Synthesis Proposal of Immunosuppressive 9,10-secosteroid A from the
Gorgonian Verrucella Umbraculum
Zhaocong Yuan
1,*
, Hanming Li
2
and Yining Han
3
1
Shenzhen College of International Education, Shenzhen, Guangdong 518043, China
2
Department of Chemical and Biomolecular Engineering, University of California, Irvine, Irvine, CA 92697, U.S.A.
3
Johnathan Academy, Vancouver, British Columbia V6M 2V9, Canada
Keywords: Natural Product, Chemical Synthesis, Diols, Molecular Structure, Aromatic Compounds.
Abstract: The proposal of a total synthesis plan of one of a series of four biologically significant 9,10-secosteroids
recently isolated from Verrucella umbraculum, together with several alternative procedures to the synthesis
plan, using widely available chemicals, is described in this work. Immunosuppressive Verrucellol A is a 9,10-
secosteroid with a bicyclic skeletal framework and an aromatic moiety. Herein, the reported proposal
commences via the synthesis of two of the three structural constituents – the α, β-unsaturated aryl ketone
vinyl, and the olefin side chain. Later, the main bicyclic ring structure is constructed via a Robinson
Annulation reaction, followed by the introduction of the olefin side chain by Grignard reductive addition.
Ultimately, an epoxidation-reductive ring opening would complete the synthesis plan.
1 INTRODUCTION
The polycyclic structures of a series of Verrucella
9,10-secosteroids resemble that of vitamin D
(Maestro, Molnár, Carlberg 2019), with the two
rightmost steroidal saturated rings preserved, and a
cleavage of C-9, C-10 bond present. The C-5, C-6 and
C-7, C-8 olefin functional groups are reduced, and
characteristically, a 3-hydroxy-10-methyl
disubstituted benzene ring is attached to the
fragmented third ring, that is, in a steroidal
framework. Variable saturated and unsaturated
substituents are connected to the five-membered ring
of the bicyclic system in this series of 9,10-
secosteroids, with irregular decorations of hydrogen
and hydroxyl groups at various regions. Specifically,
four 9,10-secosteroids, together with their twelve
derivatives, are isolated utilizing acetone extraction
and repeated column chromatography from the
Gorgonian Verrucella umbraculum collected from
the Yongxing Islands (Li, Sun, Tang, Su, Zheng,
Zhang 2021). Interestingly, in the
immunomodulation assay, the majority of the
secosteroids and their derivatives displayed an
inhibitory effect towards the proliferation of CD4
+
T
cells, or commonly known as the T helper cells.
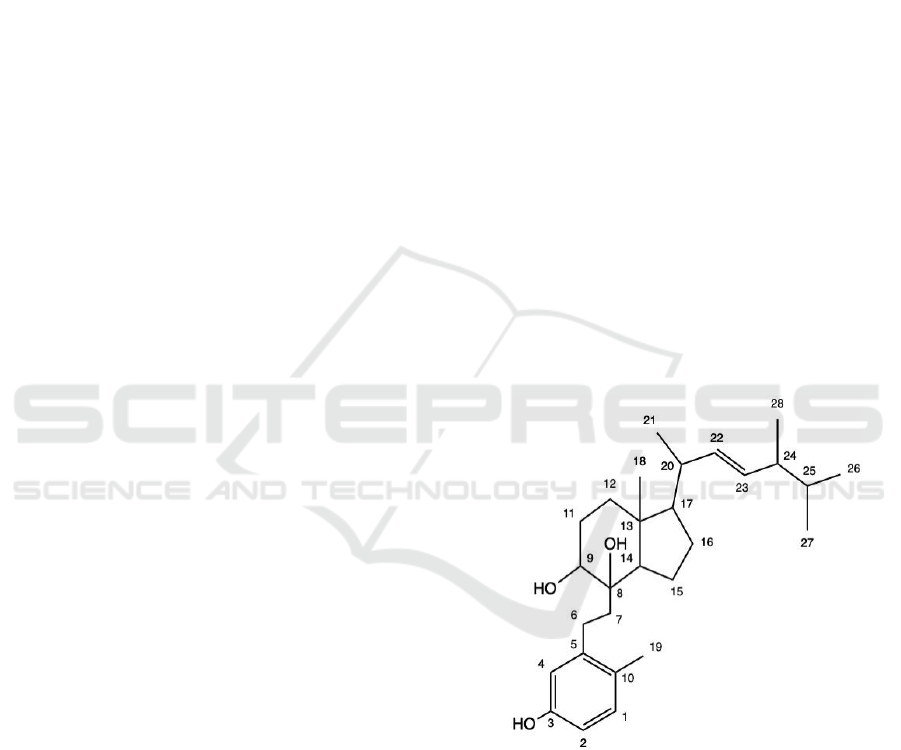
Figure.1: Target Molecule with carbon atoms numbered.
Amongst them, Verrucellol A, which features a
C-22, C-23 unsaturated hydrocarbon side chain and
an 8,9-diol moiety, displayed particular
immunosuppressive efficacy, as compared with its 9-
keto and 15-hydroxy counterparts (Li, Sun, Tang, Su,
Zheng, Zhang 2021). Therefore, the potential of using
Verrucellol A upon treating several autoimmune
diseases has been derived, including Type 1 diabetes
(Haskins, Cooke 2011), and systemic lupus
erythematosus (SLE)(He et al 2016), as both these
612
Yuan, Z., Li, H. and Han, Y.
Synthesis Proposal of Immunosuppressive 9,10-secosteroid A from the Gorgonian Verrucella Umbraculum.
DOI: 10.5220/0011249500003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 612-619
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
disorders feature CD4
+
T cells malfunction. The
existing immunosuppressive pharmaceuticals are
mainly glucocorticoids, which are sometimes prone
to a list of psychological and cognitive adverse
effects (Judd et al 2014,
Correction 2014). This
highlights the potential of using 9,10-Verrucellol A
in replacement of the existing glucocorticoid
treatments (once the drug efficacy and toxicity are
determined with further investigations), as quite
significant structural differences are present between
the two genres of immunomodulatory molecules
(Maestro, Molnár, Carlberg 2019,
Li, Sun, Tang, Su,
Zheng, Zhang 2021). Owing to the
immunosuppressive effects Verrucellol A features
towards the proliferation of CD4
+
T cells, and its
better performance than its counterparts, and the
existing adverse effects of glucocorticoids
immunomodulatory treatments, we were motivated to
propose a synthesis plan of this molecule. Several
suggestions of reaction conditions are also included
at critical procedures of the synthesis.
2 RETROSYNTHETIC ANALYSIS
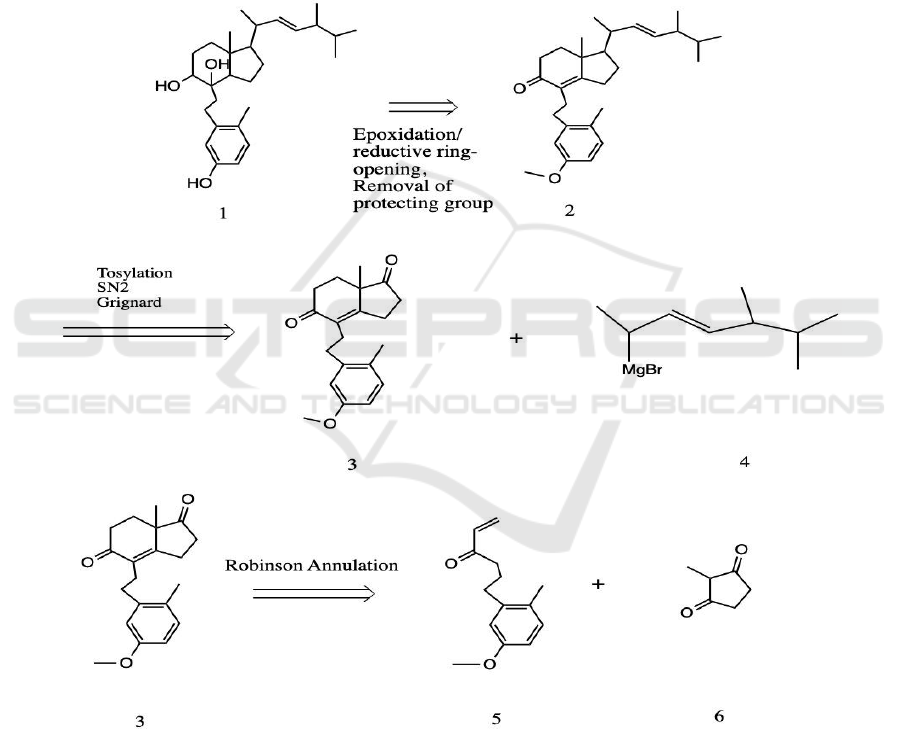
Figure 2: Retrosynthesis of 9,10-Verrucellol A.
In the analysis of Verrucella 9,10-Secosteroid’s
structure, so as to devise the retrosynthetic strategy, it
is noticed that if the diol at C-8 and C-9 on molecule
1 can be converted to an α, β-unsaturated ketone, the
structure is very close to a product of Robinson
Annulation. On the other hand, since the alcohol on
the benzene ring can be very reactive to bases, it is
decided to protect it with a methyl group until the last
step of synthesis. For the olefin side chain at the top
right, it can be added to the 5-membered ring via a
Grignard reaction. It is concluded that the alkyl group
should be added after the Robinson Annulation for a
cleaner reaction. Since molecule 6 is commercially
available, the main goal is to synthesize molecule 4
Synthesis Proposal of Immunosuppressive 9,10-secosteroid A from the Gorgonian Verrucella Umbraculum
613
and molecule 5. It is envisioned that molecule 4,
which has an olefin on it, can be assembled through a
Wittig reaction. After that, the Riley oxidation and a
substitution reaction can contribute to synthesize
molecule 2. Considering the fact that an α, β-
unsaturated ketone is quite electrophilic, it is decided
to add the terminal olefin last when constructing
molecule 5. Thus, the synthesis plan is proposed to
start with a benzene derivative 11, then extend the
carbon chain and make a Weinreb amide, which an
addition of Grignard reagent can result in the desired
molecule 5. (Schematic diagram of retrosynthetic
strategy see Figure 2.)
3 FORWARD SYNTHESIS
PROPOSAL
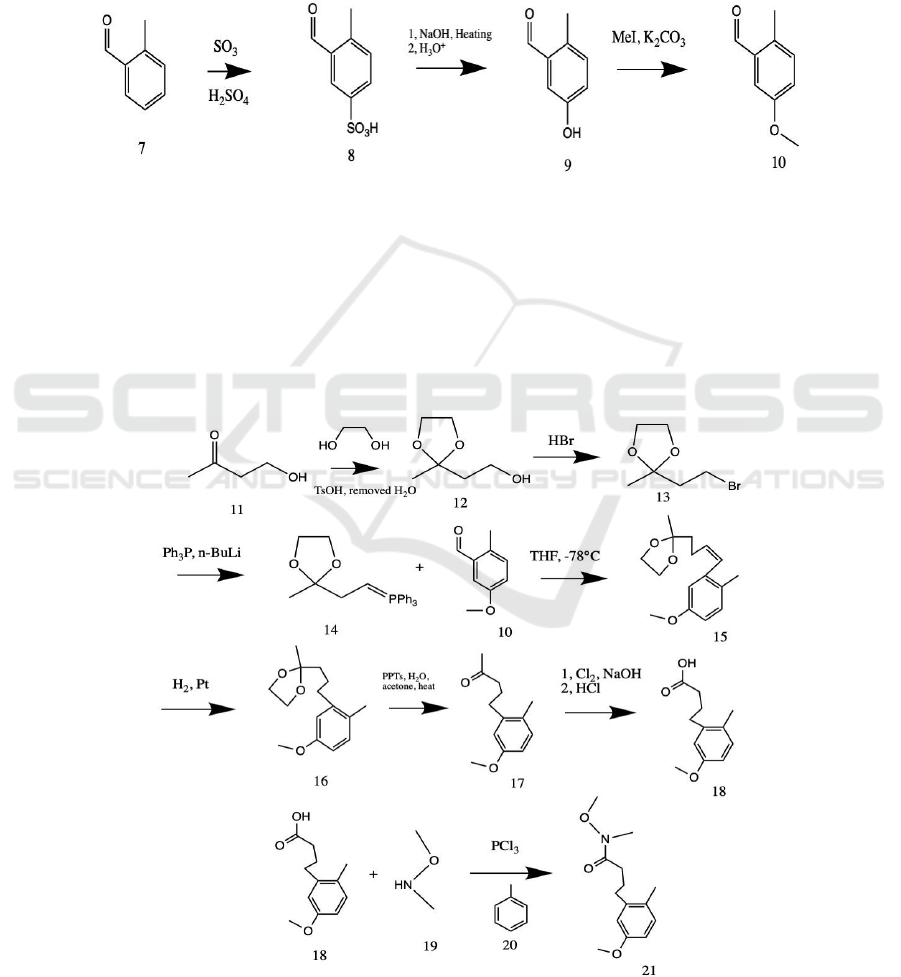
Figure 3: Synthesis of ether-protected aryl constituent 10.
The proposal of the synthesis of Verrucellol A begins
with the regioselective synthesis of ether-protected
aryl constituent 10. Due to the commercial
availability of 2-methyl benzaldehyde 7, this is
chosen as the starting material of the procedure. By
regioselectively sulfonating the meta- position with
respect to the aldehyde, followed by reflux heating
with NaOH and then an acidic workup, the meta-
regioselective hydroxylation of the benzene
derivative can be achieved. In order to prevent the
phenol 9 from further reactions with alkaline
conditions described below, the use of methyl ether
as its protection is incorporated (MeI, K
2
CO
3
,
acetone, reflux) (Greene, Wuts 1999a). This series of
processes (see Figure 3) should give the ether-
protected aryl constituent 10 in quite good yields.
Figure 4: Forward Synthesis of Weinreb Amide 21.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
614
The synthesis of ether-protected aryl ketone vinyl
5, which is vital to the construction of the six-
membered ring of the bicyclic skeleton, commences
with commercially available 4-hydroxy-2-butanone
11. In order to install the carbon chain from C-5 to C-
8 (same labelling as in Verrucellol A molecule) via a
Wittig reaction, it is necessary to substitute the
hydroxyl group of butanone 11 into a PPh
3
moiety,
making it the Wittig reagent 14. This can be
actualized by ketal protection of the carbonyl
(ethylene glycol, tosylic acid, benzene, reflux)
(Greene, Wuts 1999b), and then bromination utilizing
HBr; the bromine substituted ketal 13 can then be
treated with PPh
3
, followed by n-butyllithium
deprotonation, which yields the desired phosphonium
ylide 14. The Wittig olefination can thus be
commenced by adding the ether-protected aryl
constituent 10 to ylide 14 in THF (Maryanoff, Reitz
1989), generating the 6,7-cis unsaturated aromatic
ketal 15. This ketal 15 can then be reduced by
hydrogen gas over platinum so as to establish the
saturated C-6, C-7 σ system. Platinum has been
selected as the transition-metal catalyst as it is almost
inactive towards other reactions to olefin than
hydrogenation, such as olefin cis-trans isomerization
(Bond, Wells 1965). The saturated ketal 16 is then
subjected to deprotection at carbonyl to yield the aryl
ketone 17 (PPTs, H
2
O, acetone, heat) (Greene, Wuts
1999b). As linking the vinyl moiety to the aromatic
ketone 17 via Grignard addition with Weinreb amide
21 is intended, the conversion of aryl ketone 17 into
its carboxylic acid derivative 18 is a sound decision.
It is envisioned that this functional group
interconversion can be realized by a chloroform
oxidation (excess Cl
2
, NaOH, methyl alcohol, then
HCl workup), for comparing with other halogen
counterparts, hypochlorite facilitates the reaction
kinetics most, raising the yield and simplifying the
separation procedures rather significantly (Fuson,
Bull 1934, VanArendonk, Cupery 1931). The desired
Weinreb amide 21 can be yielded from the carboxylic
acid 18 in a one-pot, transition-metal-free reaction
with N, O-dimethylhydroxylamine 19 (PCl
3
, toluene
20)(Huang, Hu, Niu, Wang, Xu, Su, Fu 2013). This
method is no longer prone to moisture and air
sensitivity during storage, which may sometimes
become problematic (Niu, Zhang, Huang, Xu, Wang,
Hu 2009). (Schematic diagram see Figure 4.)
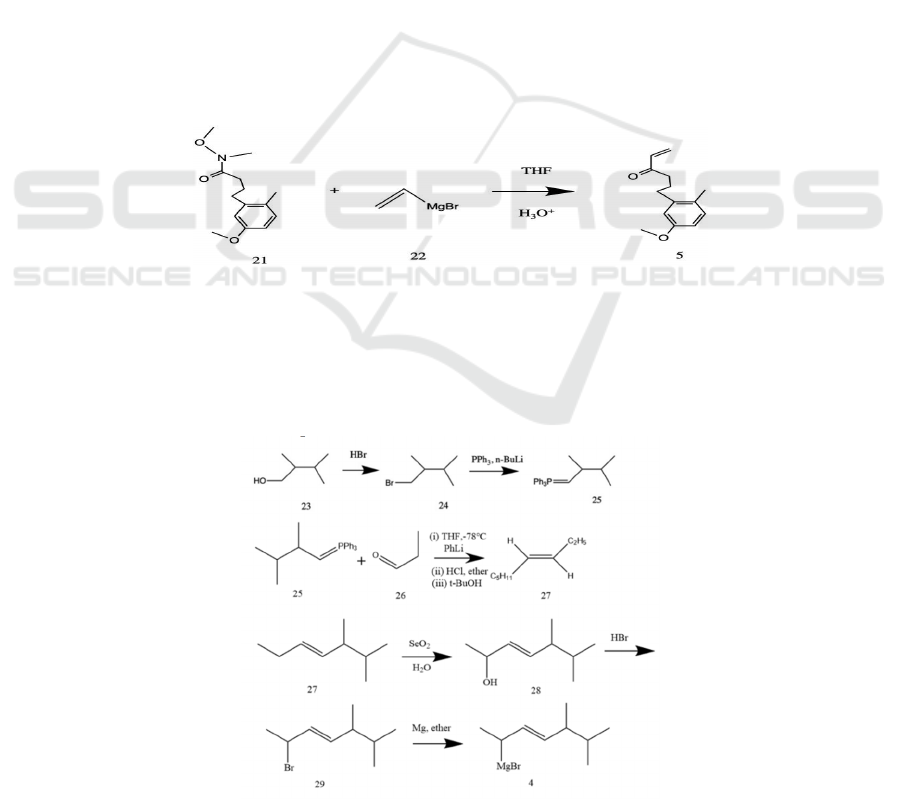
Figure.5 Grignard Addition to Weinreb Amide 21 to form molecule 5.
Then, the ether-protected aryl ketone vinyl 5 can
be easily granted by adding the vinyl Grignard 22 to
the Weinreb amide 21 in THF followed by an HCl
workup, as the formation of a stabilized O-Mg-O
chelating intermediate in this process prevents double
addition of vinyl moiety. This is an intermediate that
can be easily removed by an acidic workup to
generate the desired α, β-unsaturated ketone 5
(Nahm, Weinreb 1981). (see Figure 5)
Figure 6: The Synthesis of Grignard Reagent 4.
Synthesis Proposal of Immunosuppressive 9,10-secosteroid A from the Gorgonian Verrucella Umbraculum
615
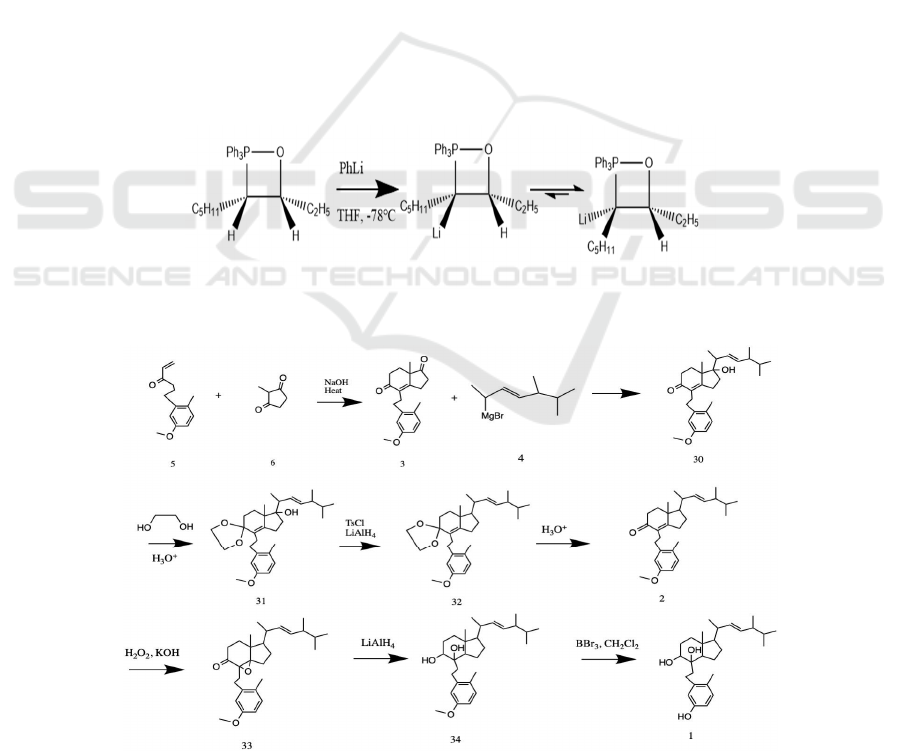
The synthesis of the olefin side chain (see Figure
6) and its attachment to the main framework could be
initiated by introducing 2,3-dimethylbutanol 23,
which is widely accessible. In an attempt to prepare a
proper ylide for future reactions, a
triphenylphosphine group could substitute the OH
group on the α carbon. A proposed method is to
substitute via HBr as such reactions take place under
relatively milder conditions. The yielded 1-bromo-
2,4-dimethylbutane 24 could subsequently react with
PPh
3
under strongly basic conditions (for example,
with the presence of n-BuLi), therefore producing the
desired ylide 25. A Schlosser-modified Wittig
reaction with propionaldehyde 26 could then be
conducted to give 5,6-dimethyl-3-heptene 27, which
features the desired E-stereoselectivity (Sondheimer,
Mechoulam 1957, Schlosser, Christmann 1966) with
a subsequent workup. With Schlosser modification,
at a low temperature and basic conditions, the
oxaphosphetane intermediate formed (Vedejs, Marth
1990, Vedejs, Snoble 1973) favors the formation of
E-stereoisomer concomitantly due to the stabilizing
effect of lithium to the ylide and the repulsion
between the bulky moieties.
The olefin side chain could be attached to a
bicyclic ring via a Grignard reaction. To prepare the
Grignard reagent 4, allylic hydroxylation by selenium
dioxide could be applied in an attempt to oxidize the
C-20 and prepare an enol. Such method introduces a
hydroxyl group selectively without any structural
rearrangement, whereas most other methods of
oxidizing allylic carbons do not have this advantage
of regioselectivity and would often prefer reacting
with the tertiary carbon, therefore leading to
undesired products. Studies have shown that it is a
sterically-driven reaction (Stephenson, Speth 1979).
There are two allylic carbons where an addition could
take place—a secondary carbon, C-20, and a tertiary
carbon, C-24. Therefore, the addition of OH group
would, presumably, take place on C-20. To complete
the preparation of the olefin side chain, the OH group
is substituted by Br so as to proceed to prepare the
Grignard reagent 4. With ether as a solvent,
magnesium reacts with 2-bromo-5,6-Dimethyl-3-
heptene 29 to give the final Grignard reagent 4
(Seyferth 1979).
Figure.7: Schlosser Modification Mechanism.
Figure.8: Robinson Annulation and Reductive Ring Opening to reach target molecule.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
616
With molecule 4 and 5 synthesized, it is ready to
assemble the target molecule. The proposed method
commences by executing a Robinson annulation with
molecule 5 and 6 and results in molecule 3 (Li 2009).
There are currently two carbonyls on molecule 3.
Since the α, β-unsaturated ketone is conjugated, its
resonance effect makes it less likely to react with
Grignard reagent 4 than the other ketone (at C-17).
Therefore, the addition of Grignard reagent 4 will be
more likely to happen at C-17. In order to eliminate
the extra OH group (at C-17) formed during Grignard
reaction, the ketone at C-9 is first protected with
ethylene glycol (ethylene glycol, tosylic acid,
benzene, reflux) (Greene, Wuts 1999b); then, the
hydroxy group at C-17 is tosylated to make it a better
leaving group, followed by the use of LiAlH
4
as a
base to substitute the tosyl group with an H, resulting
in molecule 2. In order to prevent the α, β-unsaturated
ketone from reacting with LiAlH
4
, ketal (ethylene
glycol, tosylic acid, benzene, reflux) is used to protect
it until OH group at C-17 is eliminated; molecule 32
is then subjected to de-protection (PPTs, H
2
O,
acetone, heat) (Greene, Wuts 1999b). The next step
of the synthesis is to reduce the ketone to an alcohol
and add an OH to the olefin at α-position. Such
procedure is proposed as it achieves both of them
through a reductive epoxide opening reaction. H
2
O
2
and KOH are used to construct an epoxide at the α-
position, then LiAlH
4
will be added. Since ketone is
an electron withdrawing group, it makes the olefin at
C-8 more reactive to nucleophilic epoxidation than
the olefin at C-22 with alkyl electron donating groups
around it. LiAlH
4
first reduces the carbonyl to an
alcohol, then the Al will chelate with both oxygen
atoms; therefore, when the LiAlH
4
attacks the
epoxide, it is envisioned to attack C-14, subsequently
opening the epoxide ring and forming molecule 34
(Diehl 1937). The last step of the synthesis is to de-
protect the MeO group (BBr
3
, CH
2
Cl
2
) on the
benzene ring, which gives us the target molecule
(Greene, Wuts 1999a).
4 EVALUATIONS
The purpose of this proposal is to offer an insight into
the synthesis of a potentially biologically significant
molecule, so experimentations are necessary when
realizing this scheme in laboratories. To further
elaborate the synthesis, efforts could be endeavored
to select a route that features diastereoselectivity of
9,10-Verrucellol A. Nonetheless, we will include
below several suggestions which could be taken into
account if unexpected yields are derived from
particular procedures.
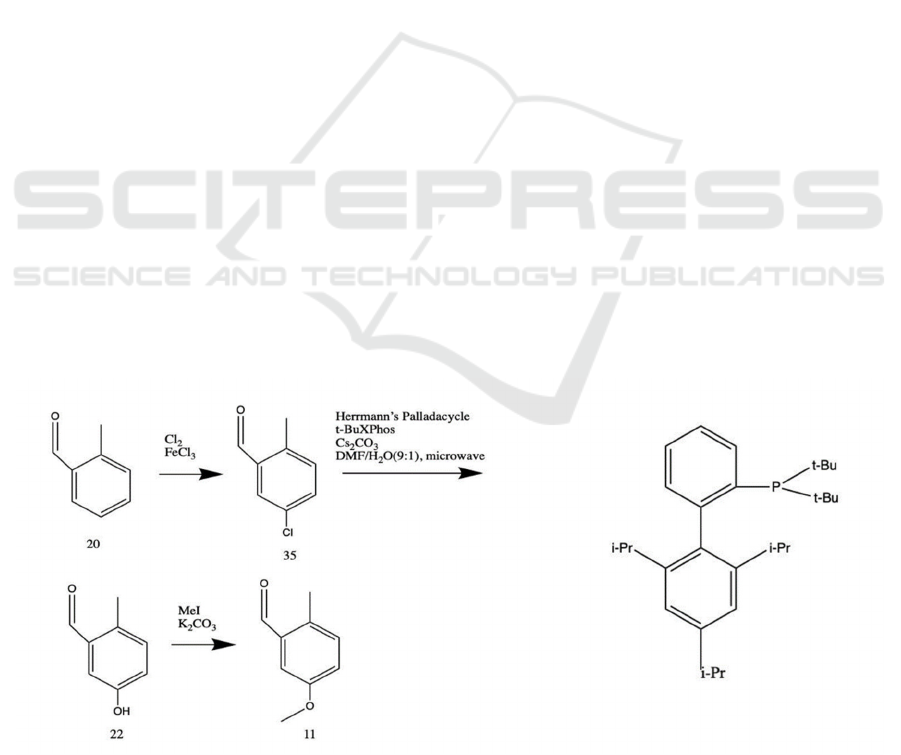
As sometimes, too reactive reagents (such as
strong acids and alkalis, high temperatures and
pressures, repetitive heating and so on) might lead to
undesired functional group interconversions, which is
problematic. One potential manifestation of this
repercussion lies with the regioselective synthesis of
ether-protected aryl constituent 11. Included in the
aforementioned synthesis scheme is the utilization of
the Dow process (sulfonation, NaOH reflux, workup)
with 2-methyl benzaldehyde 20, but from practical
perspectives, the high temperature in alkaline
conditions may degrade the desired aldehyde
functional group.
Figure.9: Pd-catalyzed synthesis of ether-protected aryl constituent 11 (left); with structure of t-BuXPhos ligand elucidated
(right).
Synthesis Proposal of Immunosuppressive 9,10-secosteroid A from the Gorgonian Verrucella Umbraculum
617

Instead, a palladium-catalyzed, microwave-
assisted alkaline hydroxylation of aryl chloride 35
(see Figure 9), with the help of Herrmann’s
Palladacycle (Herrmann, Brossmer, Reisinger,
Riermeier, ÖFele, Beller 1997) and t-BuXPhos
ligand, can be incorporated (Yu, Chen, Huang, Chern
2012). This method has been proved to maintain high
yields even with aldehyde substituents present on the
benzene ring, as milder reaction conditions are
featured. Therefore, the modified reaction cascade
would be as follows: aryl chlorination (liquid Cl
2
,
FeCl
3
), then palladium-catalyzed hydroxylation
(Herrmann’s Palladacycle, t-BuXPhos, Cs
2
CO
3
,
DMF/H
2
O (9:1), microwave) (Yu, Chen, Huang,
Chern 2012), followed by methyl ether protection
(MeI, K
2
CO
3
, acetone) (Greene, Wuts 1999a), giving
the desired ether-protected aryl constituent 11.
It should be noted that in the synthesis proposal,
it has been hypothesized that for molecule 3, the
ketone at C-17 reacts more readily with Grignard
moiety than the α, β-unsaturated ketone at C-9.
Nonetheless, if proved otherwise in experimentations
(by getting more product molecules whose bicyclic
system is linked to the olefin side chain at C-9), a
back-up synthesis route can be adopted: the α, β-
unsaturated ketone at C-9 is first protected (ethylene
glycol, tosylic acid, benzene, reflux) (Greene, Wuts
1999b), followed by reductive addition with Grignard
reagent 4 in THF, which yields molecule 31 directly.
The synthesis can then proceed in the same cascade
as shown in Figure 8.
5 CONCLUSIONS
In summary, because of its biochemical significance
and potential pharmacological efficacy, a synthesis
scheme of 9,10-Verrucellol A has been proposed,
using widely accessible starting materials and a
relatively straightforward synthesis route, which
features constructing the bicyclic skeleton with a
Robinson Annulation, followed by joining the olefin
side chain by a Grignard addition, and approaching
the structure of target molecule with several fine
adjustments. A number of proposed reaction
conditions have also been included herein. This paper
could act as a foundation for further investigations,
such as diastereoselective synthesis of 9,10-
Verrucellol A; in vivo assessments of the target’s
pharmacological efficacy could also be endeavored to
evaluate its practical use.
REFERENCES
Bond, G., & Wells, P. (1965). The Mechanism of the
Hydrogenation of Unsaturated Hydrocarbons on
Transition Metal Catalysts. Advances in Catalysis, 91–
226. https://doi.org/10.1016/s0360-0564(08)60554-4
Correction. (2014). American Journal of Psychiatry,
171(11), 1224.
https://doi.org/10.1176/appi.ajp.2014.17111correction
Diehl, H. (1937). The Chelate Rings. Chemical Reviews,
21(1), 39–111. https://doi.org/10.1021/cr60068a003
Fuson, R. C., & Bull, B. A. (1934). The Haloform Reaction.
Chemical Reviews, 15(3), 275–309.
https://doi.org/10.1021/cr60052a001
Greene, T. W., & Wuts, P. G. M. (1999a). Protection for
Phenols and Catechols. Protective Groups in Organic
Synthesis, 246–292.
https://doi.org/10.1002/0471220574.ch3
Greene, T. W., & Wuts, P. G. M. (1999b). Protection for
the Carbonyl Group. Protective Groups in Organic
Synthesis, 312–322.
https://doi.org/10.1002/0471220574.ch4
Haskins, K., & Cooke, A. (2011). CD4 T cells and their
antigens in the pathogenesis of autoimmune diabetes.
Current Opinion in Immunology, 23(6), 739–745.
https://doi.org/10.1016/j.coi.2011.08.004
He, J., Zhang, X., Wei, Y., Sun, X., Chen, Y., Deng, J., Jin,
Y., Gan, Y., Hu, X., Jia, R., Xu, C., Hou, Z., Leong, Y.
A., Zhu, L., Feng, J., An, Y., Jia, Y., Li, C., Liu, X., . .
. Li, Z. (2016). Low-dose interleukin-2 treatment
selectively modulates CD4+ T cell subsets in patients
with systemic lupus erythematosus. Nature Medicine,
22(9), 991–993. https://doi.org/10.1038/nm.4148
Herrmann, W. A., Brossmer, C., Reisinger, C. P.,
Riermeier, T. H., ÖFele, K., & Beller, M. (1997).
Palladacycles: Efficient New Catalysts for the Heck
Vinylation of Aryl Halides. Chemistry - A European
Journal, 3(8), 1357–1364.
https://doi.org/10.1002/chem.19970030823
Huang, D., Hu, Y., Niu, T., Wang, K. H., Xu, C., Su, Y., &
Fu, Y. (2013). One-Pot Transition-Metal-Free
Synthesis of Weinreb Amides Directly from
Carboxylic Acids. Synthesis, 46(03), 320–330.
https://doi.org/10.1055/s-0033-1340317
Judd, L. L., Schettler, P. J., Brown, E. S., Wolkowitz, O.
M., Sternberg, E. M., Bender, B. G., Bulloch, K.,
Cidlowski, J. A., Ronald De Kloet, E., Fardet, L., Joëls,
M., Leung, D. Y., McEwen, B. S., Roozendaal, B., van
Rossum, E. F., Ahn, J., Brown, D. W., Plitt, A., &
Singh, G. (2014). Adverse Consequences of
Glucocorticoid Medication: Psychological, Cognitive,
and Behavioral Effects. American Journal of
Psychiatry, 171(10), 1045–1051.
https://doi.org/10.1176/appi.ajp.2014.13091264
Li, J. J. (2009). Robinson Annulation. Name Reactions,
470–471. https://doi.org/10.1007/978-3-642-01053-
8_219
Li, J., Sun, Y. L., Tang, H., Su, L., Zheng, G. L., & Zhang,
W. (2021). Immunosuppressive 9,10-Secosteroids
from the Gorgonian Verrucella umbraculum Collected
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
618

in the South China Sea. Journal of Natural Products,
84(5), 1671–1675.
https://doi.org/10.1021/acs.jnatprod.1c00200
Maestro, M. A., Molnár, F., & Carlberg, C. (2019). Vitamin
D and Its Synthetic Analogs. Journal of Medicinal
Chemistry, 62(15), 6854–6875.
https://doi.org/10.1021/acs.jmedchem.9b00208
Maryanoff, B. E., & Reitz, A. B. (1989). The Wittig
olefination reaction and modifications involving
phosphoryl-stabilized carbanions. Stereochemistry,
mechanism, and selected synthetic aspects. Chemical
Reviews, 89(4), 863–927.
https://doi.org/10.1021/cr00094a007
Nahm, S., & Weinreb, S. M. (1981). N-methoxy-n-
methylamides as effective acylating agents.
Tetrahedron Letters, 22(39), 3815–3818.
https://doi.org/10.1016/s0040-4039(01)91316-4
Niu, T., Zhang, W., Huang, D., Xu, C., Wang, H., & Hu, Y.
(2009). A Powerful Reagent for Synthesis of Weinreb
Amides Directly from Carboxylic Acids. Organic
Letters, 11(19), 4474–4477.
https://doi.org/10.1021/ol901886u
Schlosser M. & Christmann K. (1966). Trans-Selective
Olefin Syntheses. Angewandte Chemie, 5(1), 126–126.
https://doi.org/10.1002/anie.196601261
Seyferth D. (1979). The Grignard Reagents.
Organometallics, 28(6), 1598–1605
https://pubs.acs.org/doi/10.1021/om900088z
Sondheimer F. & Mechoulam R. (1957). Synthesis of
Steroidal Methylene Compounds by the Wittig
Reaction. Journal of the American Chemical Society,
79(18), 5029–5033.
https://pubs.acs.org/doi/10.1021/ja01575a054
Stephenson L. & Speth. D. (1979). Mechanism of allylic
hydroxylation by selenium dioxide. The Journal of
Organic Chemistry, 44(25), 4683–4689.
https://pubs.acs.org/doi/10.1021/jo00393a045
VanArendonk, A. M., & Cupery, M. E. (1931). The
Reaction of Acetophenone Derivatives with Sodium
Hypochlorite. Journal of the American Chemical
Society, 53(8), 3184–3186.
https://doi.org/10.1021/ja01359a506
Vedejs E. & Marth C. (1990). Mechanism of Wittig
Reaction: Evidence Against Betaine Intermediates.
Journal of the American Chemical Society, 112(10),
3905–3909.
https://pubs.acs.org/doi/10.1021/ja00166a026
Vedejs E. & Snoble K. (1973). Direct Observation of
Oxaphosphetanes from Typical Wittig Reactions.
Journal of the American Chemical Society, 95(17),
5778–5780.
https://pubs.acs.org/doi/pdf/10.1021/ja00798a066
Yu, C. W., Chen, G. S., Huang, C. W., & Chern, J. W.
(2012). Efficient Microwave-Assisted Pd-Catalyzed
Hydroxylation of Aryl Chlorides in the Presence of
Carbonate. Organic Letters, 14(14), 3688–3691.
https://doi.org/10.1021/ol301523q
Synthesis Proposal of Immunosuppressive 9,10-secosteroid A from the Gorgonian Verrucella Umbraculum
619
