
Nephropathy
643

4 DISCUSSIONS
Through network pharmacology data collection,
screening, and analysis, some key genes (Figure 3d)
and signal pathways (Figure 4b) were obtained. In the
process of searching the literature, we learned that
berberine can treat DKD renal insufficiency (Niksic
L, 2005) and protect the kidneys (Lan, 2010). After
screening the core gene and the corresponding target
of berberine to take the intersection, it was finally
determined to select the only intersection gene
PTGS2. Four related signal pathways were chosen
from the first 30 pathways with significant KEGG
enrichment (Table 1). Finally, PTGS2 was
molecularly docked (Figure 5). The results showed
that the lowest score was -9.2 (kcal/mol) (Table 2),
indicating that PTGS2 and its related pathways may
be essential genes and pathways regulating berberine
treatment of DKD.
Studies have shown that the occurrence of DKD
may be related to inflammation, metabolic status,
activation of NF-κB, etc. Inflammation plays a vital
role in the pathogenesis of diabetic nephropathy,
causing kidney damage and activating some signal
channels to exacerbate inflammation reactions.
Inflammatory factors include interleukin (IL), nuclear
factor-κB (NF-κB), tumor necrosis factor-α (TNF-α),
transforming growth factor-β1 (TGF-β1), etc. Among
them, IL may be related to IL- 17 signaling pathway,
TNF-α is related to TNF signaling pathway, and NF-
κB is associated with NF -kappa B signaling pathway.
Berberine is likely to regulate these inflammatory
factors to regulate the DKD metabolic pathway to
protect the kidney and treat DKD.
Studies have shown that berberine has a relatively
apparent anti-inflammatory effect, mainly by
inhibiting the production and activity of
inflammatory factors. It can reduce the activity of
neutrophil phospholipase A2 and reduce the
production of prostaglandin E2 in inflammatory
tissues (Hu, 2014). Prostaglandin-endoperoxide
synthase (PTGS), also known as cyclooxygenase, is a
key enzyme in prostaglandin biosynthesis, closely
related to the synthesis of prostaglandin E2, and
PTGS2 is one of the two types of PTGS Inducible
may be involved in the synthesis of prostaglandin E2.
Based on this speculation, berberine may regulate
PTGS2 to regulate related metabolic pathways to
achieve anti-inflammatory effects and reduce kidney
damage.
In addition, the gene mutation analysis of PTGS2
and CCL2 in section 2.6 (Figure 6) also shows that
PTGS2 is more likely and more prone to gene
mutations in related kidney diseases than CCL2,
which also illustrate the relationship of PTGS2 and
pathogenesis of DKD is closer.
5 CONCLUSIONS
In summary, based on network pharmacology,
predictive analysis verified that PTGS2 and its related
pathways may be important genes and pathways
regulating berberine treatment of DKD. Berberine is
likely to further inhibit the synthesis and release of
some inflammatory factors (such as IL, NF-κB, TNF-
α, TGF-β1, etc.) by inhibiting PTGS2, thereby
achieving regulation of DKD metabolic pathways
(TNF signaling pathway, IL-17 signaling pathway,
NF-kappa B signaling pathway, VEGF signaling
pathway) to achieve anti-inflammatory effects, and
ultimately reduce kidney damage, protect the
kidneys, and achieve the effect of treating DKD.
In this study, a series of methods were used to find
the relationship between berberine and the DKD
disease gene PTGS2, the effective component of
Coptidis Rhizome, and to verify the feasibility of
Coptis Rhizoma (berberine) for regulating PTGS2 in
the treatment of DKD. It provides a new idea for the
research on the target and mechanism of Coptidis in
the treatment of diabetic nephropathy in the future.
It is hoped that this study can provide reference
for other researchers who are starting to develop the
mechanism of action of Coptis in the treatment of
DKD in the future.
REFERENCES
Friedman AN, Hunsicker LG, Selhub J, et al.Clinical and
nuitritional correlates of C-reactive protein in
type2diabetic nephropathy. Atherosclero-sls, 2004,
172:121-125.
Fu L; Fu Q; Li J; Tong X. Research progress on the
chemical constituents and pharmacological effects of
Rhizoma Coptidis[J]. Journal of Traditional Chinese
Medicine,2021,49(02):87-92.
Haraguchi R, Kohara Y, Matsubayashi K, et al. New
Insights into the Pathogenesis of Diabetic Nephropathy:
Proximal Renal Tubules Are Primary Target of
Oxidative Stress in Diabetic Kidney[J]. Acta
histochemica et cytochemica official journal of the
Japan Society of Histochemistry and Cytochemistry,
2020, 46(12):1723-1731.
Hu SP, Wang Y, Yu P-Y, et al. In vitro anti-inflammatory
effects of the main components of Huanglian Jiedu
Decoction[J]. China Modern Applied Pharmacy, 2014,
31, (10): 1171-1174
Lan T, Shen XY, Liu PQ, et al.Berberine ameliorates renal
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
644

injury in di-abetic C57BL/6 mice:Involvement of
suppression of SphK–S1P sig-naling pathway[J].Arch
Biochem Biophys, 2010, 502 (2) :112-120.
Li JP, Wu CZ, Yue W, et al. Research progress of new
clinical uses and new dosage forms of berberine[J].
China Pharmacy, 2016, 27(22):3154-3158.
Liu ZH. Nephrology in China[J]. Nature Reviews
Nephrology, 2013, 9 (9) :523-528
Li XS. Inflammation-related factors and diabetic
nephropathy[J]. Medicine, 2008, (02):214-216.
Lu F, Tang LQ. The role of inflammatory factors in diabetic
nephropathy-related signal pathways[J]. China
Pharmacy, 2010, 21 (18) :1706-1710.
Lu J-J; Liu S. Research progress on the effects of berberine
on diabetes and its complications[J]. Anhui Medicine,
2012, (11): 1566-1569.
Morii T, Fujita H, Narita T, et al.Association of monocyte
chemoattractant protein-1 with renal tubular damage in
diabetic nephropathy[J].J Diabetes Complications,
2003, 17 (1) :11-15.
Neal B, Perkovic V, Mahaffey KW, et al. CANVAS
Program Collaborative Group. Canagliflozin and
Cardiovascular and Renal Events in Type 2 Diabetes. N
Engl J Med, 2017, 377(7):644-657.
Niksic L, Martin PY.BMP-7 (Bone morphogenetic protein-
7): afuture for chronic renal failure? [J]. Rev Med
Suisse, 2005, 1 (8) :568-573.
Perkovic V, Jardine MJ,Neal B,et al. CREDENCE Trial
Investigators. Canagliflozin and Renal Outcomes in
Type 2 Diabetes and Nephropathy. N Engl J Med, 2019,
380(24):2295-2306.
Peng C. Pharmacology of Traditional Chinese Medicine-
(New Century Fourth Edition) -Thirteenth Five-Year
Plan for Higher Education in Chinese Medicine
Industry [M]. China Press of Traditional Chinese
Medicine, 2018.
Ritz E, Wolf G. Pathogenesis, Clinical Manifestations, and
Natural History of Diabetic Nephropathy[J].
Comprehensive Clinical Nephrology (Fourth Edition),
2010, 42(7):359-376.
Verma S, Mazer CD, Fitchett D, et al. Empagliflozin
reduces cardiovascular events, mortality and renal
events in participants with type 2 diabetes after
coronary artery bypass graft surgery: subanalysis of the
EMPA-REG OUTCOME randomised trial.
Diabetologia, 2018, 61(8):1712-1723.
WANG J, WANG L, LOU G H,et al Coptidis Rhizoma:a
comprehensive review of its traditional uses, botany,
phytochemistry ,pharmacology and toxicology [J].
Pharm Biol,2019,57(1):193.
Wang HT. Neijing [M]. Beijing: People's Health Publishing
Madness, 2011; 203.
Xing T-T. The protective effect of crocetin on kidney
damage in diabetic rats by inhibiting oxidative stress
and inflammation[C]. Jinzhou Medical University,
2021.
Yang NY; Zhang QC; Zhu HX; Fu TM; Li B; Duan JA;
Tang ZS. Research progress and utilization strategy of
resource chemical components of Coptis alkaloids[J].
Chinese Traditional and Herbal Medicine, 2019,
50(20):5080-5087.
Zhang Y.A study of ancient and modern medicine thinking
in diabetic nephropathy[C]. Beijing University of
Chinese Medicine, 2017.
Zhu BY; Huang LJ; Yu J-Y. The research progress of
chemokines and their receptors and diabetic
nephropathy[J]. Jiangsu Medicine, 2013, (02):210-213.
Based on Network Pharmacology and Molecular Docking Technology to Explore the Mechanism of Coptis in the Treatment of Diabetic
Nephropathy
645
