
Sleep and Alzheimer’s Disease: Lacking Sleep, Having Enough Sleep,
Sleeping Too Much, and Mental Activities during Daytime
Tingting Pan
The Second Foreign Language School Affiliated to Shanghai Normal University
Shanghai, 201399, China
Keywords:
Alzheimer’s Disease, Sleep Aβ Plaque, Tau Protein.
Abstract:
This work discusses how does sleep influences the incidence rate of Alzheimer’s Disease, from 3 scenarios:
lacking sleep, having enough sleep, and sleeping too much. Lack of sleep can be further divided into having
a snap during the daytime or not. Some studies proved that sleep disturbances are associated with Alzheimer’s
Disease from experiments by comparing mice with not enough sleep and with enough sleep. However, they
didn’t concern about the symptoms of oversleeping, and the mental activities during a human being’s life
compared to a mouse. Therefore, this work will try to demonstrate that sleep disturbances are associated with
the risk of developing AD inherently. Alzheimer’s Disease is incurable, but there are ways to reduce the
potential of getting it.
1 INTRODUCTION
Alzheimer's Disease is a progressive disease, with
symptoms like confusion, disorientation, poor
concentration, and change in personality, which
would become worsen over years. “There are over 55
million people worldwide living with dementia in
2020”(Alzheimer’s Disease International. (2020),
which means there are about 55 million families
suffered from it. Moreover, Alzheimer’s disease has
heredity, which means it is possible to continue this
suffering. More understanding of sleep and
Alzheimer’s Disease could promote the development
of new therapeutic approaches, and benefit all human
beings. Therefore, sleep as one of the factors of
Alzheimer’s Disease is essential to study.
Some studies proved that sleep disturbances are
associated with Alzheimer’s Disease from
experiments by comparing mice with not enough
sleep and with enough sleep. However, these
experiments are biased because they didn’t mention
the symptoms of oversleeping. Also, these
experiments are not clear about the situations of mice
in the daytime, because normal human beings would
have much more mental activities in the daytime than
mice, and these experimenters might only afford them
water and food during experiment days.
In order to determine different kinds of sleep
symptoms’ relationship to Alzheimer’s
Figure 1: Shows relationships between sleep and AD.
228
Pan, T.
Sleep and Alzheimer’s Disease: Lacking Sleep, Having Enough Sleep, Sleeping Too Much, and Mental Activities during Daytime.
DOI: 10.5220/0011248400003438
In Proceedings of the 1st International Conference on Health Big Data and Intelligent Healthcare (ICHIH 2022), pages 228-232
ISBN: 978-989-758-596-8
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

Disease, the experiment would compare the AD
pathogenesis in 4 experimental groups and 1 control
group. The results might demonstrate that sleep
disturbances are associated with the risk of
developing AD inherently and explain why people
with less sleep and less mental activities are more
vulnerable to AD progression.
Sleep can be divide into three kinds: not enough
(not enough time or poor quality), enough (about 7
hours), and oversleeping. Lack of sleep can be divide
into sleep (like siesta) or doesn’t sleep in the daytime.
However, all of them will cause the increase of
amyloid-β (Aβ) amount and result in Alzheimer’s
Disease at a later age.
Studies showed that, “People who slept six hours
or less per night in their 50s and 60s were more likely
to develop dementia later in life.” (Bryant, Erin.
2021). During the sleep, cerebrospinal fluid (CSF)
can wash away “harmful waste proteins that build up
between brain cells during waking hours.”(Hamilton,
Jon. 2013)
Figure 2: Shows change of Aβ levels in human between
wake and sleep periods. (Cedernaes, Jonathan., et al. 2017).
Therefore, if a person didn’t sleep for the whole
night, the Aβ level will increase extremely in his
bloodstream and cerebrospinal fluid (CSF).
Researchers also found people who are oversleeping
(over 9 hours a day) “were twice as likely to develop
Alzheimer’s” (British Neuroscience Association.
2017). The reason is that most people who are
oversleeping have metabolic dysfunction because of
obesity and inactivity, which can affect their sleep
quality, like diabetes which “can cause sleep loss”
(Mann, Denise. 2010). For example, people who are
overweight are more likely to have sleep apnea.
Moreover, people with Alzheimer’s Disease are
often tired during the day but with poor sleeping
quality, so it would also cause them to oversleep.
Besides, the two kinds (lack of sleep and
oversleeping) of sleep disturbances and the normal
kind of sleep (enough sleeping during the day) will all
be growing the risk of developing Alzheimer’s
Disease because of aging.
Figure 3: Shows ciucular relationshp between Obesity
(Type 2 Diabetes) and Sleep Apnea. (Framnes, Sarah., et al.
2018).
2 METHODS AND RESULTS
2.1 Animals
In this study, transgenic C57BL/6J mice are used as a
model of Alzheimer’s disease. The mice are most
commonly used as the human disease model because
of their availability of homogeneous strains and they
are easy to breed. Moreover, the C57BL/6J mice have
always been used to study sleep disturbances, because
after acute hypoxia, they showed different symptoms
of sleep disturbances, like “greater amount of
irregular breathing during rest” (Chai, Sam., et al.
2011), which would influence sleep quality.
Because of the average life span for C57BL/6J
mice is about 550 days, the experiment starts at the
ages of 10 months with 6 mice per cage (3 female and
3 male animals) as a group. Before the experiment
started, mice are ad libitum to access water and food
at 23 degrees Celsius with 12h-day/12h-night cycles.
When the experiment started, four groups of
C57BL/6J mice are used for these studies: normal
sleep with normal activity (eating, etc.) in the daytime
(n=6, 3 female and 3 male animals), abnormal sleep
with normal activities, and allowed to take a rest (like
siestas in human) during the daytime (n=6), abnormal
sleep with normal activities and not allowed to take a
rest in the daytime (n=6), and abnormal sleep with
mind training in the daytime (n=6). Non-transgenic
littermates with normal sleep and mind training in the
daytime (n=6) act as the control group because it's
closer to normal human beings’ daily routine. The
animal experiments mentioned above conform to the
requirements given by the institutional animal care
and animal use committee.
Sleep and Alzheimer’s Disease: Lacking Sleep, Having Enough Sleep, Sleeping Too Much, and Mental Activities during Daytime
229
2.2 Brain Training
To simulate normal human beings' daily routine,
brain training is necessary for the experiment. Clicker
training is introduced to certain experiment groups
for the enrichment of the cognition of the mice. The
training session is last for three weeks, and studies
showed “trained mice displayed less of this
depression-related behavior” (Leidinger, Charlotte.,
et al. 2017).
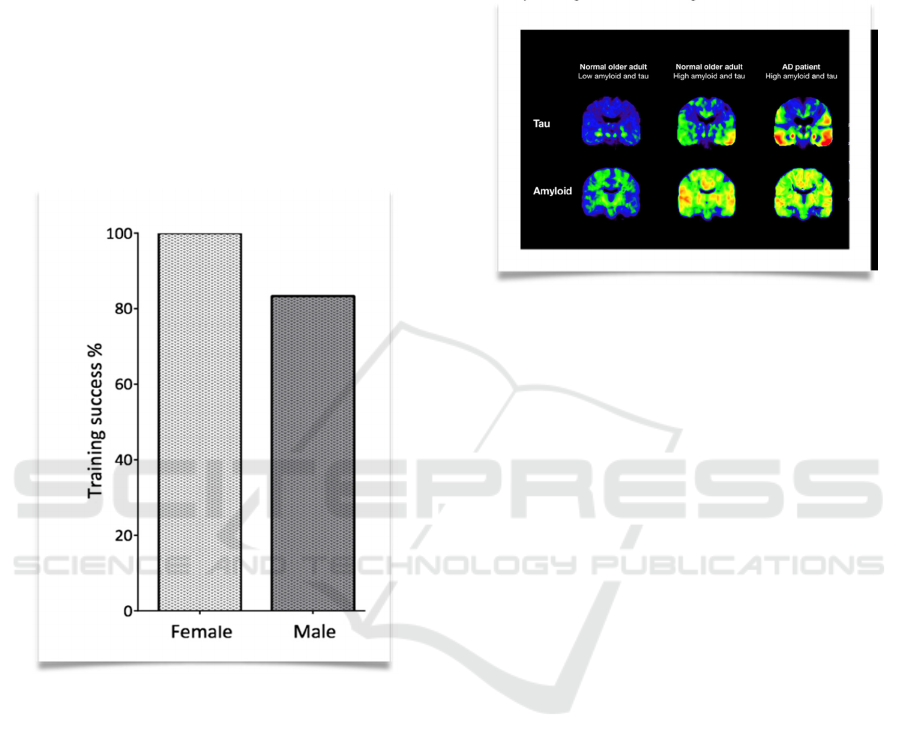
One benefit of this training is that it could be
achieved successfully in almost all the mice (“100%
of female mice and 83% of male mice”) (Leidinger,
Charlotte., et al. 2017).
Figure 4: Shows training success rate (Leidinger,
Charlotte., et al. 2017)
2.3 HistologyAβ Plaque Detection
Positron emission tomography (PET) imaging can
“measure physiological function by looking at blood
flow, metabolism, neurotransmitters, and
radiolabelled drugs” (Berger, Abi. 2003), which
include Aβ plaques of a certain region. To detect the
Aβ plaques level in five groups of mice, 18F-FC119S,
which is a radiopharmaceutical is injected into the
mouse. Later, the Aβ plaque level in the brain and
also in the cerebrospinal fluid (CSF) can be seen by
using PET scanning.
Tau protein detection:
Positron emission tomography (PET) imaging can
also be used for tau protein detection in five groups
of mice. To detect the tau protein, 18F-AV-1451 is
injected into the mice's brain as a radio-diagnostic
agent. “F-FC119S is a positron emission tomography
(PET) tracer for imaging β-amyloid (Aβ) plaques in
Alzheimer’s disease (AD)” (Oh, Se Jong., et al.,
2018). After that, the tau protein aggregation can be
seen by using PET scanning.
Figure 5: Shows PET scans for Aβ plaque and Tau protein
from normal older adult (with low Aβ plaque and Tau
protein level), normal older adult (with high Aβ plaque and
Tau protein level), and AD patient. (Yang, Sarah. (2016)
2.4 Results
Experiment groups with abnormal sleep are assumed
to have higher tau protein and Aβ levels, whereas
groups with normal sleep have less tau protein
because the tau protein and Aβ protein are normally
cleared by the glymphatic system, “a cleaning
mechanism that functions in the removal of
potentially harmful metabolites and proteins from the
brain” (Cai, XueZhu., et al. 2020), during sleep.
Take the average and standard deviation of the
data (Aβ level, tau protein level) in each group. Then,
do the following tests:
T-test:
H0: There is no difference between 5groups
H1: group 1 < group 2, 3, 4 < group 5
If P≤ 0.05, then the results reject the null
hypothesis; if P> 0.05, then the results failed to reject
the null hypothesis.
One-way ANOVA test:
H0: There is no difference among the means of the
three test groups.
H1: There is a difference among means of five test
groups, and the data of the group 2, 3, 4 lies between
group 1 and group 5.
If p≤ 0.05, then the results reject the null
hypothesis; if p>0.05, then the results fail to reject the
null hypothesis.
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
230
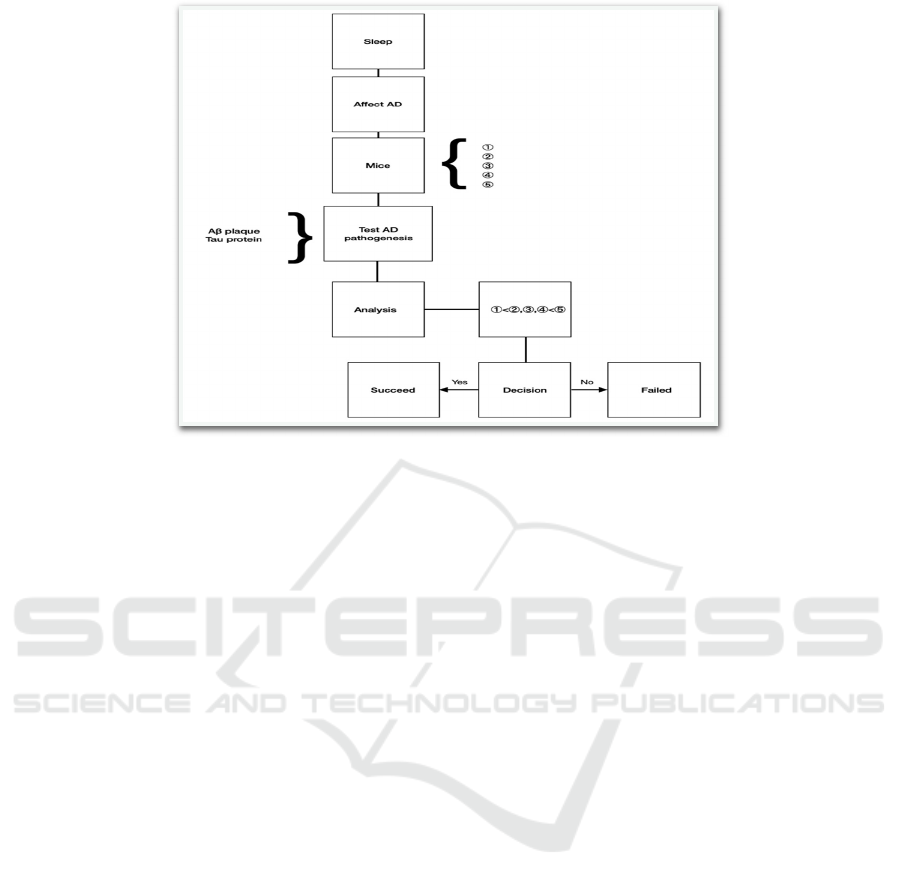
Figure 6: Shows experiment process of the work.
3 CONCLUSIONS
3.1 Experiments Review
It is no doubt that sleep can affect the risk of
Alzheimer’s Disease. Therefore, in the experiment,
four groups of transgenic C57BL/6J mice are used as
experiment groups, and the non-transgenic mice are
the control group.
①
Non-transgenic littermates with normal sleep and
mind training in the daytime (n=6, 3 female and 3
male animals) act as the control group.
②
normal sleep with normal activity (eating etc.) in
the daytime (n=6)
③
abnormal sleep with normal activities and allowed
to take a rest (like siestas in humans) during the
daytime (n=6)
④
abnormal sleep with normal activities and not
allowed to take a rest in the daytime (n=6)
⑤
abnormal sleep with mind training in the daytime
(n=6)
After the experiments, Aβ plaques level and Tau
protein are detected as AD pathogenesis. After
analyzing the data, if it matches the hypothesis that
the tau protein and Aβ plaques level in group 1 is less
than group 2, 3, 4, and less than group 5, the
experiments succeed, and if not, the experiments
failed.
3.2 Future Directions
As mentioned in the results, the glymphatic system in
the mice brain can clear amyloid-β and tau protein
during sleep, but it is only in the mice brain. The
glymphatic system in the human brain still needs to
explore.
Notably, sleep is related to inflammation in the
brain, and Alzheimer’s Disease can also lead to
inflammation. Therefore, the connection between
sleep, inflammation, and Alzheimer’s Disease is also
an essential area to be understood.
Along with aging, the sleeping time will normally
decrease in older people, how to improve their sleep
quality is a problem that remains unsolved.
Moreover, how to lengthen older people’s sleep, and
if that is helpful to reduce the risk of developing
Alzheimer’s Disease remains unknown.
The proposed model still needs further research to
improve, and here are some suggestions for decrease
the risk of developing Alzheimer’s Disease.
1.
Sleep about 8 hours a day, not less than 6 hours
and no more than 9 hours.
2.
Siesta should be less than 1 hour a day
3.
Focus on sleep quality as well, can use eye patch
and sleep aromatherapy, etc. to improve sleep
quality.
Sleep and Alzheimer’s Disease: Lacking Sleep, Having Enough Sleep, Sleeping Too Much, and Mental Activities during Daytime
231

REFERENCES
Alzheimer’s Disease International. (2020) Dementia
statistics. https://www.alzint.org/about/dementia-facts-
figures/dementia-statistics/.
Berger, Abi. (2003) Positron emission tomography.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1126
321/.
British Neuroscience Association. (2017)
OVERSLEEPING LINKED TO DEMENTIA.
https://www.bna.org.uk/mediacentre/news/oversleepin
g-linked-to-dementia/.
Bryant, Erin. (2021) Lack of sleep in middle age may
increase dementia risk. https://www.nih.gov/news-
events/nih-research-matters/lack-sleep-middle-age-
may-increase-dementia-risk.
Cai, XueZhu., et al. (2020) Imaging the effect of the
circadian light–dark cycle on the glymphatic system in
awake rats. https://www.pnas.org/content/117/1/668.
Cedernaes, Jonathan., et al. (2017) Candidate mechanisms
underlying the association between sleep-wake
disruptions and Alzheimer's disease.
https://www.sciencedirect.com/science/article/pii/S10
87079216000186#fig1.
Chai, Sam., et al. (2011) Morphological differences of the
carotid body among C57/BL6 (B6), A/J, and CSS
B6A1 mouse strains.
https://europepmc.org/article/pmc/pmc4455900.
Framnes, Sarah., et al. (2018) The Bidirectional
Relationship Between Obstructive Sleep Apnea and
Metabolic Disease.
https://www.frontiersin.org/articles/10.3389/fendo.201
8.00440/full.
Hamilton, Jon. (2013) Brains Sweep Themselves Clean Of
Toxins During Sleep.
https://www.npr.org/sections/health-
shots/2013/10/18/236211811/brains-sweep-
themselves-clean-of-toxins-during-
sleep?t=1628856998176.
Leidinger, Charlotte., et al. (2017) Introducing Clicker
Training as a Cognitive Enrichment for Laboratory
Mice.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5408
971/.
Mann, Denise. (2010) The Sleep-Diabetes Connection.
https://www.webmd.com/diabetes/features/diabetes-
lack-of-sleep.
Oh, Se Jong., et al., (2018) Early Detection of
Aβ Deposition in the 5xFAD Mouse by Amyloid PET.
https://www.hindawi.com/journals/cmmi/2018/527201
4/.
Yang, Sarah. (2016) PET scans reveal key details of
Alzheimer’s protein growth in aging brains.
https://news.berkeley.edu/2016/03/02/pet-scans-
alzheimers-tau-amyloid/.
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
232
