
Targeting Myeloid Cells for Potential Cancer Therapies
Jingwen Pan
1,*
, Yida Zhang
2,#
, Wenkai He
3,#
and Yian Chen
4
1
Department of Bioengineering University of California San Diego, CA, U.S.A.
2
Truro High School Truro, Cornwall, U.K.
3
Shanghai Southwest Weiyu Middle School, Shanghai, China
4
IB program Beijing National Day School, Beijing, China
#
These two authors contributed equally to this work and should be considered as co-second authors
Keywords: Cancer, Myeloid Cells, CXCL12, Oral Squamous Cell Carcinoma, Positron Emission Tomography.
Abstract: Cancer is one of the major health concerns facing the global society. Though significant improvement in
cancer therapies have been done, further investigation into alternative treatment approaches are expected to
improve clinical outcomes. Myeloid cells are key components that shape the tumor microenvironment. The
distribution and recruitment of myeloid cells account for tumor progression and metastasis. Previous studies
provided evidence suggesting that the CXCL12/CXCR4 pathway is responsible for recruiting tumor-
supportive M2-type macrophages in the oral squamous cell carcinoma (OSCC) model, promoting
proliferation and spreading of OSCC cancer cells. CXCL12/CXCR4 pathway also actively affect other types
of tumors, like breast cancer. Thus, modifying CXCL12/CXCR4 interaction might be a potential target for
cancer treatment. Due to the lack of existing therapies targeting this pathway, we propose a potential treatment
targeting the CXCL12/CXCR4 pathway that binds monoclonal antibodies to CXCL12 ligands to eliminate its
expression in tumor sites. Positron emission tomography (PET) imaging enables the monitoring and
verification of the outcome of this novel design.
1 INTRODUCTION
Cancer, a collection of diseases characterized by an
abnormal cell that divides and spread into
surrounding tissues uncontrollably, is one of the
primary causes of death worldwide (World Health
Organization, 2021). Prevailing types of cancer
include oral squamous cell carcinoma (OSCC), breast
cancer, and melanoma. OSCC is the most common
cancer type that contributes to 95% of head and neck
cancers largely due to alcohol abuse and smoking
(Taghavi and Yazdi, 2015). Though advanced
treatments have been developed, the percentage of
patients who are alive five years after starting
treatment is still unsatisfactory due to the high
recurrence and metastasis rate (Taghavi and Yazdi,
2015; National Cancer Institute). Breast cancer is one
of the most often diagnoses among female cancer
patients and the leading cause of cancer mortality in
women (Akram, Iqbal, Daniyal, Khan, 2017). In
2012, around 1.7 million new cases were diagnosed
globally, accounting for 25% of all female cancer
cases (Breast cancer | world cancer research fund
international, 2021). Melanoma is a form of skin
cancer with a high incidence of metastasis that could
significantly reduce the survival rate (Davis, Shalin,
Tackett, 2019). Further understanding and
development of cancer mechanisms and treatments
are needed to boost patients' chances for survival and
recovery.
Myeloid cells are a vital component in the tumor
microenvironment (TME). They are derived from a
common myeloid progenitor in the human bone
marrow (Figure 1) (A., T. (n.d.), 2021). Tumor
growth is enhanced by myeloid cells since they
promote tumor angiogenesis, accelerate cancer cell
migration, and weaken the immune system (Schmid,
Varner, 2010).
Pan, J., Zhang, Y., He, W. and Chen, Y.
Targeting Myeloid Cells for Potential Cancer Therapies.
DOI: 10.5220/0011239000003438
In Proceedings of the 1st International Conference on Health Big Data and Intelligent Healthcare (ICHIH 2022), pages 173-180
ISBN: 978-989-758-596-8
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
173
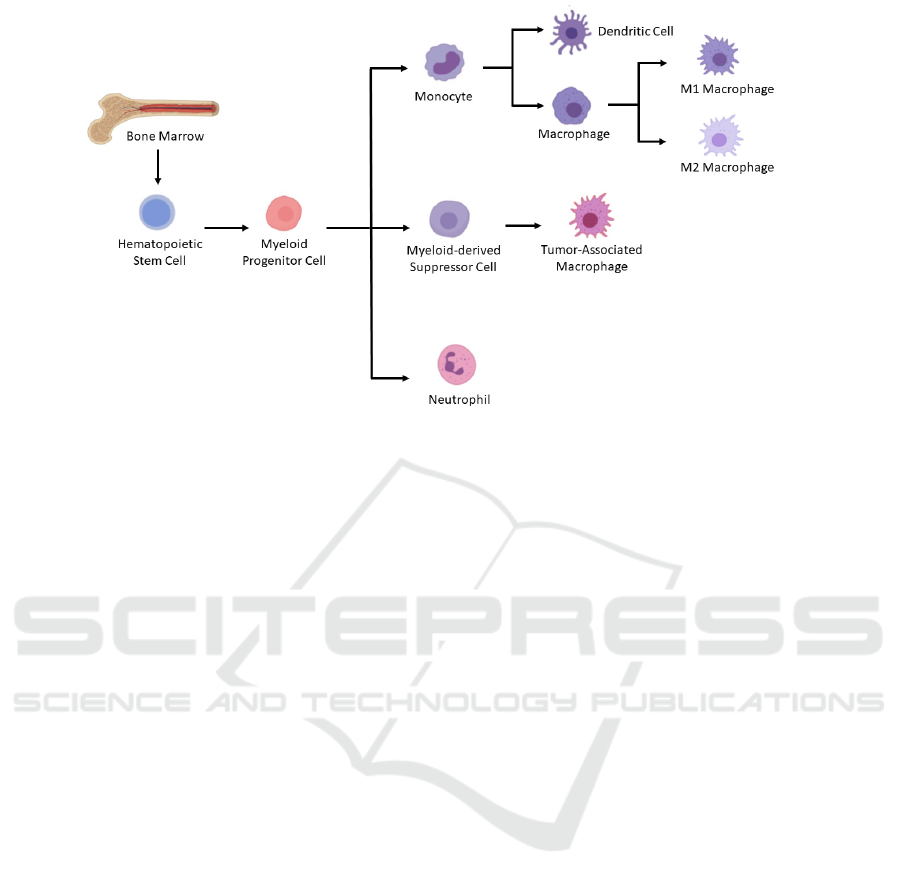
Figure 1. Differentiation of myeloid lineage cells. In the bone marrow, myeloid progenitor cells are derived from
hematopoietic stem cells. And then myeloid progenitor cells differentiate into monocyte and neutrophils. Under pathological
conditions like cancer, myeloid-derived suppressor cells, which could further differentiate into tumor-associated
macrophages, would be generated as well. Macrophages could be induced to derive into either M1 or M2 macrophages. Figure
modified from (A., T. (n.d.). 2021).
Myeloid lineage cells comprise a heterogeneous
group of cells, including but not limited to
macrophages, neutrophils, and myeloid-derived
suppressor cells (MDSC). Tumor-associated
macrophages (TAMs) are the most copious tumor-
infiltrating myeloid cells that work in the innate
immune system and assist the initiation of adaptive
immunity (A., T. (n.d.). 2021). Based on their
functions in tumors and activations, macrophages are
classified into two main subsets: classically-activated
(M1) macrophages that show tumor-suppressive
functions and alternatively activated (M2)
macrophages that suppress the immune response and
facilitate tumor growth and invasion (Schmid, Varner
2010, Dandekar, Kingaonkar, Dhabekar 2011) . Since
TAMs have been proved to contribute to
immunosuppression and tumor invasion, the
mechanism of how TAMs are recruited to the tumor
sites is crucial to the development of potential cancer
immunotherapies (Dandekar, Kingaonkar, Dhabekar
2011).
Myeloid-derived suppressor cells (MDSC)
constitute a more recently discovered immature
myeloid cell population featured by the ability to
inhibit immune responses (Lv, Wang, Huang, 2019).
MDSC are widely considered as pro-tumorigenic in
solid tumors (Lv, Wang, Huang, 2019). These cells
demonstrate the pathological state of activation of
monocytes and relatively immature neutrophils.
Their prominent characteristic is the ability to inhibit
T cells' normal functioning, and consequently
promote the pathogenesis of various diseases (Veglia,
Perego, Gabrilovich, 2018).
Neutrophils are a type of granulocyte and belong
to the category of leukocytes. Granulocytes can be
classified by their performance under Wright's stain,
which is a hematologic stain that used for
distinguishing among blood cell types, into three
categories: neutrophils, eosinophils, and basophils, of
which neutrophils are the most common phagocytic
cells (Morris 2018, Wright stain 2020). The bone
marrow does not produce neutrophils directly at the
onset of infection, but first produces myeloid and
promyelocytes, which later differentiate into
neutrophils. After receiving the corresponding signal,
neutrophils travel through the bloodstream to the
infection and proceed to rapidly surround and engulf
the foreign material covered by complement and
antibodies (Brostjan, Oehler 2020).
Cancer-associated fibroblasts (CAFs), a type of
stroma cells that remodel the extracellular matrix, are
abundant in the tumor mesenchyme (Liu et al 2019,
Ping et al 2021). Accumulating studies indicate that
CAFs hinder the function of anti-tumor immunity via
their interaction with natural killer (NK) cells and T
cells (Li et al 2019). Furthermore, CAFs are
responsible for directing immunosuppressive cells
onto the tumor owing to their ability to secrete
various growth factors and proinflammatory
cytokines, such as CXCL12 (Liu et al 2019).
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
174

CAF-derived CXCL12 is a type of small molecule
chemokines that are distributed in assorted tissues.
Similar to other chemokines, CXCL12 along with its
chemokine receptor CXCR4 are key factors that
mediate the metastasis and proliferation of cancer
cells (Righetti 2019, Mollica Poeta, V., Massara, M.,
Capucetti, A., & Bonecchi, R. 2019). Recent studies
revealed that the activation of the CXCL12/CXCR4
pathway positively correlates with the recruitment of
TAMs to the tumor sites and the number of
monocytes differentiated into M2 macrophages
(Dandekar, Kingaonkar, Dhabekar 2011).
2 PREVIOUS MYELOID
CELLS-RELATED STUDIES
2.1 Myeloid Cell Profiles and
Immunotherapy Resistance
Mechanism
Kim et al. provide evidence that intrinsic tumor
pathways and mutual regulation between the
neutrophils and macrophages contribute to the
development of dichotomous myeloid (Mollica
Poeta, Massara, Capucetti, & Bonecchi 2019). The
dichotomous distribution between the macrophages
and neutrophils is observed by mouse breast cancer
models, found out that breast cancer can be divided
into 'hot tumors' (with rich immune cells) and 'cold
tumors.' And the 'hot' tumor could be further divided
into neutrophil-enriched subtypes (also known as the
NES) and macrophage-enriched subtype (MES)
group. The theory had been confirmed in human
breast cancer by analyzing the TNBC database (Kim
et al 2019).
Further, authors derived transcriptomic data sets
from various murine syngeneic mammary tumor
models and compared them to human breast cancer
data (Kim et al 2019). Kim et al. demonstrate that
human and murine cancers share many similarities.
But it is hard to control the immune
microenvironments with only one changing variable
in clinical trials (Kim et al 2019).
When breast cancer of NES and MES were
inoculated on both sides of mice, the different
subtypes will maintain their neutrophil and
macrophage frequencies, demonstrating the
formation of different myeloid subtypes are caused
by intrinsic tumor factors (Kim et al 2019). Thus, the
internal tumor factors could contribute to myeloid
cell profiles.
Some macrophages in different MES have high
signaling pathways that promote immune
suppression and promote tumor formation. In
contrast, others have increased the expression of
signaling pathways that promote the inflammatory
response (Kim et al 2019). And an increase in
monocytes is caused by the decrease in neutrophils
for NES.
Moreover, the ICB therapy had been found to
have a good effect on enriching MES in macrophages
with high expression of promoting inflammatory
responder signaling pathways. The ICB therapy has a
moderate impact on the enrichment of MES in
macrophages with increased expression of promoting
suppression of immune signaling pathways but could
improve the efficacy by combining CCR2KO and
ICB therapy. And no effect on NES at all as the
reduction of neutrophils hasn't enhanced the
effectiveness of ICB therapy for NES (Kim et al
2019).
The immunotherapy resistance mechanisms show
the importance of the immune microenvironment to
tumor heterogeneity. And provide a potential route in
improving the effectiveness of immunotherapy (Kim
et al 2019).
2.2 Promote Macrophage Recruitment
via the CXCL12/CXCR4 Pathway
CXCL12/CXCR4 pathway is one of the potential
mechanisms to actively recruits abundant
macrophages, especially pro-tumor M2
macrophages, that achieves the macrophage-enriched
feature.
The mechanism of how TAMs, especially tumor-
promoting M2 macrophages, migrate and accumulate
in the OSCC via the interaction between CAF,
CXCL12/ CXCR4 pathway, CSC, and M2
macrophages is demonstrated in a previous study (Li
et al 2019). In the research conducted by Li et al., an
in vivo test investigated the distribution and
phenotype of TAM in OSCC. Overexpression of
macrophages was observed in all OSCC samples
compared to normal and dysplasia specimens.
Besides, masses of these macrophages are pro-tumor
M2 (Li et al 2019). Using the human monocytic cell
line THP-1, resultant data shown that CAFs were
most efficient at recruiting THP-1 monocytes.
Among CAF-derived chemokines, the disparity
between expressions in normal cells and CAF cells
was the most significant for the CXCL12 chemokine
(24 folds) (Li et al 2019). All data from chemotactic
experiments suggested that CXCL12 was the
predominant chemoattractant to drive monocyte
Targeting Myeloid Cells for Potential Cancer Therapies
175

migration and recruitment (Li et al 2019). By labeling
two subtypes of TAM, tumor-suppressive M1 and
tumor-supportive M2, with distinct markers, the
genotype of macrophages that were differentiated
from THP-1 monocytes with the induction of CAF
was identified as M2 macrophages (Li et al 2019).
Besides, the cell viability assay demonstrated that M2
cells are crucial to promote proliferation and hinder
apoptosis of Cal-27 cancer cells (Li et al 2019). By
culturing Cal-27 cells with M2 macrophages, it was
confirmed that polarized M2 cells induce OSCC to
obtain the cancer stem cell-like characteristic. M2-
treated Cal 27 cells were further confirmed that M2
macrophages had high resistance to the
chemotherapy drug Vincristine (Li et al 2019). After
conducting both transwell invasion assay and wound-
healing assay to investigate the mobility of Cal 27
cells, the results revealed that M2 macrophages lead
to expedited metastasis of OSCC via epithelial-
mesenchymal transition (EMT) (Li et al 2019). In
short, CXCL12/ CXCR4 pathway is critical in
recruiting M2 macrophage polarization in OSCC,
resulting in enhanced cancer cell proliferation,
migration, and chemotherapy drug resistance.
2.3 Modified Labeling Method for
Visualization and Quantification of
MDSCs
Positron emission tomography (PET) is a nuclear
medicine technique that measures the metabolism of
cells in body tissues using tiny quantities of
radioactive chemicals called radiopharmaceuticals to
aid in the visualization of biochemical changes in the
body (Positron Emission Tomography (PET). (n.d.).
Thus, PET imaging is a powerful technique that
enables the visualization and monitoring of myeloid
cell distribution in TME (Hoffmann et al 2019). As
mentioned above, myeloid-derived suppressor cells
(MDSCs), a type of myeloid cells, are key
participants within the tumor microenvironment
(TME). Hoffman et al. applied an improved
intracellular cell labeling approach to quantify in
vitro-cultured MDSC motility in primary and
metastatic cancers in vitro (Hoffmann et al 2019).
Researchers labeled in vitro produced MDSCs from
polymorphonuclear (PMN-) and monocytic (M-)
subsets using a "
64
Cu-labeled 1,4,7-
triazacyclononane-tri acetic acid (NOTA)-treated
CD11b-specific monoclonal antibody (mAB)"
(Hoffmann et al 2019). Subsequent to transferring
them into primary and metastatic MMTV-PyMT
breast cancer and B16/F10 melanoma mouse models
respectively, PET and magnetic resonance images
can be acquired for visualizing and quantifying
MDSCs migrations (Hoffmann et al 2019). The
researchers also indicate that the
64
Cu-NOTA-
CD11b-mAB could be internalized within only 3
hours, resulting in moderately stable radiolabeling
with minimal detrimental influence on cell survival
and functionality. Furthermore, it was suggested by
researchers that CD11b-specific mAB can simply
adapt to label additional myeloid cells, including
monocytes, macrophages, or neutrophils, for in vivo
molecular imaging (Hoffmann et al 2019).
3 DISCUSSION
Besides OSCC, CXCL12/CXCR4 also impacts other
types of cancers. Breast cancer remained the most
frequent cancer among women globally, and it had
been revealed that the CXCL12 and CXCR4
signaling has been implicated in practically every
facet of breast cancer carcinogenesis (Zlotnik,
Burkhardt, & Homey 2011). Chemokines are
chemotactic cytokines and could be divided into
different subgroups. The subgroup that interacts with
their receptors could influence tumor growth and
metastasis (Mollica Poeta et al, 2019b, Eckert et al
2018). Several specific chemokine receptors are
found on both immune and tumor cells. And their
presence on cancer cells could aid in cancer diagnosis
(Jacquelot, Duong, Belz, Zitvogel 2018).
CXCR4 is a crucial signal in breast cancer
metastasis as it expresses high levels of CXCR4
ligand CXCL12 in many organs, including liver,
bones, lungs, and lymph nodes (Janssens, Struyf, &
Proost, 2018, Koizumi, Hojo, Akashi, Yasumoto, &
Saiki 2007, Balkwill 2004). By the presence of the
CXCR4 positive cells in breast cancer patients' lymph
nodes, the pDCs will secrete TNF, which induces
CXCR4 expression in their body, producing a high
expression of CXCL12 (Okuyama Kishima et al
2015). Besides, the CXCR4/CXCL12 pathway also
plays a vital role in the prevention of lung metastasis.
The CXCR2 ligands will recruit the CXCR2
+
neutrophils into the TME, where they will interact
with cancer cells and promote the expression of genes
implicated in metastasis (Yu et al 2016).
As described above, the CXCL12/CXCR4
pathway has a considerable effect on tumor
development, and therefore targeting the pathway
could illustrate a potent method to create innovative
therapy in cancer treatment. CXCR4 combines with
its ligand CXCL12 and then becomes able to activate
the downstream signaling pathway to boost cancer
development. Among the current workable therapies
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
176

targeting on CXCL12/CXCR4 pathway, CXCR4
inhibitors are the major direction of massive research,
because it is thought that CXCR4 antagonism can
prevent cancer from growing (Zhou et al 2018).
Plerixafor, also called "AMD3100", is the only
CXCR4 antagonist currently in clinical use (Zhou et
al 2018). Plerixafor is a tiny bicyclam molecule with
antiretroviral properties that could bind to CXCR4
(Zhou et al 2018). Since December 2008, it has been
made available to non-lymphoma Hodgkin's (NHL)
and multiple myeloma (MM) patients in the United
States (Zhou et al 2018). The vital function for
Plerixafor to prevent cancer development is
generated by its ability to block the signaling pathway
of CXCR4 after binding to CXCR4. However, this
drug still has some deficiencies. Plerixafor lacks
CXCR4 specificity. Research has demonstrated that
Plerixafor only competes for CXCL12 binding to
CXCR4 when high quantities of Plerixafor are
present, indicating that Plerixafor is a low-affinity
CXCR4 ligand. Plerixafor promotes CXCL12
binding to CXCR7 and triggers the CXCL12/CXCR7
signaling pathway (Kalatskaya 2009). Therefore, it is
necessary to find other efficient treatment targeting
on CXCL12/CXCR4 pathway.
4 POTENTIAL NOVEL
TREATMENT
Owing to the lack of well-developed treatment
targeting the CXCL12/CXCR4 interaction approved
for clinical use, we herein propose a potential
treatment that is based on the use of anti-CXCL12
monoclonal antibody (mAB) for OSCC. Fig.2
illustrates that CXCL12 mAB would bind to
CXCR12; thus, preventing CXCL12 from interacting
with CXCR4 receptor. It's expected to see reduced
migration and expression of M2 macrophages in
tumor sites with the anti-CXCL12 treatment.
Figure 2. The CXCL12 mAB is expected to bind to
CXCL12, which would prevent it from interacting with its
receptor CXCR4 located on the cell surface. Figure
modified from (Cancilla, Rettig & DiPersio 2020).
By using animal models, in vitro experiments are
able to represent the whole organism with
physiological relevance and the inherent complexity
of a living system (Katt, Placone, Wong, Xu &
Searson 2016). Due to the fact that mice are cheap,
easy to breed, and biologically similar to humans,
mice are the desired animal models to be used in this
proposed design (Bryda 2013). OSCC-bearing Balb/c
mice models can be established from the murine
squamous cell carcinoma cell line, SCC7, which is
indicated to result in stable syngeneic OSCC models
in a previous study (Li 2020). Due to its ability to
recognize CXCL12 in both human and mice models,
monoclonal antibody (mAB) MAB350 (R&D
Systems, Inc) is selected to test the hypothesis that
neutralizing CXCL12 ligands is capable of reducing
M2 macrophage recruitments and treating OSCC in
the living organism (Human/Mouse CXCL12/SDF-1
antibody. 2020). The negative control is achieved
with no injection of MAB350 into mice, while the
experimental group is peritumoral injected with anti-
CXCL12 antibodies. Peritumoral injection would
prevent artificial injury to surrounding tissues by the
needle (Yoshida 2018).
The effect of injected mAB can be identified and
visualized via PET imaging. Tumor-associated
macrophages with the specific pro-tumor M2
phenotype could be cultured in vitro following the
instructions mentioned in Rey-Giraud et al.'s study. In
brief, freshly isolated monocytes would be cultured
in XVivo 10 media with M-CSF for six days to allow
for the generation of monocyte-derived M2
macrophages (Rey-Giraud, Hafner & Ries 2012). The
fact that CD11b is a common surface protein
expressed on both human and murine macrophages
makes CD11b a great target for radiolabeling
(Dziennis et al 1995). The procedure to radiolabel in
vitro-generated M2 macrophages is adapted from
Sabrina et al. In short, M2 macrophages are incubated
with [
64
Cu]NOTA-αCD11b-mAb in PBS media for
half an hour at 37℃ (Hoffmann et al 2019).
Radiolabeled M2 macrophages are then transferred
into OSCC-bearing mice models for PET imaging.
For 2hr, 4hr, and 12hr after injection of anti-
CXCL12 mAB, a PET scan would be acquired for
each mice model (InveonTM user manual: Inveon
scanners and inveon acquisition workplace 1.5 with
service pack 1. 2011). By quantitatively analyzing
and comparing the signal of the PET images, we
might be able to examine the migration of labeled M2
macrophages to the OSCC tumor sites. Since the
expression of M2 macrophages is confirmed to
correlate with tumor progression positively, lower
levels of M2 macrophages are expected in anti-
Targeting Myeloid Cells for Potential Cancer Therapies
177

CXCL12 mAb-treated mice models (Li et al 2019).
The negative control is estimated to have a much
higher expression of M2 macrophages than all
experimental models. Results that match our
expectations could verify our hypothesis that
potential cancer treatment targeting on
CXCL12/CXCR4 pathway; specifically, the use of
anti-CXCL12 antibodies is a feasible therapy.
5 CONCLUSIONS
Briefly, based on different characteristics of various
myeloid cells and previous myeloid cells-related
studies, the CXCL12/CXCR4 pathway was used as
an entry point to identify viable therapies for this
pathway, namely the use of Plerixafor (Righetti 2019,
Kim et al 2019). Due to drawbacks of Plerixafor, it is
necessary to continue searching for alternative
therapeutic approaches targeting the
CXCL12/CXCR4 pathway. Herein, a potential
cancer treatment pathway is proposed: by developing
CXCL12 inhibitors, the migration and expression of
M2 macrophages at the tumor site are reduced. This
treatment can be done as a further test in humans after
the effects are confirmed in animal models.
REFERENCES
A., T. (n.d.). Immunology: What cells have a myeloid
lineage and how are they identified? Cell Signaling
Technology. Retrieved March 3, 2021, from
https://blog.cellsignal.com/immunology-what-cells-
have-a-myeloid-lineage-and-how-are-they-identified
A., T. (n.d.). Immunology: What cells have a myeloid
lineage and how are they identified? Cell Signaling
Technology. Retrieved August 28, 2021, from
https://blog.cellsignal.com/immunology-what-cells-
have-a-myeloid-lineage-and-how-are-they-identified
Akram, M., Iqbal, M., Daniyal, M., & Khan, A. U. (2017).
Awareness and current knowledge of breast cancer.
Biological research, 50(1), 33.
https://doi.org/10.1186/s40659-017-0140-9
Balkwill, F. (2004). Cancer and the chemokine network.
Nature Reviews Cancer, 4(7), 540–550.
https://doi.org/10.1038/nrc1388
Breast cancer | world cancer research fund international.
(2021, May 19). WCRF International.
https://www.wcrf.org/dietandcancer/breast-
cancer/#:%7E:text=Breast%20cancer%20is%20the%2
0most,per%20100%2C000%20in%20Northern%20A
merica.
Brostjan, C., & Oehler, R. (2020). The role of neutrophil
death in chronic inflammation and cancer. Cell Death
Discovery, 6(1). https://doi.org/10.1038/s41420-020-
0255-6
Bryda E. C. (2013). The Mighty Mouse: the impact of
rodents on advances in biomedical research. Missouri
medicine, 110(3), 207–211.
Cancer. (2021, March 3). World Health Organization.
https://www.who.int/news-room/fact-
sheets/detail/cancer
Cancilla, D., Rettig, M. P., & DiPersio, J. F. (2020).
Targeting CXCR4 in AML and ALL. Frontiers in
Oncology, 10. https://doi.org/10.3389/fonc.2020.01672
Dandekar, R. C., Kingaonkar, A. V., & Dhabekar, G. S.
(2011). Role of macrophages in malignancy. Annals of
maxillofacial surgery, 1(2), 150–154.
https://doi.org/10.4103/2231-0746.92782
Davis, L. E., Shalin, S. C., & Tackett, A. J. (2019). Current
state of melanoma diagnosis and treatment. Cancer
biology & therapy, 20(11), 1366–1379.
https://doi.org/10.1080/15384047.2019.1640032
Dziennis, S., Van Etten, R., Pahl, H., Morris, D., Rothstein,
T., Blosch, C., Perlmutter, R., & Tenen, D. (1995). The
CD11b promoter directs high-level expression of
reporter genes in macrophages in transgenic mice
[published erratum appears in Blood 1995 Apr
1;85(7):1983]. Blood, 85(2), 319–329.
https://doi.org/10.1182/blood.v85.2.319.319
Eckert, F., Schilbach, K., Klumpp, L., Bardoscia, L.,
Sezgin, E. C., Schwab, M., Zips, D., & Huber, S. M.
(2018). Potential role of CXCR4 targeting in the
context of radiotherapy and immunotherapy of cancer.
Frontiers in Immunology, 9.
https://doi.org/10.3389/fimmu.2018.03018
Hoffmann, S. H. L., Reck, D. I., Maurer, A., Fehrenbacher,
B., Sceneay, J. E., Poxleitner, M., Öz, H. H.,
Ehrlichmann, W., Reischl, G., Fuchs, K., Schaller, M.,
Hartl, D., Kneilling, M., Möller, A., Pichler, B. J., &
Griessinger, C. M. (2019). Visualization and
quantification of in vivo homing kinetics of myeloid-
derived suppressor cells in primary and metastatic
cancer. Theranostics, 9(20), 5869–5885.
https://doi.org/10.7150/thno.33275
Human/Mouse CXCL12/SDF-1 antibody. (2020, August
25). R&D Systems.
https://www.rndsystems.com/products/human-mouse-
cxcl12-sdf-1-antibody-79018_mab350#product-details
InveonTM user manual: Inveon scanners and inveon
acquisition workplace 1.5 with service pack 1. (2011).
Siemens Medical Solutions USA, Inc.
https://cancer.wisc.edu/research/wp-
content/uploads/2018/02/inveon_iaw15_manual.pdf
Jacquelot, N., Duong, C. P. M., Belz, G. T., & Zitvogel, L.
(2018). Targeting chemokines and chemokine receptors
in melanoma and other cancers. Frontiers in
Immunology, 9.
https://doi.org/10.3389/fimmu.2018.02480
Janssens, R., Struyf, S., & Proost, P. (2018). Pathological
roles of the homeostatic chemokine CXCL12. Cytokine
& Growth Factor Reviews, 44, 51–68.
https://doi.org/10.1016/j.cytogfr.2018.10.004
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
178

Kalatskaya, I., Berchiche, Y. A., Gravel, S., Limberg, B. J.,
Rosenbaum, J. S., & Heveker, N. (2009). AMD3100 is
a CXCR7 ligand with allosteric agonist properties.
Molecular pharmacology, 75(5), 1240–1247.
https://doi.org/10.1124/mol.108.053389
Katt, M. E., Placone, A. L., Wong, A. D., Xu, Z. S., &
Searson, P. C. (2016). In vitro tumor models:
Advantages, disadvantages, variables, and selecting the
right platform. Frontiers in Bioengineering and
Biotechnology, 4.
https://doi.org/10.3389/fbioe.2016.00012
Kim, I. S., Gao, Y., Welte, T., Wang, H., Liu, J., Janghorban,
M., Sheng, K., Niu, Y., Goldstein, A., Zhao, N., Bado,
I., Lo, H. C., Toneff, M. J., Nguyen, T., Bu, W., Jiang,
W., Arnold, J., Gu, F., He, J., . . . Zhang, X. H. F. (2019).
Immuno-subtyping of breast cancer reveals distinct
myeloid cell profiles and immunotherapy resistance
mechanisms. Nature Cell Biology, 21(9), 1113–1126.
https://doi.org/10.1038/s41556-019-0373-7
Koizumi, K., Hojo, S., Akashi, T., Yasumoto, K., & Saiki,
I. (2007). Chemokine receptors in cancer metastasis
and cancer cell-derived chemokines in host immune
response. Cancer Science, 98(11), 1652–1658.
https://doi.org/10.1111/j.1349-7006.2007.00606.x
Li, Q., Dong, H., Yang, G., Song, Y., Mou, Y., & Ni, Y.
(2020). Mouse Tumor-Bearing models as preclinical
study platforms for oral squamous cell carcinoma.
Frontiers in Oncology, 10.
https://doi.org/10.3389/fonc.2020.00212
Li, X., Bu, W., Meng, L., Liu, X., Wang, S., Jiang, L., Ren,
M., Fan, Y., & Sun, H. (2019). CXCL12/CXCR4
pathway orchestrates CSC-like properties by CAF
recruited tumor associated macrophage in OSCC.
Experimental Cell Research, 378(2), 131–138.
https://doi.org/10.1016/j.yexcr.2019.03.013
Liu, T., Han, C., Wang, S., Fang, P., Ma, Z., Xu, L., & Yin,
R. (2019). Cancer-associated fibroblasts: an emerging
target of anti-cancer immunotherapy. Journal of
Hematology & Oncology, 12(1).
https://doi.org/10.1186/s13045-019-0770-1
Lv, M., Wang, K., & Huang, X. J. (2019). Myeloid-derived
suppressor cells inhematological malignancies: friends
or foes. Journal of Hematology & Oncology, 12(1).
https://doi.org/10.1186/s13045-019-0797-3
Mollica Poeta, V., Massara, M., Capucetti, A., & Bonecchi,
R. (2019). Chemokines and chemokine receptors: New
targets for cancer immunotherapy. Frontiers in
Immunology, 10.
https://doi.org/10.3389/fimmu.2019.00379
Mollica Poeta, V., Massara, M., Capucetti, A., & Bonecchi,
R. (2019b). Chemokines and chemokine receptors:
New targets for cancer immunotherapy. Frontiers in
Immunology, 10.
https://doi.org/10.3389/fimmu.2019.00379
Morris, S. Y. (2018, September 29). Understanding
neutrophils: Function, counts, and more. Healthline.
https://www.healthline.com/health/neutrophils
NCI Dictionary of Cancer Terms. (n.d.). National Cancer
Institute.
https://www.cancer.gov/publications/dictionaries/canc
er-terms/def/five-year-survival-rate
Okuyama Kishima, M., Oliveira, C. E. C. D., Banin-Hirata,
B. K., Losi-Guembarovski, R., Brajão De Oliveira, K.,
Amarante, M. K., & Watanabe, M. A. E. (2015).
Immunohistochemical expression of CXCR4 on breast
cancer and its clinical significance. Analytical Cellular
Pathology, 2015, 1–6.
https://doi.org/10.1155/2015/891020
Ping, Q., Yan, R., Cheng, X., Wang, W., Zhong, Y., Hou, Z.,
Shi, Y., Wang, C., & Li, R. (2021). Cancer-associated
fibroblasts: Overview, progress, challenges, and
directions. Cancer Gene Therapy. Published.
https://doi.org/10.1038/s41417-021-00318-4
Positron Emission Tomography (PET). (n.d.). Johns
Hopkins Medicine.
https://www.hopkinsmedicine.org/health/treatment-
tests-and-therapies/positron-emission-tomography-pet
Rey-Giraud, F., Hafner, M., & Ries, C. H. (2012). In vitro
generation of monocyte-derived macrophages under
serum-free conditions improves their tumor promoting
functions. PloS one, 7(8), e42656.
https://doi.org/10.1371/journal.pone.0042656
Righetti, A., Giulietti, M., ŠAbanović, B., Occhipinti, G.,
Principato, G., & Piva, F. (2019). CXCL12 and its
isoforms: Different roles in pancreatic cancer? Journal
of Oncology, 2019, 1–13.
https://doi.org/10.1155/2019/9681698
Schmid, M. C., & Varner, J. A. (2010). Myeloid cells in the
tumor microenvironment: Modulation of tumor
angiogenesis and tumor inflammation. Journal of
Oncology, 2010, 1–10.
https://doi.org/10.1155/2010/201026
Taghavi, N., & Yazdi, I. (2015). Prognostic factors of
survival rate in oral squamous cell carcinoma: clinical,
histologic, genetic and molecular concepts. Archives of
Iranian medicine, 18(5), 314–319.
Veglia, F., Perego, M., & Gabrilovich, D. (2018). Myeloid-
derived suppressor cells coming of age. Nature
Immunology, 19(2), 108–119.
https://doi.org/10.1038/s41590-017-0022-x
Wright stain. (2020, January 5). Lab Tests Guide.
https://www.labtestsguide.com/wright-stain
Yoshida, H., Yoshimura, H., Matsuda, S., Ryoke, T.,
Kiyoshima, T., Kobayashi, M., & Sano, K. (2018).
Effects of peritumoral bevacizumab injection against
oral squamous cell carcinoma in a nude mouse
xenograft model: A preliminary study. Oncology
Letters. Published.
https://doi.org/10.3892/ol.2018.8399
Yu, P. F., Huang, Y., Xu, C. L., Lin, L. Y., Han, Y. Y., Sun,
W. H., Hu, G. H., Rabson, A. B., Wang, Y., & Shi, Y. F.
(2016). Downregulation of CXCL12 in mesenchymal
stromal cells by TGFβ promotes breast cancer
metastasis. Oncogene, 36(6), 840–849.
https://doi.org/10.1038/onc.2016.252
Zhou, Y., Cao, H. B., Li, W. J., & Zhao, L. (2018). The
CXCL12 (SDF-1)/CXCR4 chemokine axis: Oncogenic
properties, molecular targeting, and synthetic and
natural product CXCR4 inhibitors for cancer therapy.
Targeting Myeloid Cells for Potential Cancer Therapies
179

Chinese Journal of Natural Medicines, 16(11), 801–
810. https://doi.org/10.1016/s1875-5364(18)30122-5
Zlotnik, A., Burkhardt, A. M., & Homey, B. (2011).
Homeostatic chemokine receptors and organ-specific
metastasis. Nature Reviews Immunology, 11(9), 597–
606. https://doi.org/10.1038/nri3049
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
180
