
Medicines and Vaccines in Dealing with Covid-19
Xianyi Zheng
a
Li Po Chun United World College of Hong Kong, HKSAR, China
Keywords:
SARS-CoV-2, Covid-19, Medecines, Vaccines.
Abstract: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)(Wikipedia 2021) has several components,
including spike protein and membrane protein, and has several variants. Currently, remdesivir and
dexamethasone are two common medicines that are used for treatments of the Covid-19 disease. It has been
shown that the recovery time for patients who were treated with these two drugs was much shorter than
patients who did not treat with them and the mortality of patients who were treated with them was lower than
patients who did not. Also, several vaccines, including vaccines produced by Moderna, vaccines produced by
BioNTech/ Pfizer, and vaccines produced by Oxford/ AstraZeneca have shown efficacies in preventing
infection of the virus. The efficacies of these three vaccines towards original SARS-CoV-2 were all above
70%, for which vaccine produced by Moderna, vaccine produced by BioNTech/ Pfizer was around 94.5%
(Moderna, Inc. 2020) and 95%)(Polack et al. 2020), and that of Oxford/ AstraZeneca was around
70%(NEEDHAM 2020). However, when dealing with SARS-CoV-2 variants, the efficacies of these three
vaccines decreased. In conclusion, although different medicines and vaccines have been used to prevent the
exacerbation of the Covid-19 pandemic, the emerge of SARS-CoV-2 variants is still a challenge as these
variants decrease the efficacies of vaccines and increase the infection rate.
1 INTRODUCTION
A new type of coronavirus (severe acute respiratory
syndrome coronavirus 2) was rapidly spread in
Wuhan, China, in late 2019. Although many
governments and non-government organizations put
a large number of financial resources into tackling the
pandemic, it is hard to observe the turning point of
this pandemic until now, and the future course of this
virus is still unknown. Many companies and
institutions have been trying to invent vaccines and
medicines based on the structure of COVID-19, and
some of them have major breakthroughs on vaccines
and medicines. This paper would illustrate the basic
structure of coronavirus and give a broad overview of
the current medicines and the development of
vaccines. Topics discussed in the paper give the
newest update to understand the current situation of
the pandemic, which is essential to the fundamental
development of different research.
a
https://orcid.org/0000-0003-1467-1342
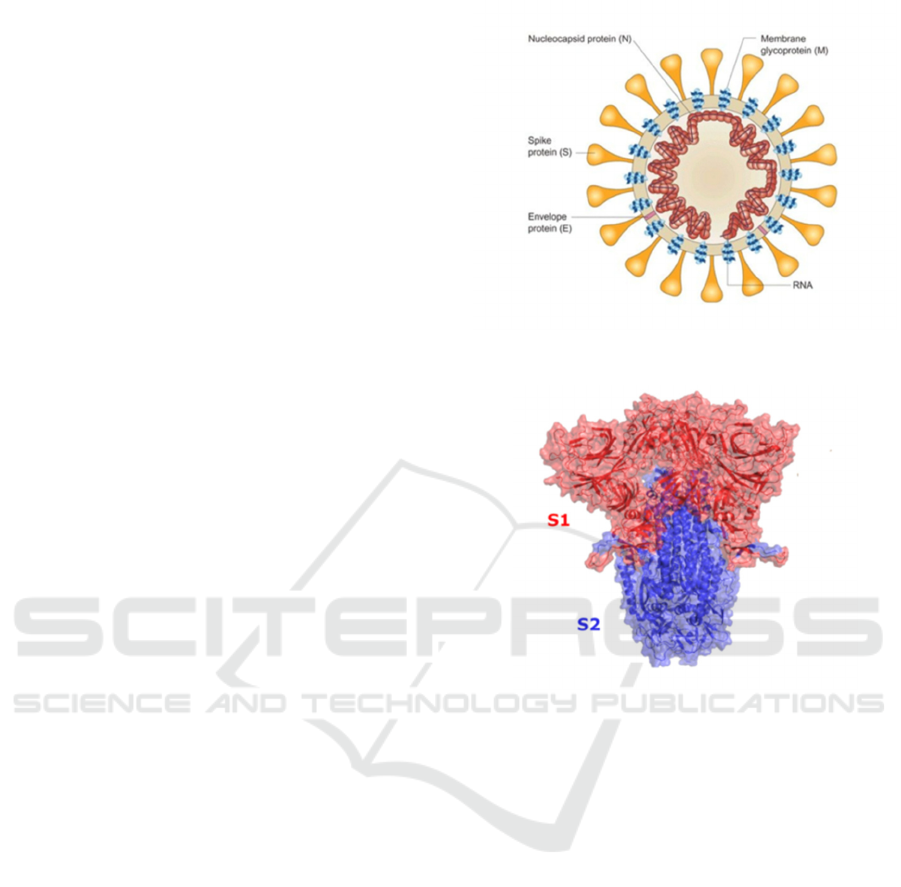
2 STRUCTURE OF SARS-COV-2
SARS-CoV-2 is a single-stranded RNA-enveloped
virus (figure 1), and like other viruses, it cannot
survive without a host cell. This virus has been
reported that it has more than 95% homology with the
coronavirus in the bat’s body and more than 70%
similar to the SARS-CoV. RNA of the virus gives its
structure and enables it to replicate. It has structural
proteins, such as the S (Spike) protein, the E
(Envelope) protein, the M (Membrane), and N
(Nucleocapsid) proteins, and non-structural proteins,
such as 3-chymotrypsin-like protease, papain-like
protease, and RNA-dependent RNA
polymerase(Huang, Yang, Xu, Xu, and Liu 2020).
2.1 Spike Protein
The spike protein gives the virus a “corona” structure.
For spike protein (figure 1), it is responsible for a
virus to attach to the membrane of the host cell, and
it has two functional subunits (figure 2), which are S1
and S2. The S1 subunit consists of the N-terminal
Zheng, X.
Medicines and Vaccines in Dealing with Covid-19.
DOI: 10.5220/0011248000003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 601-611
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
601
domain (NTD) and receptor-binding domain (RBD)
(Huang, Yang, Xu, Xu, Liu 2020), which allows it to
bind to the host cell receptor. The S2 subunit
comprises of fusion peptide (FP), heptad repeat 1
(HR1), central helix (CH), connector domain (CD),
heptad repeat 2 (HR2), transmembrane domain (TM),
and cytoplasmic tail (CT) (Huang, Yang, Xu, Xu, and
Liu 2020) (Figure 3), which allows it to mediate the
fusion of viral and cellular membranes. The receptor-
binding domain (RBD) recognizes a specific receptor
called the ACE2 (angiotensin-converting enzyme
receptor 2). It has shown that the binding of ACE2
receptor with RBD is at least the same affinity and
potentially as much as 20 times greater affinity than
the SARS virus. Such high infinity could be one of
the explanations for the reasons why it spreads so
easily.
2.2 Membrane Protein
The membrane protein (figure 1) is the most abundant
protein on the viral surface and defines the shape of
the viral envelope. It likes a central organizer for
coronavirus assembly and interacts with the other
structural proteins on the viral membrane.
2.3 Other Components and
Nucleocapsid Protein
The viral envelope (figure 1), a fatty layer, is
underneath the surface proteins derived from the host
cell membrane. When it contacts with soap, it will
break down and die, which suggests that
handwashing with soap is essential to prevent the
spread of this virus.
Underneath this layer is a capsid, a protein shell
that encloses the virus’s genetic material. Inside this
capsid, nucleocapsid proteins (figure 1) can be found.
These proteins are bound to the virus’s single strand
of RNA, which is the place where genetic information
is held to allow the virus to replicate. Nucleocapsid
protein is multifunctional. It essentially inhibits a lot
of host cells defense mechanisms and assists the viral
RNA in replicating itself and, therefore, in creating
new viral particles.
Figure 1: (LubioScience 2020). Structure of SARS-CoV-2.
Figure 2: (Mansbach, Chakraborty, Nguyen, Montefiori,
Korber, Gnanakaran 2021). Structure of spike protein.
3 SYMPTONS OF DISEASE
3.1 Transmission of COVID-19
Infection
People of all ages can be infected by this virus.
Infection is transmitted through a droplet of saliva or
snivel generated during talking, coughing, or
sneezing of symptomatic patients but can also occur
from asymptomatic people. Also, contact surface
spread, which means that directly touching the
surface which has viruses on it, is possible for virus
transmission, and it has been reported that this type of
virus can be transmitted through the air.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
602

3.2 Symptoms
Typically, COVID-19 symptoms begin one to
fourteen days after exposure to the virus. On average,
it takes 5–6 days from when someone is infected with
the virus for symptoms to
Figure 3: (Mansbach, Chakraborty, Nguyen, Montefiori,
Korber, Gnanakaran 2021): Detail structure of spike
protein.
show; however, it can take up to 14 days. Around one
in five infected individuals do not develop any
symptoms. Most people have mild symptoms, and the
most common mild symptoms of COVID-19 are
fever, dry cough, and fatigue(Wikipedia 2021). Also,
other mild symptoms that are less common but still
can affect some people, including loss of taste or
smell, nasal congestion, conjunctivitis (also known as
red eyes), sore throat, headache, muscle or joint pain,
different types of skin rash, nausea or vomiting,
diarrhea, and chills or dizziness(World Health
Organization 2021). However, some may have severe
symptoms, including shortness of breath, loss of
appetite, confusion, persistent pain or pressure in the
chest, high temperature(above 38 °C) (World Health
Organization 2021). Other less common severe
symptoms include irritability, confusion, reduced
consciousness (sometimes associated with seizures),
anxiety, depression, sleep disorders, and more severe
and rare neurological complications such as strokes,
brain inflammation, delirium, and nerve damage
(Wikipedia 2021). Some people even develop acute
respiratory distress syndrome (ARDS). ARDS can be
precipitated by cytokine storms, multi-organ failure,
septic shock, and blood clots (Wikipedia 2021).
Longer-term damage to organs (in particular, the
lungs and heart) has been observed (Wikipedia 2021).
4 VARIANTS OF SARS-COV-2
Variants of SARS-CoV-2 are caused by the mutation
of the genetic sequence of the virus. The mutation of
the genetic sequence of a virus can affect its property,
such as transmissibility. Up to August 2021, there are
many variants of SARS-CoV-2, and four of them:
Alpha, Beta, Gamma, and Delta, are listed in
currently designated variants of concern according to
the World Health Organisation(World Health
Organization 2021). Apart from these four variants,
lambda variant, another variant of SARS-CoV-2, is
also under concern.
4.1 Alpha
The Alpha variant, also known as lineage B.1.1.7,
was first documented in the United Kingdom in
September 2020(World Health Organization 2021). It
has been found that the transmissibility of this variant
is around 29%, substantially higher than the original
one. One of the most important differences between
the original virus and the Alpha one is the amino acid
in position 501. In the Alpha variant, the amino acid
position 501 is tyrosine instead of asparagine. Such
changes in amino acids cause changes in the receptor-
binding domain (RBD), which changes the specificity
of the binding between human ACE2 receptors and
RBD(Wikipedia 2021). This allows viruses to
become more infectious.
4.2 Beta
The beta variant, also known as lineage B.1.351, was
first recorded in South Africa in May 2020 (World
Health Organization 2021). It has also been proved
that the transmission rate of this variant is higher than
the original one. There is a total of eight mutations in
the spike proteins in this virus, including K417N (a
change from lysine to asparagine in amino acid
position 417), E484K (a change from glutamic acid to
lysine in amino acid position 484), and N501Y (a
change from asparagine to tyrosine in amino acid
position 501) (Wikipedia 2021). Similar to the
situation in the alpha variant, these mutations cause
changes in the receptor-binding motif (RBM) of the
receptor-binding domain (RBD), which allows the
beta variants to spread faster.
4.3 Gamma
The gamma variant, known as lineage P.1, is one of
the variants of SRAS-CoV-2. It was first recorded in
Brazil in November 2020(World Health Organization
2021). This variant has ten amino acids mutations,
including N501Y (a change from asparagine to
tyrosine in amino acid position 501), E484K (a
change from glutamic acid to lysine in amino acid
position 484), and K417T (a change from lysine to
threonine in amino acid position 417) (Wikipedia
2021). It has been shown that the transmission rate is
Medicines and Vaccines in Dealing with Covid-19
603

approximately 38%, which is higher than that of the
alpha variant.
4.4 Delta
The delta variant, known as lineage B.1.617.2, has the
highest transmissibility of all discovered variants. It
was first identified in India in October 2020
according to the WHO(World Health Organization
2021). Mutations, D614G (an aspartic acid-to-glycine
substitution at amino acid position 614), T478K (a
threonine-to-lysine substitution at amino acid
position 478), L452R (a leucine-to-arginine
substitution at amino acid position 452), and P681R
(a proline-to-arginine substitution at amino acid
position 681), are found in delta variants (Wikipedia
2021). The common symptoms of the delta variant
have changed. Headaches, sore throat, a runny nose,
and fever are common symptoms of this
variant(healthline 2021).
4.5 “Delta Plus”
“Delta plus” variant is a delta variant with K417N
mutation (a lysine-to-asparagine substitution at
amino acid position 417) (Wikipedia 2021).
4.6 Lambda
Lambda variant, known as lineage C.37, was first
detected in Peru in December 2020. G75V, T76I,
L452Q, F490S, D614G, and T859N mutations and a
7-amino-acid deletion in the NTD (RSYLTPGD246-
253N) were found in the spike protein of the lambda
variant(Kimura et al. 2021). It has been shown that
this variant is more infectious, and the deletion in
NTD is responsible for neutralizing antibiotics
(Kimura et al. 2021).
5 CURRENT MEDICINES
Two common medicines – remdesivir and
dexamethasone -- that are used for the covid-19
treatment would be discussed below.
5.1 Remdesivir
Remdesivir is one of the most common medicines
that is used for the treatment of COVID-19 because it
has been shown in research that it is efficacious.
Remdesivir is an intravenous nucleotide prodrug of
an adenosine analog. Remdesivir binds to the viral
RNA-dependent RNA polymerase, inhibiting viral
replication through premature termination of RNA
transcription(NIH 2021).
5.1.1 Analysis of the Research of Remdesivir
In the research, there were 1114 patients who were
assessed for eligibility: 1062 of them underwent
randomization; 541 of them were allocated to the
remdesivir group, and 521 were placed to the placebo
group(Beigel et al. 2020). The primary analysis was a
stratified log-rank test of the time to recovery with
remdesivir as compared with placebo, with
stratification by disease severity(Beigel et al. 2020).
5.1.2 Primary Outcome
Patients in the remdesivir group (median: 10 days;
rate ratio of recovery: 1.29) recovered faster than
patients in the placebo group (median: 15 days)
(Beigel et al. 2020). The 95% confidence interval (CI)
and the probability of extreme cases were 1.12-1.49
and <0.001 (Beigel et al. 2020). Among patients who
were hospitalized and required any supplementary
oxygen, the rate ratio for recovery was 1.45 (95% CI,
1.18 to 1.79) (Beigel et al. 2020); among patients who
were hospitalized and did not require any
supplementary oxygen but required ongoing medical
care and those who were hospitalized and required
noninvasive ventilation or use of high-flow oxygen
devices, the rate ratio of recovery were 1.29 (95% CI,
0.91 to 1.83) and 1.09 (95% CI, 0.76 to 1.57),
respectively(Beigel et al. 2020). The rate ratio for
recovery was 0.98 (95% CI, 0.70 to 1.36) for those
receiving mechanical ventilation or ECMO at
enrollment(Beigel et al. 2020). Patients who
underwent randomization during the first 10 days
after the onset of symptoms had a rate ratio for
recovery of 1.37 (95% CI, 1.14 to 1.64), which was
higher than that of those who underwent
randomization more than ten days after the onset of
symptoms (1.20; 95% CI, 0.94 to 1.52) (Beigel et al.
2020). P value in this experiment is very small, which
means that the probability of extreme cases in this
research is very small. Also, high confidence interval
(CI), 95% (Beigel et al. 2020), ensures the accuracy
of the primary outcome in the research of remdesivir.
5.1.3 Key Secondary Outcome
Mortality of patients in the remdesivir group was
numerically lowered than those in the placebo group,
but the difference was not significant (hazard ratio,
0.55; 95% CI, 0.36 to 0.83) (Beigel et al. 2020). The
mortality by 14 days was 6.7% and 11.9% in the
remdesivir and placebo groups, respectively(Beigel et
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
604

al. 2020). The mortality rate by day 29 was estimated
to be 11.4% in remdesivir group and 15.2 in placebo
group, respectively (hazard ratio, 0.73; 95% CI, 0.52
to 1.03) (Beigel et al. 2020). Patients in the remdesivir
group (24.6%) who had serious adverse events were
lower than those in the placebo group (31.6%);
patients in the remdesivir group who suffered from
serious respiratory failure (8.8%) were lower than
those in the placebo group (15.5%)(Beigel et al.
2020). However, patients in the remdesivir group who
suffered from pyrexia were higher than those in the
placebo group. Other comparisons of safety outcomes
are shown in Table 1. Therefore, it can be observed
that the possibility of serious adverse events occurred
in the remdesivir group is much less than that in the
placebo group.
5.2 Dexamethasone
Dexamethasone is a corticosteroid that has been
recommended by the National Health Service in the
UK and the National Institutes of Health (NIH) in the
US for the treatment of the Covid-19. It is used to treat
those who are very ill, and it has been shown that
patients who were treated with dexamethasone
recovered faster because dexamethasone can
modulate inflammation-mediated lung injury caused
by the SRAS-CoV-2 and thereby reduce progression
to respiratory failure and death(Engl 2021).
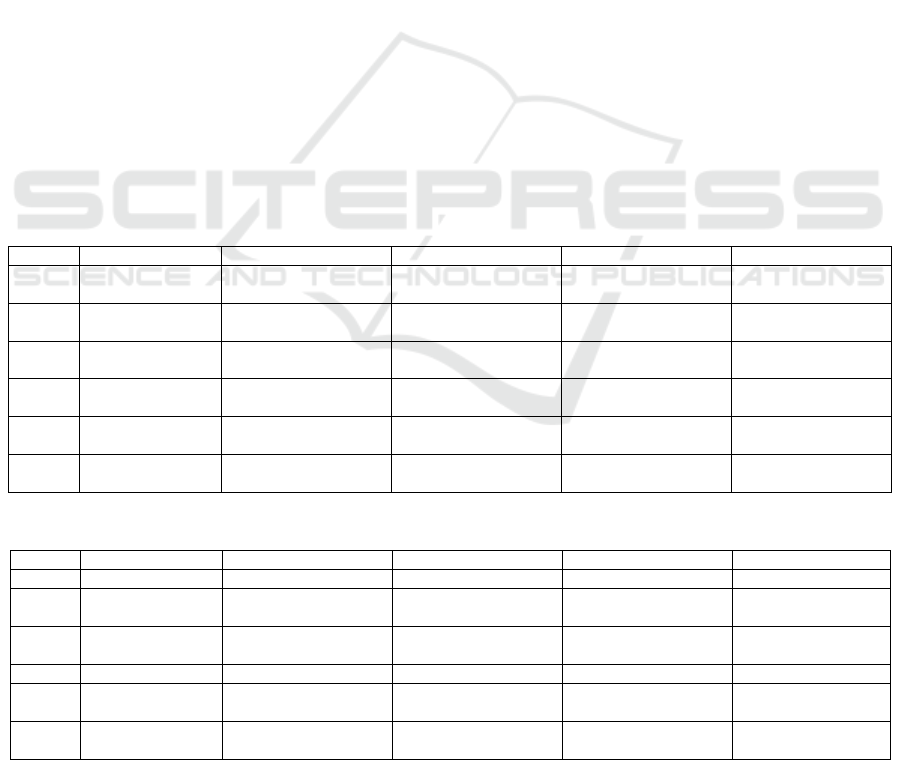
Table 1: A Table showing the negative effects of
remdesivir. All the data is collected from Remdesivir for
the Treatment of Covid-19 — Final Report (Beigel et al.
2020).
Events Remdesivir
group
Placebo group
Total serious
adverse event
occurred
131 out of 531
patients
(24.6%)
163 out of 516
patients (31.6%)
Serious respiratory
failure adverse
events
47 patients
(8.8%)
80 patients
(15.5%)
Acute respiratory
failure,
hypotension, viral
pneumonia, and
acute kidney injury
Less common More common
Death related to
treatment
assignmen
t
No No
Anemia or
decreased
hemoglobin
43 events
(7.9%)
47 events (9.0%)
Acute kidney
injury, decreased
estimated
glomerular
filtration rate or
creatinine
40 events
(7.4%)
38 events (7.3%)
clearance, or
increased blood
creatinine
Pyrexia 27 events
(5.0%)
17 events (3.3%)
Hyperglycemia or
increased blood
glucose level
22 events
(4.1%)
17 events (3.3%)
Increased
aminotransferase
levels including
alanine
aminotransferase,
aspartate
aminotransferase,
or both
22 events
(4.1%)
31 vents
(5.9%)
5.2.1 Samples of the Research of
Dexamethasone
In this research, a total of 6425 patients underwent
randomization, where 2104 of them were assigned to
receive dexamethasone, and 4321 of them received
usual care(Engl 2021). The mean age of the patients
in this research was 66.1±15.7 years(Engl 2021). 36%
of them were female, and 18% were Black, Asian, or
from a minority ethnic group(Engl 2021).
5.2.2 Primary Outcome and Secondary
Outcome
At 28 days, mortality of those who received treatment
of dexamethasone (22.9%) was lower than that of
those who received usual treatments (25.7%) (rate
ratio, 0.83; 95% confidence interval [CI], 0.75 to
0.93; P<0.001) (Engl 2021). Among patients
receiving invasive mechanical ventilation, the
incidence of death in the dexamethasone group
(29.3%) was lower than that in the usual care group
(41.4%) (rate ratio, 0.64; 95% CI, 0.51 to 0.81) (Engl
2021). However, no obvious effect of dexamethasone
could be seen among patients who were not receiving
any respiratory support at randomization(Engl 2021).
5.2.3 Secondary Outcome and Other
Prespecribe Clinical Outcomes
Patients who received dexamethasone as treatment
(median: 12 days) had a shorter duration of
hospitalization than those who received usual care
(median: 13days) and had a greater probability of
discharging alive(Engl 2021). The percentage of
patients who received Invasive mechanical
ventilation or died in the dexamethasone group (26%)
was lower than that of those in the usual group
(27.6%) (rate ratio: 0.93; 95% CI, 0.85–1.01) (Engl
2021). The percentage of patients who were not
receiving invasive mechanical ventilation at
Medicines and Vaccines in Dealing with Covid-19
605

randomization and progressed to received invasive
mechanical ventilation later was lower in the
dexamethasone group than in the usual care group
(risk ratio, 0.79; 95% CI, 0.64 to 0.97) (Engl 2021).
The percentage of those who were receiving invasive
mechanical ventilation at randomization and did not
require invasive mechanical ventilation later was
higher in the dexamethasone group than in the usual
care group (rate ratio, 1.47; 95% CI, 1.20 to 1.78)
(Engl 2021). The percentage of the patients who were
not receiving renal- replacement therapy (renal
dialysis or hemofiltration) at randomization and had
to use later within 28 days was lower in the
dexamethasone group than in the usual care group
(risk ratio, 0.61; 95% CI, 0.48 to 0.76) (Engl 2021).
6 VACCINES
There are many covid-19 vaccines in the market, and
this paper will focus on three vaccines developed by
three renowned institutions -- Moderna, Pfizer/
BioNTech, and Oxford/ AstraZeneca. These
companies all had major breakthroughs in COVID-19
vaccine development in 2020. Vaccines developed by
these institutions have already been purchased by
different governments and used in different countries.
They all showed high efficacies for the prevention of
original SRAS-CoV-2. However, due to the
development of the pandemic and the evolvement of
different variants, the efficacies of these vaccines are
challenged.
6.1 Vaccine Produced by Moderna
Moderna, a biotechnology company, specializes in
the mRNA vaccine for COVID-19. It shows that a
total of 30,000 people in the United States have
participated in its COVID-19 vaccine clinical trial,
for which 79.4% are White, 10% are African
American, 5% are Asian, and <5% other
races/ethnicities (CDC 2021). For the age and sex
breakdown of those people, 52.6% are male, 47.4%
are female, 25% are 65 years and older, and 75% of
people are between 18-64 years old(CDC 2021).
According to data published by CDC, most people
who participated in the trials (82%) were considered
to have an occupational risk of exposure, with 25% of
them being healthcare workers(CDC 2021). People in
the clinical trials, 22.3% had at least one high-risk
condition, which included lung disease, heart disease,
obesity, diabetes, liver disease, or HIV
infection(CDC 2021). Four percent (4%) of
participants had two or more high-risk
conditions(CDC 2021). Of the 15,000 vaccinated
volunteers, only 11 were infected with the virus, and
no one developed severe symptoms(Moderna, Inc.
2020). Among the 15,000 placebo-vaccinated
volunteers, 185 were infected with the virus, of which
30 developed into severely ill patients, and one died
because of the disease(Moderna, Inc. 2020). It is
pointed out in the Moderna’s latest vaccine phase III
clinical trial data submitted to the FDA that the
vaccine efficacy is 94.5% (P<0.0001) (Moderna, Inc.
2020) in preventing infection with Covid-19 and can
prevent 100% of the severe symptoms of Covid-19.
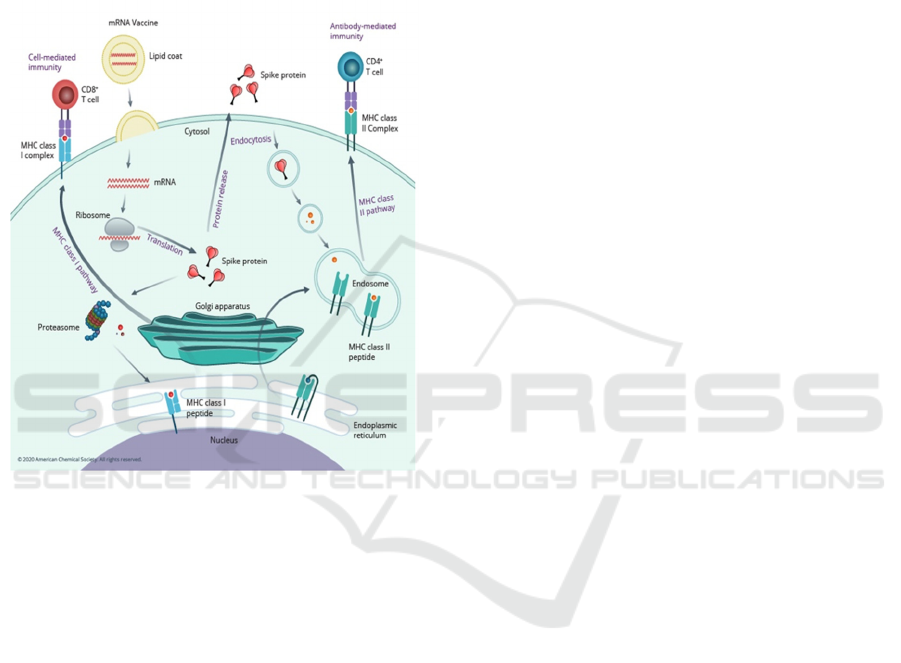
By using the genetic information of COVID-19,
the sequence of spike proteins in the virus is identified
and encoded into mRNA. When humans received the
vaccines, the mRNA is taken into immune cells.
When mRNA is inside immune cells, the cells use it
to make the protein. After the protein is made, the
cells break down the mRNA. Next, the cells display
these protein pieces on their surface, which allows the
immune systems to recognize these proteins and
begin building an immune response by making
antibodies. These antibodies can prevent the body
from getting an infection in the future. The benefit of
mRNA vaccines, like all vaccines, is those vaccinated
gain this protection without ever having to risk the
serious consequences of getting sick with COVID-19.
(Figure 4)
6.2 General Information about the
Injection of Vaccine Produced by
Moderna
People receive vaccines in the muscle of the upper
arm. Normally they receive two doses, the time of
which was a month (28 days) apart (CDC 2021). The
vaccine also does not contain eggs, preservatives, and
latex(CDC 2021). The vaccine is recommended for
people who are more than or equal to 18-year-old
(CDC 2021). Also, pain, swelling, and redness were
the most common side effects in the arm where
people got the injection, and chills, tiredness, and
headache frequently happened throughout the rest of
the body (CDC 2021). These side effects usually start
within the first two days after getting the vaccine
(CDC 2021). They might feel like flu symptoms and
might even affect people's ability to do daily
activities, but normally they will go away in a few
days (CDC 2021).
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
606

6.3 Vaccine Produced by Pfizer/
BioNTech
BioNTech is another company that specialized in the
mRNA vaccine for COVID-19. About 82% of people
who participated in the research are White, 9.8% are
African American, 4.4% are Asian, and <3% other
races/ethnicities (CDC 2021). For the age and sex
breakdown, 50.6% of the people are male, 49.4% are
female, and 21.4% are 65 years and older (CDC
2021). Also, the most frequent underlying medical
conditions for those people were obesity (35.1%),
diabetes (8.4%), and pulmonary disease (7.8%) (CDC
2021). Among 36,523 participants who had no
evidence of existing or prior SARS-CoV-2 infection,
8 cases of Covid-19 with onset at least seven days
after the second dose were observed among vaccine
recipients and 162 among placebo recipients. This
case split corresponds to 95.0% vaccine efficacy
(95% confidence interval [CI], 90.3 to 97.6) (Polack
et al. 2020). Among participants with and those
without evidence of prior SARS CoV-2 infection, 9
cases of Covid-19 at least seven days after the second
dose were observed among vaccine recipients and
169 among placebo recipients, corresponding to
94.6% vaccine efficacy (95% CI, 89.9 to 97.3)
(Polack et al. 2020). In general, local reactions were
mostly mild-to-moderate in severity and resolved
within 1 to 2 days. No deaths were considered by the
investigators to be related to the vaccine(CDC 2021).
By using the genetic information of COVID-19,
the sequence of spike protein in the virus is identified.
This sequence is encoded into mRNA. mRNA
formulated in LNP enters the cell. When the whole
things enter the cell, mRNA is released. Then, spike
protein is made and processed. APCs present spike
protein fragments, and these can activate the
formation of T cells and B cells. The CD8+ cytotoxic
T cells can eliminate virus-infected cells and
potentially increase the length of protection. The B
cells will become virus-neutralizing antibodies.
These can bind spike proteins and prevent virus
infection of human cells. In addition, the memory T
and B cells provide immune memory to ensure long-
term protection against the virus.
6.4 General Information about the
Injection of Vaccine Produced by
Pfizer/ BioNTech
People receive vaccines in the muscle of the upper
arm. Normally, they receive two doses, the time of
which was 21 days apart. The vaccine does not
contain eggs, preservatives, and latex(CDC 2021).
This vaccine is available for people aged 12 years
older (CDC 2021). The side effects of this vaccine are
similar to those produced by Moderna.
6.5 Information about People Who
Cannot Receive mRNA Vaccines,
Both Vaccines Produced by
Moderna or Pfizer/ BioNTech
People who have severe allergic reactions
(anaphylaxis) or an immediate allergic reaction to any
ingredient in an mRNA COVID-19 vaccine should
not get this mRNA vaccine (CDC 2021). Also, people
who have severe or immediate allergic reactions
(anaphylaxis) after getting the first dose of the
vaccine should not get another dose of this mRNA
COVID-19 vaccine (CDC 2021). In both situations
mentioned above, a reaction within 4 hours of getting
vaccinated, including symptoms such as hives,
swelling, or wheezing (respiratory distress), is
regarded as an immediate allergic reaction (CDC
2021). In addition, people who are allergic to PEG or
polysorbate should not get an mRNA COVID-19
vaccine (CDC 2021). Although polysorbate is not an
ingredient in either mRNA COVID-19 vaccine, it is
closely related to PEG, which is in the vaccines
(CDC 2021). (Figure 4)
6.6 Vaccine Produced by Oxford/
AstraZeneca
The vaccine produced by Oxford/ AstraZeneca is a
viral vector vaccine. It is announced that the vaccines
had average effectiveness of 70.4 percent, which
splits into 90 percent in one dosing regimen and 62
percent in the other (NEEDHAM 2020). This came
after clinical trials enrolled over 24,000 participants
from across the UK, Brazil, and South Africa. Further
trials will include 60,000 participants from the United
States, Kenya, Japan, and India (NEEDHAM 2020).
Viral vector-based vaccines use the body’s own cells
to produce antigens. In the case of SRAS-CoV-2,
spike proteins are antigens. Modified viruses (the
vector) are used to deliver genetic code for antigen,
so spike proteins found on the surface of the virus are
delivered into human cells to instruct the body’s own
cells to make spike proteins. Then, an immune
response can be triggered by producing immune T
cells and B cells as the immune system identifies that
spike proteins do not belong to the human body. This
can prevent the body from being infected in the
future.
Medicines and Vaccines in Dealing with Covid-19
607
6.7 Advantages and Disadvantages of
Different Vaccines
First of all, for safety, all three vaccines mentioned
above are safe, which means that these vaccines are
non-infectious because the real viruses are not
injected into human bodies, and only the formation of
spike protein stimulates the response of the immune
system.
Figure 4: (Worldometer 2022). How do mRNA vaccines
work.
For the efficacy, vaccines produced by Moderna
and Pfizer/ BioNTech have higher efficacy towards
original SRAS-CoV-2, both of which are around
95%(Asipilin 42195 2020) shown in the clinical trial,
compared to vaccines produced by Oxford/
AstraZeneca (around 70.4% (Asipilin 42195 2020).
In terms of the efficacy towards different variants,
patients who have received a second dose of the
vaccines, be it vaccines produced by
Pfizer/BioNTech, Moderna, or Oxford/ AstraZeneca,
have a higher percentage of preventing infection of
the virus (Table 2, Table 3, and Table 4). Vaccines
produced by Pfizer/BioNTech (above 90%) and
Moderna (around 90%) have higher efficacy towards
alpha variant; Moderna’s vaccines have higher
efficacy when dealing with the beta variant and
gamma variant; Oxford’s / AstraZeneca’s vaccines
have lower efficacy when dealing with delta variant
compared to that of Pfizer/BioNTech. Other data can
refer to Table 2, Table 3, and Table 4 below.
For the production of vaccines, all of them can be
produced faster than conventional vaccines because
the time to cultivate the virus can be saved. In terms
of the distribution of vaccines, vaccines produced by
Pfizer/ BioNTech can only store in a fright, which is
2-8°C for five days(Asipilin 42195 2020). However,
vaccines produced by Moderna and Oxford/
AstraZeneca can be stored in a fright which is 2-8°C
for a long time, which are one month and six months
respectively . Normally, the storage temperature for
vaccines produced by Pfizer/ BioNTech is -70°C and
this for vaccines produced by Moderna is -20°C, and
this for vaccines produced by Oxford/ AstraZeneca is
2.22-7.78°C (Asipilin 42195 2020).
In addition, for the price of different vaccines,
vaccines produced by Oxford/ AstraZeneca are the
cheapest, costing only 3.5USD per dose (Asipilin
42195 2020). Also, vaccines produced by Moderna
and Pfizer/ BioNTech cost 35USD and 20USD per
dose. It is obvious that vaccines produced by Pfizer/
BioNTech are the most expensive ones (Asipilin
42195 2020).
6.8 Other Types of Vaccines
There are two other common types of vaccines in the
world: inactivated vaccine and protein subunit
vaccine. For inactivated vaccine, the inactivated virus
can no longer replicate or reproduce (Johns Hopkins
University 2020). The immune system is exposed to
viral proteins, but the inactivated virus does not cause
disease. Inactivated viruses stimulate the body's
immune system to produce antibodies, so when
people are exposed to natural viruses, antibodies
work to fight the virus (Johns Hopkins University
2020). The production of inactivated vaccines
requires the ability to cultivate or breed a large
number of viruses. Since the virus cannot replicate
outside the host cell, the vaccine virus needs to be
cultured in a continuous cell line or tissue (Johns
Hopkins University 2020). Several inactivated
vaccines are currently widely used, including
vaccines against influenza, polio, hepatitis A and
rabies viruses. The inactivated virus can no longer
replicate or reproduce (Johns Hopkins University
2020). Bharat Biotech is one of the companies which
is developing this type of vaccine for COVID-19.
For subunit vaccine, it contains fragments of
protein from the pathogen. The protein selected must
be likely to produce a strong and effective immune
response. In the case of SRAS-CoV-2, spike proteins
on the surface are always selected. When these
fragments enter our body, the immune system can try
to produce immune cells and antibodies to defend
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
608
these fragments. As these fragments are incapable of
causing diseases, some side effects can be minimized.
However, a disadvantage of this vaccine is that the
antigens used to elicit an immune response may lack
pathogen-associated molecular patterns(Gavi 2021).
These patterns can be read by immune cells and
recognized as danger signals, so their absence may
result in a weaker immune response (Gavi 2021).
Also, because the antigens do not infect cells, subunit
vaccines mainly only trigger antibody-mediated
immune responses, which means the immune
response may be weaker than with other types of
vaccines (Johns Hopkins University 2020). To deal
with this problem, subunit vaccines are sometimes
delivered alongside adjuvants, and another booster
dose may be required (Gavi 2021). There are also
upsides to this type of vaccine. They are cheaper to
produce and more stable.
7 CURRENT SITUATION OF THE
PANDEMIC
Although people can be vaccinated to prevent the
SARS-CoV-2, there is no downward trend of the
covid cases and death cases indicating the alleviation
of the problem. This is due to the emerging of new
variants, and the covid vaccines are developed by
targeting the original unmutated virus. However,
different governments have encouraged residents to
get vaccines and have imposed strict policies to
prevent the spread of the virus. These actions can
potentially lower the risk of being infected.
8 CONCLUSION
In conclusion, the spike protein in the SARS-CoV-2
virus is responsible for the transmission of the virus,
and different variants arisen because of the mutation
of this protein. Also, remdesivir and dexamethasone
are the two medicines that are currently used in the
treatment, which shows efficacies in shortening the
recovery time and lowering the mortality rate. In
terms of the covid vaccines, it is clear that all three
vaccines are recorded drops in their efficacies
because of the emerging of different variants.
Therefore, the development of vaccines to deal with
different covid variants and prevention of further
mutation of the virus are vitally important to fight the
virus.
Table 2: A table showing the efficacy of vaccines produced by Pfizer/BioNTech.
Doses Severity of illness Alpha varian
t
Beta Varian
t
Gamma Variant Delta varian
t
1 Asymptomatic 38% (29–45%)
(Wikipedia 2021)
17% (10–23%)
(Wikipedia 2021)
Not found 30% (17–41%)
(Wikipedia 2021)
1 Symptomatic 27% (13–39%)
(Wikipedia 2021)
43% (22–59%)
[(Wikipedia 2021)
43% (22–59%)
(Wikipedia 2021)
33% (15–47%)
(Wikipedia 2021)
1 Hospitalization 83% (62–93%)
(Wikipedia 2021)
0% (0–19%)
(Wikipedia 2021)
56% (−9 to 82%)
(Wikipedia 2021)]
94% (46–99%)
(Wikipedia 2021)
2 Asymptomatic 92% (90–93%)
(Wikipedia 2021)
75% (71–79%)
(Wikipedia 2021)
Not found 79% (75–82%)
(Wikipedia 2021)
2 Symptomatic 92% (90–93%)
(Wikipedia 2021)
88% (61–96%)
[(Wikipedia 2021)
88% (61–96%)
(Wikipedia 2021)
83% (78–87%)
(Wikipedia 2021)
2 Hospitalization 95% (78–99%)
(Wikipedia 2021)
100% (74–100%)
(Wikipedia 2021)
100% (74–100%)
(Wikipedia 2021)
96% (86–99%)
(Wikipedia 2021)
Table 3: A table showing the efficacy of vaccines produced by Moderna.
Doses Severity of illness Alpha varian
t
Beta Varian
t
Gamma Variant Delta varian
t
1 Asymptomatic Not foun
d
Not foun
d
Not foun
d
Not foun
d
1 Symptomatic 61% (56–66%)
(Wikipedia 2021)
43% (22–59%)
(Wikipedia 2021)]
43% (22–59%)
(Wikipedia 2021)
Not found
1 Hospitalization 59% (39–73%)
(Wikipedia 2021)
56% (−9 to 82%)
(Wikipedia 2021)
56% (−9 to 82%)
(Wikipedia 2021)
Not found
2 Asymptomatic Not foun
d
Not foun
d
Not foun
d
Not foun
d
2 Symptomatic 90% (85–94%)
(Wikipedia 2021)
88% (61–96%)
(Wikipedia 2021)
88% (61–96%)
(Wikipedia 2021)
Not found
2 Hospitalization 94% (59–99%)
(Wikipedia 2021)
100% (Wikipedia
2021)
100% (Wikipedia
2021)
Not found
Medicines and Vaccines in Dealing with Covid-19
609
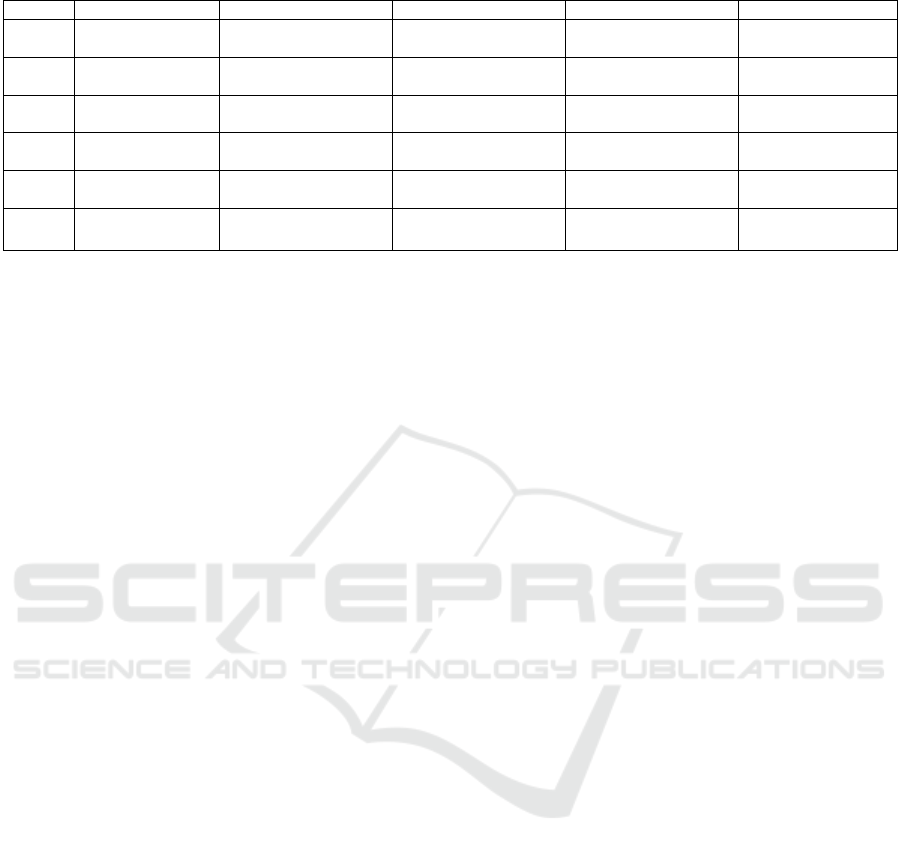
Table 4: A table showing the efficacy of vaccines produced by Oxford/ AstraZeneca.
Doses Severity of illness Alpha varian
t
Beta Varian
t
Gamma Variant Delta varian
t
1 Asymptomatic 37% (32–42%)
(Wikipedia 2021)
Not found Not found 18% (9–25%)
(Wikipedia 2021)
1 Symptomatic 39% (32–45%)
(Wikipedia 2021)
Not found 33% (26–40%)
(Wikipedia 2021)
33% (23–41%)
(Wikipedia 2021)
1 Hospitalization 76% (61–85%)
(Wikipedia 2021)
Not found 55% (47–62%)
(Wikipedia 2021)
71% (51–83%)
(Wikipedia 2021)
2 Asymptomatic 73% (66–78%)
(Wikipedia 2021)
Not found Not found 60% (53–66%)
(Wikipedia 2021)
2 Symptomatic 81% (72–87%)
(Wikipedia 2021)
10% (−77 to 55%)
(Wikipedia 2021)
78% (69–84%)
(Wikipedia 2021)
61% (51–70%)
(Wikipedia 2021)
2 Hospitalization 86% (53–96%)
(Wikipedia 2021)
Not found 88% (78–93%)
(Wikipedia 2021)
92% (75–97%)
(Wikipedia 2021)
REFERENCES
Asipilin 42195: Comparisons of different advantages and
disadvantages of vaccines [Chinese]. Available from:
http://mp.weixin.qq.com/s?__biz=MzUzMzkxOTc4N
A==&mid=2247485529&idx=1&sn=5ea5d4c26f8ff2d
5e7c066f64eb4ab91&chksm=fa9de91ecdea60082b9ab
81e29dc34be0b1c53cf6a89dc0973137bd71c224e5992
c53bf73f9b#rd (accessed Aug. 18, 2021).
“COVID-19,” Wikipedia. Aug. 01, 2021. Accessed: Aug.
18, 2021. [Online]. Available:
https://en.wikipedia.org/w/index.php?title=COVID-
19&oldid=1036654407
CDC, “Information about the Pfizer-BioNTech COVID-19
Vaccine,” Centers for Disease Control and Prevention,
Jun. 24, 2021. https://www.cdc.gov/coronavirus/2019-
ncov/vaccines/different-vaccines/Pfizer-
BioNTech.html (accessed Aug. 13, 2021).
“COVID-19 Delta Variant: What You Need to Know.”
https://www.healthline.com/health-news/the-covid-19-
delta-variant-heres-everything-you-need-to-
know#What-symptoms-does-the-Delta-variant-cause?
(accessed Aug. 05, 2021).
CDC, “Information about the Moderna COVID-19
Vaccine,” Centers for Disease Control and Prevention,
Jun. 11, 2021. https://www.cdc.gov/coronavirus/2019-
ncov/vaccines/different-vaccines/Moderna.html
(accessed Aug. 12, 2021).
CDC, “Understanding mRNA COVID-19 Vaccines,”
Centers for Disease Control and Prevention, Mar. 04,
2021. https://www.cdc.gov/coronavirus/2019-
ncov/vaccines/different-vaccines/mrna.html (accessed
Aug. 12, 2021).
“COVID Live Update: 206,980,203 Cases and 4,358,550
Deaths from the Coronavirus - Worldometer.”
https://www.worldometers.info/coronavirus/ (accessed
Aug. 14, 2021).
“Dexamethasone in Hospitalized Patients with Covid-19,”
N. Engl. J. Med., vol. 384, no. 8, pp. 693–704, Feb.
2021, doi: 10.1056/NEJMoa2021436.
I. Kimura et al., “SARS-CoV-2 Lambda variant exhibits
higher infectivity and immune resistance,” bioRxiv, p.
2021.07.28.454085, Jul. 2021, doi:
10.1101/2021.07.28.454085.
J. H. Beigel et al., “Remdesivir for the Treatment of Covid-
19 — Final Report,” N. Engl. J. Med., vol. 383, no. 19,
pp. 1813–1826, Nov. 2020, doi:
10.1056/NEJMoa2007764.
Johns Hopkins University: Detailed explanation of the
types, advantages and disadvantages of the COVID-19
vaccines. [Chinese]. 3 December 2020. Available from:
https://mp.weixin.qq.com/s/hWZ0YBL4tgpyB6BaQe
VSXQ
“Moderna’s COVID-19 Vaccine Candidate Meets its
Primary Efficacy Endpoint in the First Interim Analysis
of the Phase 3 COVE Study | Moderna, Inc.”
https://investors.modernatx.com/news-releases/news-
release-details/modernas-covid-19-vaccine-candidate-
meets-its-primary-efficacy/ (accessed Aug. 08, 2021).
“Moderna COVID-19 vaccine,” Wikipedia. Aug. 12, 2021.
Accessed: Aug. 14, 2021. [Online]. Available:
https://en.wikipedia.org/w/index.php?title=Moderna_
COVID-19_vaccine&oldid=1038439218
“Oxford–AstraZeneca COVID-19 vaccine,” Wikipedia.
Aug. 13, 2021. Accessed: Aug. 14, 2021. [Online].
Available:
https://en.wikipedia.org/w/index.php?title=Oxford%E
2%80%93AstraZeneca_COVID-
19_vaccine&oldid=1038621367
“Page not found (404),” LubioScience.
https://www.lubio.ch/blog/neutralizing-antibodies.
(accessed Aug. 18, 2021).
“Pfizer–BioNTech COVID-19 vaccine,” Wikipedia. Aug.
13, 2021. Accessed: Aug. 14, 2021. [Online].
Available:
https://en.wikipedia.org/w/index.php?title=Pfizer%E2
%80%93BioNTech_COVID-
19_vaccine&oldid=1038650584
R. A. Mansbach, S. Chakraborty, K. Nguyen, D. C.
Montefiori, B. Korber, and S. Gnanakaran, “The
SARS-CoV-2 Spike variant D614G favors an open
conformational state,” Sci. Adv., vol. 7, no. 16, p.
eabf3671, Apr. 2021, doi: 10.1126/sciadv.abf3671.
“Runtime Error.” https://www.who.int/news-room/q-a-
detail/coronavirus-disease-covid-19. (accessed Aug.
18, 2021).
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
610

“Remdesivir,” COVID-19 Treatment Guidelines.
https://www.covid19treatmentguidelines.nih.gov/thera
pies/antiviral-therapy/remdesivir/ (accessed Aug. 06,
2021).
“SARS-CoV-2 Alpha variant,” Wikipedia. Jul. 29, 2021.
Accessed: Aug. 04, 2021. [Online]. Available:
https://en.wikipedia.org/w/index.php?title=SARS-
CoV-2_Alpha_variant&oldid=1036066787
“SARS-CoV-2 Beta variant,” Wikipedia. Jul. 28, 2021.
Accessed: Aug. 04, 2021. [Online]. Available:
https://en.wikipedia.org/w/index.php?title=SARS-
CoV-2_Beta_variant&oldid=1035925918
“SARS-CoV-2 Gamma variant,” Wikipedia. Jul. 28, 2021.
Accessed: Aug. 04, 2021. [Online]. Available:
https://en.wikipedia.org/w/index.php?title=SARS-
CoV-2_Gamma_variant&oldid=1035998057
“SARS-CoV-2 Delta variant,” Wikipedia. Aug. 04, 2021.
Accessed: Aug. 04, 2021. [Online]. Available:
https://en.wikipedia.org/w/index.php?title=SARS-
CoV-2_Delta_variant&oldid=1037048949
“Safety and Efficacy of the BNT162b2 mRNA Covid-19
Vaccine | NEJM.”
https://www.nejm.org/doi/full/10.1056/NEJMoa20345
77?fbclid=IwAR1YoOCQ3DNr0GjMQv4hFXVvu4L
8SI0fAMkbJdJdh-
AfrLOHBmSVSbo7oTM#.X9QvTNjPnRc.facebook
(accessed Aug. 08, 2021).
“Tracking SARS-CoV-2 variants.”
https://www.who.int/emergencies/emergency-health-
kits/trauma-emergency-surgery-kit-who-tesk-
2019/tracking-SARS-CoV-2-variants (accessed Aug.
18, 2021).
“The top five coronavirus vaccine candidates explained |
WIRED UK.”
https://www.wired.co.uk/article/coronavirus-vaccines-
frontrunners (accessed Aug. 08, 2021).
“What are protein subunit vaccines and how could they be
used against COVID-19?”
https://www.gavi.org/vaccineswork/what-are-protein-
subunit-vaccines-and-how-could-they-be-used-
against-covid-19 (accessed Aug. 14, 2021).
Y. Huang, C. Yang, X. Xu, W. Xu, and S. Liu, “Structural
and functional properties of SARS-CoV-2 spike
protein: potential antivirus drug development for
COVID-19,” Acta Pharmacol. Sin., vol. 41, no. 9, pp.
1141–1149, Sep. 2020, doi: 10.1038/s41401-020-0485-
4.
Medicines and Vaccines in Dealing with Covid-19
611
