
Gingipain Causes Tau Tangle Formation in Alzheimer’s Disease
Brains via Regulation of TREM-1
Yiyi Zhu
Department of life sciences, Imperial College London, London, U.K.
Keywords: STREM-1, Gingipain, Tau, Alzheimer’s Disease.
Abstract: Tau phosphorylation is widely believed as an indicator of Alzheimer’s Disease (AD). Recent evidence
suggests that the periodontal pathogen, Porphyromonas gingivalis, is associated with AD development by
increasing the level of tau protein in the brain. Gingipain, the major virulence factor of the bacteria, has been
described to be involved in the cleavage of the Triggering Receptor Expressed on Myeloid cells 1 (TREM-
1). The soluble TREM-1 (sTREM-1) generated was shown to result in the activation of tau phosphorylation.
This work aims to uncover the underlying mechanism by which P. gingivalis causes AD by studying the
relationship between the level of gingipain, TREM-1 and tau in the brain of mice infected with P. gingivalis.
Through this study, a novel mechanism of AD formation may be proposed and can be exploited to generate
therapies against the disease.
1 INTRODUCTION
Alzheimer’s disease (AD) is one of the leading causes
of dementia worldwide. The widely accepted cause of
AD is the loss of neurons caused by the formation of
neurofibrillary tangles from tau phosphorylation
(Niikura, Tajima, Kita 2006, Ryder 2020). However,
the mechanism behind this process is not clear, and
recent studies have shown that the gram-negative
bacteria Porphyromonas gingivalis responsible for
periodontal infections has been detected in the brain
of AD patients, which suggest a role of the bacteria in
the pathogenesis of the disease (Dominy et al 2019).
The major virulence factor produced by the
bacteria is a family of conserved proteases called
gingipain n (Dominy et al 2019). This family of
protease consists of lysine-gingipain (Kgp), arginine-
gingipain A (RgpA), and arginine-gingipain B
(RgpB), which were found in the brain of 90% of the
AD patients (Haditsch 2020). These proteases are
localized to the hippocampus and result in the
secretion of pro-inflammatory cytokines that cause
damage to the brain (Ilievski et al 2018).
Experiments using human polymorphonuclear
neutrophils have demonstrated a role of Rgp in the
shredding of the triggering receptor expressed on
myeloid cells 1 (TREM1), which level is found to be
higher in AD patients (Sao et al 2018). TREM1
enhances cellular response by the recruitment of more
inflammatory cells (Bouchon, Dietrich, Colonna
2000). A study using human plasma demonstrated a
positive relationship between the level of sTREM1
and tau protein in AD patients, thus confirming the
role of sTREM1 in AD development (Jiang et al
2019).
The specific mechanism by which P. gingivalis
induce AD has not been elucidated, and the
connection between gingipain caused TREM-1
shredding and tau protein phosphorylation in AD
patients has not been made. Here I proposed a model
to investigate whether P. gingivalis can pass into the
brain from oral infection by assessing the level of
gingipain in the brain of mice. Then, the level of
sTREM-1 in the brain will be determined to confirm
the effect of P. gingivalis on TREM-1 shredding
(Figure 1). To investigate the contribution of
sTREM-1 in the formation of phosphorylated tau
(phospho-tau), Trem-1 deficient mice will be used
and compared with wild type mice for the level of
phospho-tau. Lastly, as Rgp is shown to be
responsible for TREM-1 shredding, the effect of a
Rgp inhibitor will be investigated to prevent mice
infected with P. gingivalis from AD.
626
Zhu, Y.
Gingipain Causes Tau Tangle Formation in Alzheimer’s Disease Brains via Regulation of TREM-1.
DOI: 10.5220/0011250000003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 626-631
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
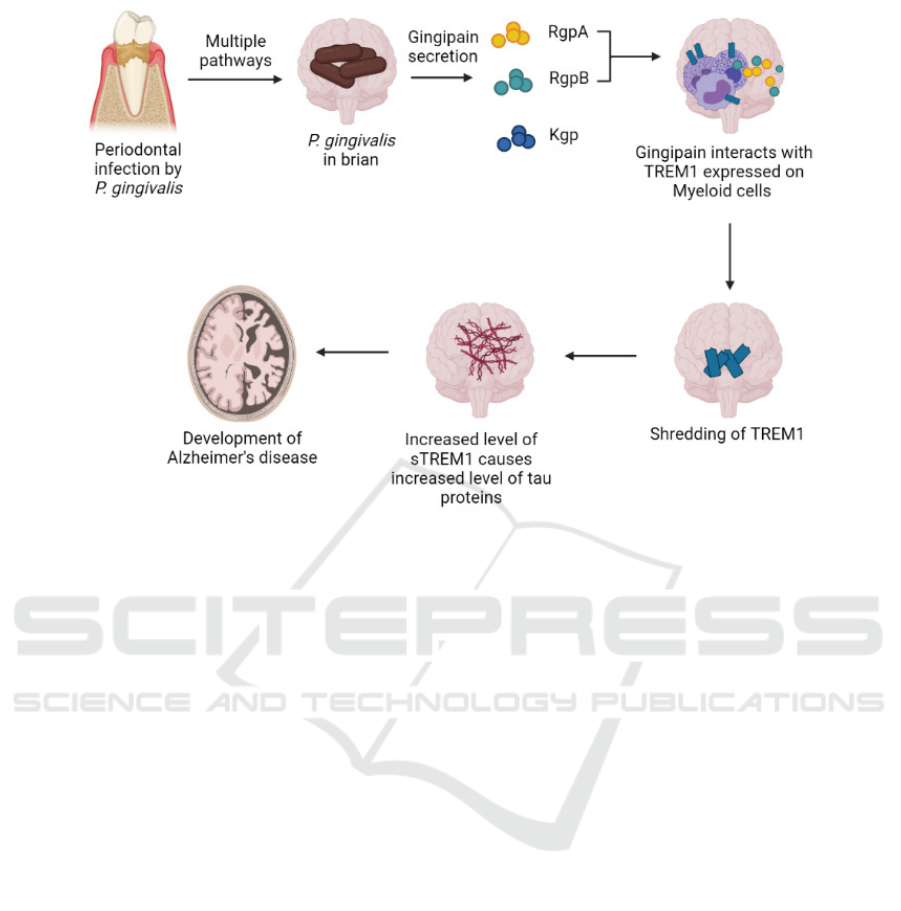
Figure 1: Proposed model for the development of Alzheimer’s disease induced by P. gingivalis (Created with
BioRender.com). P. gingivalis present at periodontal infections enters the brain via an unknown route, but several possible
mechanisms have been proposed (Dominy et al 2019). Once inside the brain, gingipains are secreted and cleaves the TREM1
receptor present on myeloid cells into sTREM1, which then circulates in CSF and amplifies inflammatory responses. sTREM1
also cause rise in tau concentration and increased phosphorylation, which results in neuronal loss that contribute to AD
development.
2 MATERIALS AND METHODS
2.1 Growth of P. Gingivalis
P. gingivalis (W83, ATCC® BAA308™) will be
grown on tryptic soy agar plates under anaerobic
condition in an anerobic gas chamber at 37°C for 3
days (ATCC® Medium 2722). P. gingivalis will then
be harvested from the agar by scrapping and
suspended in PBS + 2% methylcellulose at a
concentration of 1 × 10
10
cells/ml after washing with
phosphate-buffered saline (PBS). Cell concentration
will be determined by using a counting chamber
under a microscope.
2.2 Animal
Female 5XFAD mice (#34840) from the Jackson
Laboratory will be purchased and used for infection
with P. gingivalis. Mice needs to be maintained under
specific pathogen free conditions and fed with
LabDiet® 5K52 formulation (6% fat) with tap water.
The control and bacterially infected mice will be
maintained in separate cages and kept under a regular
12h dark/light cycle with a temperature and humidity
of 22°C and 60, respectively.
2.3 Generation of Trem-1 Knockout
Mice
The generation of Trem-1 knockout mice will follow
the procedure outlined by Weber et al, 2014 (Weber
et al 2014). Briefly, a vector based on KS loxP ftr Neo
BS cloning vector need to be constructed in order to
delete exon 2 of the Trem-1 gene. Deletion blocks
TREM-1 activity as exon2 encodes the extracellular
region and the ligand-binding site. Additional
restriction sites (AseI and AvaI), and the positive
selection markers, PuroR and Neomycin, is added for
the selection. A Tk counterselection cassette is also
included. The vector needs to be electroporated into
mice embryonic stem cells using a BAC plasmid.
Further mating of the genetically modified mice with
mice carrying Cre recombinase is required to obtain
Trem-1 knockout mice.
Gingipain Causes Tau Tangle Formation in Alzheimer’s Disease Brains via Regulation of TREM-1
627

2.4 P. gingivalis Oral Infection on Mice
Forty-three weeks old 5XFAD female mice were
used for oral infection with P. gingivalis. The mice
will be anaesthetized with ketamine (100mg/kg) and
xylazine (10mg/kg) by intraperitoneal injection. The
eyes will be lubricated with ophthalmic ointment to
prevent drying. Anaesthetized mice will then be tied
around the upper maxillary left and right second
molar with silk ligature (FS 5080, Dolphin Sutures).
100ml of the bacterial solution will be applied to the
buccal surface of the maxillae of the mice in infection
groups. This procedure needs to be repeated every
other day for six weeks. 100ml of PBS + 2%
methylcellulose will be applied to the control groups
on the same days.
Figure 2.
2.5 Effect of P. gingivalis Infection on
the Level of Gingipain in Brain
To study the level of gingipain in the brain of infected
mice, 20 infected and 20 controls of 43 weeks old
female 5XFAD mice were used. Mice brains need to
be prepared as outlined by Clark et al, 2011 (Clark et
al 2011). Briefly, brains extracted were sliced using a
cryostat into 40µm thick sections and fixed onto
slides. Then, immunohistochemistry will be
performed following the procedure described by
Dominy et al, 2019, where the Rgp antibody 18E6
(University of Georgia) needs to be added to the
samples and the result will be visualized using
UltraView Universal DAB Detection System
(Ventana Medical Systems) (Dominy et al 2019).
2.6 Effect of P. gingivalis Infection on
the Level of TREM-1 in Brain
Immune cells will be isolated from the brain of 20
infected and 20 control mice to determine TREM-1
levels. The cells will undergo flow cytometry using
the procedure stated by Liu et al, 2019 (Liu et al
2019). Briefly, cells extracted will be suspended in
Hank’s balanced salt solution (ThermoFisher), and
TREM-1 antibodies (R&D, clone 174031) will be
added. Flowjo (Tree Star Inc.) will then be used to
analyze the results.
2.7 Effect of P. gingivalis Infection on
the Level of Strem-1 in Brain
To investigate the level of sTREM-1 in mice brains,
cerebrospinal fluid (CSF) from 20 infected and 20
control 43 weeks old female 5XFAD mice were used.
CSF will be extracted into polypropylene tubes using
the method proposed by Sakic, 2019 (Šakić 2019).
Then, the samples were analyzed using the TREM1
mouse ELISA kit (EMTREM1, ThermoFisher)
following the protocol.
2.8 Effect of TREM-1 Knockout on the
Level of Total/Phospho-Tau
The level of tau was investigated in 20 infected
TREM-1(+/+) and 20 infected TREM-1(-/-) 5XFAD
mice. Mice will be anaesthetized by intraperitoneal
injection with 4% chloral hydrate (10 mL/kg), and the
level of total tau protein present in the brain of the
mice can be determined using the Tau (total) mouse
ELISA kit (KMB7011, ThermoFisher) following the
protocol using brain homogenate. The level of
phospho-tau protein present can be determined using
the Tau (Phospho) [pS199] Mouse ELISA kit
(KMB7041, ThermoFisher) following the protocol
using brain homogenate. Sample obtained were
analyzed using a spectrophotometer, and the
absorbance is read at 450nm to determine the
concentration.
2.9 Effect of Gingipain Inhibitor on the
Level of Strem-1 and Phospho-Tau
To test the effect of gingipain inhibitor in treating
AD, 40 43 weeks old female 5XFAD mice were
infected with P. gingivalis. Twenty of them received
Rgp inhibitor (A18522, Adooq Bioscience) in DMSO
every day starting from week two by intravenous
injection (10mg/kg). Control mice received only
DMSO. Determine the level of sTREM-1 and
phospho-tau in the brain of the mice as above.
2.10 Data Analysis
All statistical analysis of the data obtained will be
performed using RStudio version 1.2.5042. One-way
analysis of variance (ANOVA) will be performed to
assess whether the difference between the sample
means of the groups were significant. A Bonferroni
test will then be performed to reduce false-positive
results. Data will be considered significant at P<0.05.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
628
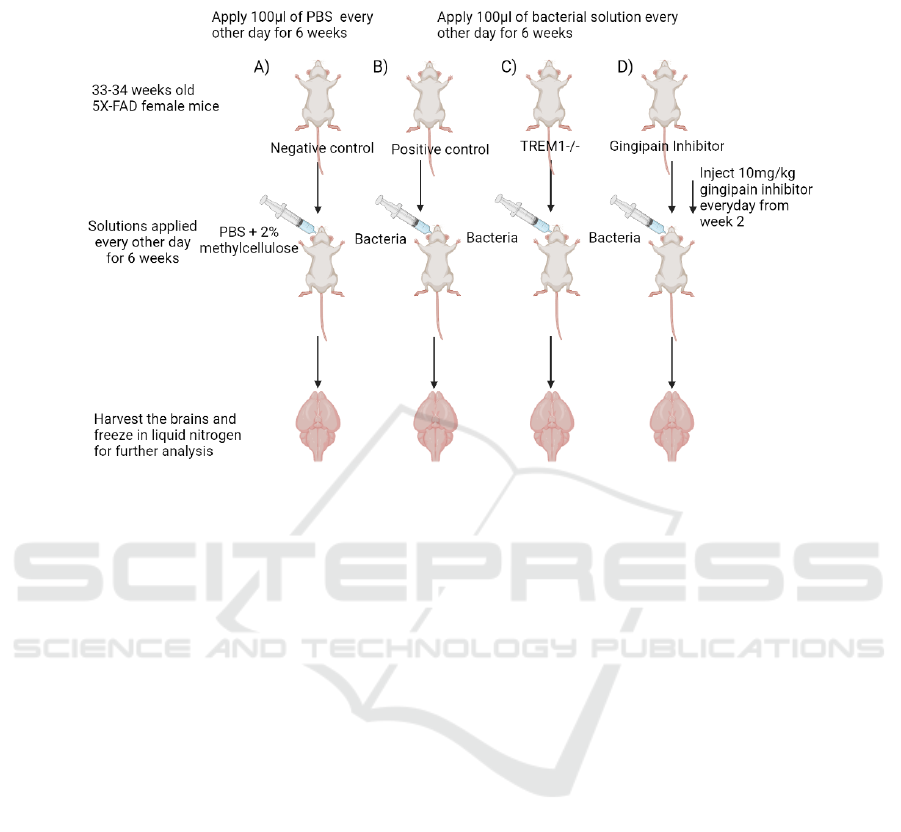
3 CONCLUSION
Figure 2: Experimental setup for sample collection of different groups (Created with BioRender.com). A) Negative control
group, receiving only the solvent used to suspend the P. gingivalis cells. Act as a reference for the level of gingipain, TREM1
and tau protein in brain. B) Positive control, mice are infected with P. gingivalis to confirm that the infection will lead to an
increase in TREM1 and tau proteins in brain. C) Mice with TREM1 knockout, to investigate whether TREM1 influences the
level of tau protein in the brain. D) Mice treated with gingipain inhibitor, to investigate whether the effect is due to the
presence of gingipain or not and also act as a possible treatment method for the infection.
This set of experiments could propose a new
mechanism by which AD can be induced. Suppose
the result meets the hypothesis: the bacterially
infected mice have a higher level of gingipain,
sTREM-1 and phosphorylated tau in the brain than
control. In that case, it can be concluded that orally
infected P. gingivalis is able to migrate into the brain
and secretes gingipain that cleaves TREM-1 from cell
surfaces. The increase in the level of sTREM-1
triggers the accumulation and phosphorylation of tau,
which is a sign of AlD. Suppose the inhibitor-treated
mice demonstrated a lower level of sTREM-1 and
phosphor-tau in the brain than positive control mice.
It could be concluded that Rgp is responsible for the
disease, and inhibition of this virulence factor could
prevent AD development. However, other adverse
effects of the replication bacteria inside the brain
have not been determined and may contribute to the
neurodegeneration seen in AD patients, thus
requiring further characterization. This study only
investigates the effect of Rgp, and the role of Kgp in
the pathogenesis of the bacteria would need to be
determined to understand the complete mechanism
by which P. gingivalis causes AD.
Although this set of experiments did not
investigate the route by which P. gingivalis passes
into the brain, but many models have been proposed
and the mechanism of entry may contribute to the
development of AD symptoms. For example, P.
gingivalis may gain entry into the brain via direct
damage to the endothelial cells of the blood-brain
barrier (BBB) via its gingipains. As an earlier study
has demonstrated the ability of gingipains secreted by
the bacteria to induce apoptosis in endothelial cells
(Sheets, Potempa, Travis, Casiano, Fletcher 2005).
The damage to BBB are often seen as an early marker
for the development of AD, and contributes to
neurodegeneration that led to dementia. Thus, both
the effect of the damage of BBB and cleavage of
TREM1 need to be considered to fully characterize P.
gingivalis infection on causing AD.
Overall, the experiments provided a pathway to
investigate a novel cause of AD and presented a
possible treatment method.
Gingipain Causes Tau Tangle Formation in Alzheimer’s Disease Brains via Regulation of TREM-1
629
Figure 3: Steps of making brain sections for immunohistochemistry.
REFERENCES
Bouchon, A., Dietrich, J., & Colonna, M. (2000). Cutting
Edge: Inflammatory Responses Can Be Triggered by
TREM-1, a Novel Receptor Expressed on Neutrophils
and Monocytes. The Journal of Immunology, 164(10),
4991.
Clark, S., Duangdao, D., Schulz, S., Zhang, L., Liu, X., Xu,
Y., Reinscheid, R. (2011). Anatomical characterization
of the neuropeptide S system in the mouse brain by in
situ hybridization and immunohistochemistry. Journal
of Comparative Neurology, 519(10), 1867-1893.
Dominy, S., Lynch, C., Ermini, F., Benedyk, M., Marczyk,
A., Konradi, A., Nguyen, M., Haditsch, U., Raha, D.,
Griffin, C., Holsinger, L., Arastu-Kapur, S., Kaba, S.,
Lee, A., Ryder, M., Potempa, B., Mydel, P., Hellvard,
A., Adamowicz, K., Potempa, J. (2019).
Porphyromonas gingivalis in Alzheimer’s disease
brains: Evidence for disease causation and treatment
with small-molecule inhibitors. Science Advances. 5.
eaau3333.
Haditsch, U., Roth, T., Rodriguez, L., Hancock, S., Cecere,
T., Nguyen, M., Arastu-Kapur, S., Broce, S., Raha, D.,
Lynch, C. C., Holsinger, L. J., Dominy, S. S., Ermini,
F. (2020). Alzheimer's Disease-Like
Neurodegeneration in Porphyromonas gingivalis
Infected Neurons with Persistent Expression of Active
Gingipains. Journal of Alzheimer's disease: JAD,
75(4), 1361–1376.
Ilievski, V., Zuchowska, P. K., Green, S. J., Toth, P. T.,
Ragozzino, M. E., Le, K., Aljewari, H. W., O'Brien-
Simpson, N. M., Reynolds, E. C., Watanabe, K. (2018).
Chronic oral application of a periodontal pathogen
results in brain inflammation, neurodegeneration and
amyloid beta production in wild type mice. PloS one,
13(10), e0204941.
Jiang, T., Gong, P. Y., Tan, M. S., Xue, X., Huang, S.,
Zhou, J. S., Tan, L., Zhang, Y. D. (2019). Soluble
TREM1 concentrations are increased and positively
correlated with total tau levels in the plasma of patients
with Alzheimer's disease. Aging clinical and
experimental research, 31(12), 1801–1805.
Liu, Q., Johnson, E. M., Lam, R. K., Wang, Q., Bo Ye, H.,
Wilson, E. N., Minhas, P. S., Liu, L., Swarovski, M. S.,
Tran, S., Wang, J., Mehta, S. S., Yang, X., Rabinowitz,
J. D., Yang, S. S., Shamloo, M., Mueller, C., James, M.
L., Andreasson, K. I. (2019). Peripheral TREM1
responses to brain and intestinal immunogens amplify
stroke severity. Nature immunology, 20(8), 1023–
1034.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
630

Niikura, T., Tajima, H., Kita, Y. (2006) Neuronal cell death
in Alzheimer’s disease and a neuroprotective factor,
humanin. Current neuropharmacology, 4(2): 139-147
Ryder, M. I. (2020). Porphyromonas gingivalis and
Alzheimer disease: Recent findings and potential
therapies. Journal of Periodontology, 91(S1), 45-49.
Šakić, B. (2019). Cerebrospinal fluid collection in
laboratory mice: Literature review and modified
cisternal puncture method. Journal of Neuroscience
Methods, 311, 402-407.
Sao, T., Yoshino, Y., Yamazaki, K., Ozaki, Y., Mori, Y.,
Ochi, S., Yoshida, T., Mori, T., Iga, J. I., Ueno, S. I.
(2018). TREM1 mRNA Expression in Leukocytes and
Cognitive Function in Japanese Patients with
Alzheimer's Disease. Journal of Alzheimer's disease:
JAD, 64(4), 1275–1284.
Sheets, M., Potempa, J., Travis, J., Casiano, A., & Fletcher,
M. (2005). Gingipains from Porphyromonas gingivalis
W83 induce cell adhesion molecule cleavage and
apoptosis in endothelial cells. Infection and immunity,
73(3), 1543–1552.
https://doi.org/10.1128/IAI.73.3.1543-1552.2005
Weber, B., Schuster, S., Zysset, D., Rihs, S., Dickgreber,
N., Schürch, C., Riether, C., Siegrist, M., Schneider, C.,
Pawelski, H., Gurzeler, U., Ziltener, P., Genitsch, V.,
Tacchini-Cottier, F., Ochsenbein, A., Hofstetter, W.,
Kopf, M., Kaufmann, T., Oxenius, A., Reith, W.,
Saurer, L.,Mueller, C. (2014). TREM-1 deficiency can
attenuate disease severity without affecting pathogen
clearance. PLoS pathogens, 10(1), e1003900.
Gingipain Causes Tau Tangle Formation in Alzheimer’s Disease Brains via Regulation of TREM-1
631
