
Research Progress of Air-water Interface Stability in Food Foam
System
Xinghui Wu
1,2 a
, Mingjie Xia
1,2
, Shufang Cao
1,2
, Wenqi Cai
1,2
, Li Li
1,2
and Lina Yang
1,2,*
1
College of Food Science and Engineering, Bohai University, Jinzhou, 121013, Liaoning, China
2
Grain and Cereal Food Bio-efficient Transformation Engineering Research Center of Liaoning Province, Jinzhou,
121013, Liaoning, China
*
Corresponding author
Keywords: Foam System, Polysaccharide Hydrocolloid, Interfacial Stability, Surfactant.
Abstract: The foam dispersion system is widely used in many kinds of food processing fields and give good texture and
sense for food. This paper summarized this formation mechanism, the effect mechanism of internal factors
(surface tension, surface viscoelasticity) and external factors (temperature, pH, surfactant, ionstability) on
stability of foam system, and the progress of effect of polysaccharide hydrocolloids co-regulating protein on
stability of foam system. The aims was to provide theory support for developing natural surfactant and obtain
expected foam texture in food system. In order to promote the application of polysaccharides hydrocolloid
surfactant in the field of foam food processing.
1 INTRODUCTION
In daily life, the macro behavior of many foods, such
as their stability, rheological properties and structure,
is related to the state of structural units. As one of the
most common structural units in multiphase food
system, the foam system plays a vital role in beer,
inflatable candy, ice cream, baking products and other
foods. The foam system not only gives the superiority
of food organization structure and appearance, but
also benefits chewing and food flavor transmission.
At the same time, the addition of air can also reduce
energy intake to a certain extent and meet modern
people's pursuit of healthy diet (Liu 2009). Because
foam is a typical thermodynamic instability system of
air-liquid two phase, the high specific surface area
and surface free energy of air-liquid interface lead to
unstable foam (Li 2020). Therefore, how to obtain
stable (metastable) foam system in actual food
production and processing is a problem that needs to
be further studied.
a
https://orcid.org/0000-0001-8481-667X
2 FORMATION AND
INSTABILITY OF FOAM
SYSTEM IN FOOD
2.1 Formation of Foam System
The foam in food is a dispersive system composed of
bubbles separated by liquid membrane, where liquid
or semisolid is a continuous phase and gas is
dispersed phase (Diao et al. 2021). The foam
structure is shown in Figure 1. Foam formation
requires four conditions: gas, water, surfactant and
energy. Full contact between gas phase and water
phase is a necessary condition for foaming. Energy
increase the interfacial area between the two phases.
The function of surfactant is to adsorb on the air-
liquid interface to form an elastic liquid film with a
certain thickness, reduce the surface tension and
maintain the stability of the air-liquid interface.
100
Wu, X., Xia, M., Cao, S., Cai, W., Li, L. and Yang, L.
Research Progress of Air-water Interface Stability in Food Foam System.
DOI: 10.5220/0011232900003438
In Proceedings of the 1st International Conference on Health Big Data and Intelligent Healthcare (ICHIH 2022), pages 100-106
ISBN: 978-989-758-596-8
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

Figure 1: Scheme of the foam structures (Li 2020).
2.2 Instability of Foam System
Because of the high surface free energy of the foam
system in the air-water boundary, the foam surface is
unstable. According to the principle of Gibbs free
energy, in order to reduce the interfacial area, the
surface free energy will decrease spontaneously,
resulting in the loss of water film, polymerization and
disproportionation of the bubble film, which will
cause the instability of the foam system (Murray &
Ettelaie 2004). As shown in Figure 2:
Drainage of water is the process of separation
of air-water two phase in foam system due to
different density (Conroy et al. 2013). Loss of
fluid can result in gradual drying of foam;
Polymerization is the phenomenon that the film
between two adjacent bubbles breaks, resulting
in the merger of two bubbles (Murray et al.
2006).
Disproportionation is the transfer of air in small
bubbles to large bubbles, resulting in the
disappearance of small bubbles (Langevin et al.
2017).
These three phenomena exist simultaneously in
the foam system and contain each other. But no
matter which mechanism is dominant, the final
equilibrium state of the foam system will be separated
into an independent water phase and air phase (Diao
et al. 2021).
Figure 2: This caption has more than one line so it has to be
set to justify.
3 FACTORS AFFECTING THE
STABILITY OF FOOD FOAM
SYSTEM
Evenly distributed foam often makes food texture
fine, lubricated, with a certain brightness, but also
enhances the flavor components divergent and
observable (Li et al. 2020). It constitutes the main
sensory attraction of consumers. Stable beer foam can
not only give consumers the sense of enjoyment, but
also reduce the overflow of beer flavor substances. It
prevents the direct contact of oxygen and beer in the
air and oxidize, so as to ensure the quality of beer
(Han 2017). Good foaming and foam stability help to
form the texture of ice cream and extend the storage
period of ice cream (Lian et al. 2020). In inflatable
chocolate, small and stable foam will make the
chocolate more lubricate and delicate, and it will also
be very different in color (Liu2009). Therefore, the
stability of the foam system is critical to the quality
of food. However, foam stability is easily affected by
internal and external factors such as surface tension,
surface viscoelasticity, temperature, pH and
surfactant.
3.1 Formation of Foam System
3.1.1 Surface Tension
With the formation of foam, the surface area of liquid
increases and the surface potential energy increases
(Zhan et al. 2020). The lower surface tension is
favorable for improving the foam stability. The
Laplasse equation shows that the smaller the surface
tension and the smaller the pressure difference, the
more stable the foam is. Xiao Xia (Xiao 2013)
randomly selected 7 kinds of wine samples from the
market to determine the foam retention and surface
tension. The results showed that the surface tension
of the wine with good foam stability was poor. At the
same time, as the storage period lengthened, the foam
retention gradually decreased, and the surface tension
gradually increased.
3.1.2 Surface Viscoelasticity
Surface viscosity refers to the viscosity of the
molecular layer of foaming agent on the liquid
membrane, which is produced by the interaction
between hydrophilic groups and water (Li 2014). The
greater the adsorption force of surfactant molecules
on the air-liquid interface, the higher the surface
viscosity and the better the elasticity of the
Research Progress of Air-water Interface Stability in Food Foam System
101

membrane. The surface viscosity directly determines
the drainage rate of the liquid film, thereby affecting
the stability of the foam to a certain extent. The
greater the surface viscosity, the smaller the drainage
velocity, and the more stable the foam is (Cilurzo et
al. 2019). However, for the production and
processing of ice cream, if the viscosity of the system
is too high, the ice cream material will be too viscous.
During the freezing process, it is difficult for a large
number of air to be evenly distributed in ice cream,
resulting in low expansion rate and poor taste of the
product (Zhan et al. 2020).
Surface elasticity refers to the ratio of the stress
on the surface to its strain. It means the tendency or
ability of the surface to return to the initial state after
deformation under external force (Wu 2017).
Therefore, the surface elasticity of the foam liquid
film can also be considered as ‘self repairing’ ability
to resist external interference. When the liquid film of
the foam is disturbed, this ‘self repair’ capability is
embodied in two aspects:
With the increase of the area of the foam liquid
film and the decrease of the surface density of
the surfactant, the local surface tension
increases, and the surface tension gradient
formed has the tendency to shrink the liquid
film, which is called the Gibbs effect;
Under the action of surface tension gradient,
surfactant molecules migrate from regions with
higher density to regions with lower density,
which is called Marangoni effect (Wang &
Guo 2007).
Generally speaking, Gibbs elasticity is more
suitable for measuring the stability of static foam,
while Marangoni elasticity is often used to measure
the stability of dynamic foam. Zhang (Zhang 2012)
found that the interaction between Tween 20 and
BSA and the formation of gel like interface network
structure enhanced the surface elasticity of the
adsorption film, increased the ability to resist external
interference, reduced the liquid film drainage rate,
and further improved the stability of the foam.
Because there is no adsorbed surfactant on the
surface, the pure aqueous film cannot adjust the
surface tension gradient through the movement of
surfactant or the contraction of the film when
disturbed by the outside world, so it does not have the
ability of ‘self repair’ (Li 2014).
3.2 Influence of External Factors
3.2.1 Temperature
The change of temperature significantly affects the
properties of liquid film. Most of the foam is unstable
at high temperature. As the temperature increases, the
surface viscosity of the liquid film decreases, and the
discharge rate increases, resulting in the decrease of
foam stability (Li 2014). Han (Han 2017) explored
the effect of different pretreatment temperatures on
the stability of beer foam. It was found that the
stability of beer foam decreased with the increase of
temperature, and the higher the temperature, the
worse the foam stability. The reason may be that the
solubility of CO
2
in beer decreases after the
temperature rises, and the dissolution of CO
2
after
ultrasonic oscillation is faster, resulting in larger
volume of individual bubbles formed by beer
foaming, and the decrease of surface viscosity and
foam stability of beer foam.
3.2.2 pH
The change of pH affects the ionization of the foam
system. Ionization is closely related to the interaction
in the whole solution, the adsorption of molecules at
the air-water interface and the interaction at the
interface. Li et al. (Li, et al. 2017) found that the egg
white protein produced excessive negative charges in
alkaline solution, which increased the static repulsion
between protein molecules and weakened the stability
of the foam system. However, for egg yolk protein,
the increase of pH is helpful to the dissociation of
insoluble yolk protein particle aggregates, improve its
adsorption capacity at the air-water interface, reduce
the surface tension, and help to enhance the stability
of the foam system. The results are quite different
from those of the egg white foam system in alkaline
solution. Kuropatwa et al. (Kuropatwa et al. 2009)
found that whey protein and egg white protein
complex at pH 9.0 had higher foaming and foam
stability. This is because the electrostatic interaction
of proteins has occurred before foaming, and the
protein of the composite system is adsorbed at the air-
water interface. Under alkaline conditions, expanded
β-Lactoglobulin monomer can increase the
interaction of egg white protein whey protein
complex. Compared with neutral conditions, the
foaming property and foam stability of the composite
system increased. However, Li et al. (Li 2009) tested
the foam stability of benzyl phosphate under different
pH. Compared with the results of pH=5.5 and
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
102

pH=7.2, it was found that the stability of pH=5.5
foam was good.
3.2.3 Surface Active Agent
In order to make foam easily generated and stable, it
is necessary to add stabilizer to the system, usually
surfactants. Its existence not only makes foaming
easy, but also makes foaming speed faster than bubble
breaking speed, thus obtaining stable foam (Li 2020).
As shown in Fig. 3, the foam generated by pure water
will burst quickly after reaching the interface.
However, the foam formed by surfactant solution can
be stabilized. This is because a large number of
surfactants can adsorb to the air water interface,
reduce the surface tension, and form a viscoelastic
interfacial film around the bubble through non
covalent molecular interactions and covalent two
sulphide crosslinking, thus forming and stabilizing
the foam (Perez et al. 2006). Gao (Gao 2020)
compared the pure rice flour bread with the rice flour
bread added with pullulan polysaccharide, and found
that the addition of pullulan polysaccharide improves
the characteristics of rice flour bread, such as high
hardness, poor elasticity and difficult to chew. The
reason may be that pullulan as a surfactant, combined
with water, form a more compact viscoelastic colloid
in the continuous phase, increase foam stability, and
better preserve the gas generated during fermentation.
Schmidt et al. (Schmidt et al. 2010) studies found that
the interfacial activity of the compound formed by
rapeseed protein and pectin was higher than that of
pure rapeseed protein, and the thicker interfacial film
could be formed at the air water interface, resulting in
the decrease of water permeability and the rise of the
stability of the foam.
Figure 3: Rise of bubbles in pure aqueous phase and
surfactant solution (Cilurzo et al. 2019).
3.2.4 Ion
The effect of inorganic salts on foam stability is
mainly through interaction with surfactant (Yu et al.
2010). The addition of inorganic salts will introduce
ions which with charge opposite to surfactant
headgroup. When the amount of inorganic salt is
small, the charged ions will be adsorbed on the
surface of the surfactant base, reducing the
electrostatic repulsion between the adjacent
surfactants, making them close to each other, thus
reducing the interfacial tension. This will help to
enhance the stability of the foam (Teng et al. 2005).
However, when the content of inorganic salt is too
high, the combination of excess inorganic salt ions
and surfactants may lead to the failure of surfactant or
destroy the electric double layer structure, which is
not conducive to the stability of foam (Liu 2011). Wu
Gang (Wu 2017) studied the mechanism of the effect
of inorganic salts on the foam stability of surfactant
and its composite system. It was found that the
addition of sodium salt had a negative effect on the
stability of surfactant foam, and the higher the
concentration of sodium salt, the more unfavorable to
foam stability. Low concentration of calcium salts
and magnesium salts had a favorable effect on foam
stability, and magnesium salt had greater impact than
calcium salts. When high concentration, the stability
of surfactant foam would also be adversely affected.
The higher the concentration, the worse the foam
stability.
4 POLYSACCHARIDE
HYDROCOLLOID REGULATES
THE STABILITY OF FOAM
SYSTEM
Because of its excellent foaming properties,
solubility, emulsification and gelation, the protein has
been widely used as a surfactant in the production and
processing of foam food (Narsimhan & Nin 2018).
However, individual protein molecules are not ideal
candidates for stable foaming systems because they
cannot fully adjust the high Laplace pressure
difference at the air-water interface (Shankaran &
Chinnaswamy 2019). Since most high molecular
weight polysaccharides are hydrophilic, they do not
have much adsorption tendency at the air-water
interface. However, they greatly enhance the stability
of the dispersion system through their thickening or
gelling properties. Therefore, in the food industry,
polysaccharides are used to synergistic protein
maintain the stability of the foam system and
emulsion system (Dickinson 2003). A classic
example is the use of pectin to stabilize casein
micelles in yogurt. Due to electrostatic interaction,
negatively charged pectin molecules are adsorbed on
Research Progress of Air-water Interface Stability in Food Foam System
103
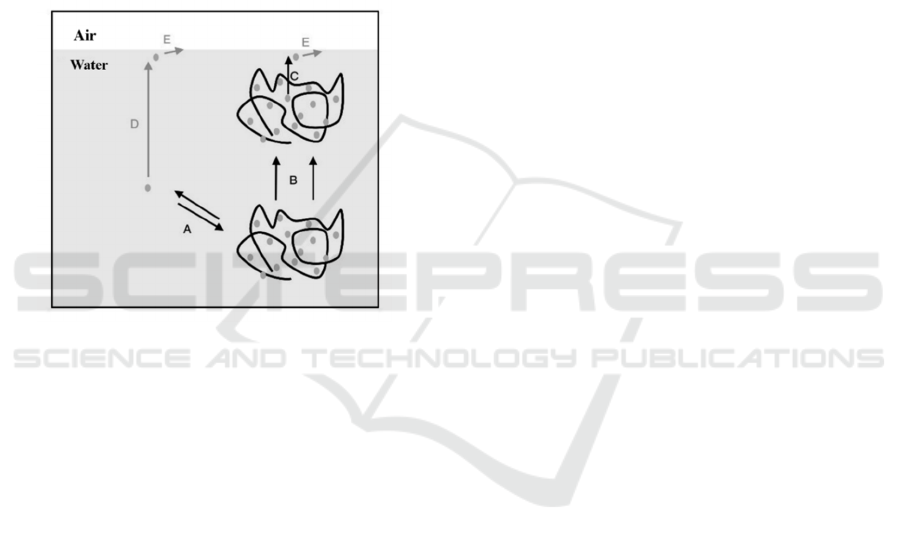
casein micelles and prevent casein from acid-induced
aggregation due to electrostatic and spatial repulsion
(see Fig. 4) (Renate et al. 2005). Among them,
polysaccharides act as two roles. One is to control the
rheological properties of the continuous phase. The
other is to increase the thickness of the adsorption
film, which reduces the rate of thinning of the bubble
film and increases the stability of the foam (Sadahira,
et al. 2018). The addition of polysaccharide water
colloid significantly improves the viscosity of gluten
free dough and hinders the escape of gas during
fermentation, so as to improve the quality of bread
and prolong the shelf life of bread, which solves the
dietary problems faced by celiac patients to a certain
extent (Niño-Medina et al. 2019).
Figure 4: Model for polysaccharide controlled protein
adsorption at the air/water interface (Renate et al. 2005) (A
: partition free protein and protein bound to
polysaccharide, B : diffusion of protein/polysaccharide
complexes in bulk, C:availability of complexed protein
for interface, D:diffusion of free protein in bulk, E:
kinetic barrier for protein adsorption)
From the existing research, the application of
polysaccharide hydrocolloid in the stabilization of
food foam system is based not only on its electrostatic
interaction with protein, but also by the covalent
grafting products formed by Maillard reaction with
proteins, which play an important role in modifying
the functional properties of proteins (Diao et al.
2021). The amino group on the protein and the
carboxyl group of reducing sugar bind to each other
can significantly improve the solubility and oxidation
resistance of protein, and further improve the stability
of foam system (Yu 2016). In the process , the
amino group on the protein and the carboxyl group of
reducing sugar bind to each other can significantly
improve the solubility and oxidation resistance of
protein, and further improve the stability of foam
system (Yu 2016). At present, many studies have
reported that proteins are modified by various food
grade substrates to obtain a stable foaming system.
Ovalbumin pullulan cement shows better foaming
performance and surface activity than natural protein
(Sheng et al. 2020). A large number of studies have
shown that the increase of protein foaming ability by
glycosylation reaction is mainly due to the covalent
connection between sugars and proteins, which
increases the hydrophilic groups in proteins, which
may lead to the increase of protein solubility, or the
formation of protein melting spherical structure
caused by mild heat treatment, which improves the
hydrophobicity of protein surface (An et al. 2014,
Murray 2007).
Because proteins usually have high surface
activity, most protein microgel particles also have
surface activity, and their Pickering particles can
adsorb efficiently at the air-water interface and
require very high desorption energy. Therefore, it has
higher viscoelasticity compared with the interfacial
film formed by single protein adsorption. Microgel
particles can also be composed of polysaccharides
with gel characteristics. Although most
polysaccharides are non surface active substances,
they can be combined with other components, such as
proteins or lipids, so as to achieve good adsorption
effect (Li 2020). From the perspective of food,
protein and polysaccharides have great advantages as
microgel particles, which can control the size of
surface active particles reasonably by controlling the
size of complex biopolymers or their mixtures (Chen
et al. 2017). In the presence of protein /
polysaccharide complexes, the foam stability
increases due to the formation of viscoelastic colloids
in continuous phases (Zhan et al. 2019). The protein
/ polyphenol complex which whey protein with gallic
acid (GA) and epigallocatechin gallate (EGCG) have
a similar strengthening effect (Cao & Xiong 2017).
Despite the above findings, the foam formation
properties of the composites are not always consistent
with their foam stability. For example, tannic acid
improves foam stability, but it inhibited the foam
formation of sodium caseinate (Zhan et al. 2018).
Therefore, high performance food hydrocolloids
exhibiting excellent foaming and foam stability are
still being constantly excavated.
5 CONCLUSIONS
In the food industry, the stability of the foam system
will directly affect the quality of the interface leading
foods. Therefore, controlling the stability of foam
system, regulating protein aggregation behavior and
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
104

understanding the relevance between surfactant and
foam system play an important role in guiding the
development of foam food industry. Future research
on food foam system can focus on establishing a
relationship model of protein (polysaccharide)
molecular polymer foam system, and clarify the
mechanism between the three. It aims at precisely
regulating protein nano polymer in order to obtain an
ideal foam system, and provide a theoretical basis for
the application of foam food in the food industry.
ACKNOWLEDGEMENTS
This study was supported by National Natural
Science Foundation of China (Grant No. 31901680).
REFERENCES
An, Y. Cui, B. Wang, Y. (2014). Functional properties of
ovalbumin glycosylated with carboxymethyl cellulose
of different substitution degree. J. Food Hydrocoll. 40,
1-8.
Briceno-Ahumada, Z. & Langevin, D. (2017). On the
influence of surfactant on the coarsening of aqueous
foams. J. Adv Colloid Interfac. 244, 124-131.
Cao, Y.Y. & Xiong, Y.L.L. (2017). Binding of Gallic Acid
and Epigallocatechin Gallate to Heat-Unfolded Whey
Proteins at Neutral pH Alters Radical Scavenging
Activity of in Vitro Protein Digests. J. J Agr Food
Chem. 65(38), 8443-8450.
Chen, X.W. Yang, D.X. Zou, Y. (2017). Stabilization and
functionalization of aqueous foams by Quillaja
saponin-coated nanodroplets. J. Food Res Int. 99, 679-
687.
Cilurzo, F. Critello, C.D. Paolino, D. (2019). Polydocanol
foam stabilized by liposomes: Supramolecular
nanoconstructs for sclerotherapy.J. Colloid Surface B.
175, 469-476.
Conroy, M.W. Taylor, J.C. Farley, J.P.(2013). Liquid
drainage from high-expansion (HiEx) aqueous foams
during and after filling of a container. J. Colloid Surface
A. 426, 70-97.
Diao, Y.C. Zhang, Y.P. Zhang, W. N.(2021). Research
progress of protein nanomaterials foam system .J. Food
Science: 1-11,
http://kns.cnki.net/kcms/detail/11.2206.TS.20201211.
1647.026.html.
Dickinson, E. (2003). Hydrocolloids at interfaces and the
influence on the properties of dispersed systems. J.
Food Hydrocoll. 17(1),25-39.
Gao, Y.Q. (2020). Effects of different processing methods
on the quality of rice dough and bread. D. Shenyang
Normal University.
Han, Y.P. (2017). Study on the mechanism of malt protein
Z stabilizing beer foam. D. Jiangnan University.
Kuropatwa, M. Tolkach, A. Kulozik, U. (2009). Impact of
pH on the interactions between whey and egg white
proteins as assessed by the foamability of their
mixtures.J. Food Hydrocoll. 23(8), 2174-2181.
Lian, J.W. Zhang, J.J. Han, D.(2020) Effect of the dual
protein on the quality of ice cream. J. Sci. Technol.
Food Ind. 41(17), 8-12.
Li, Y.Y. (2014). Study on influencing factors and plugging
ability of foam stability .D. China University of
Petroleum (East China)
Li, J. Wang, C. Li, X. (2017). Effects of pH and NaCl on
the physicochemical and interfacial properties of egg
white/yolk. J. Food Biosci. 23, 115-120.
Li, M.S. Zhao, G.H. Ye, F.Y. (2020). Progress in nano-and
micro-particle-stabilized foams and their implications
in food technology. J. Food and Fermentation
Industries. 46( 13) , 270-279.
Liu, L.N. (2009). Research on the technology of aerated
chocolate. D. Jiangnan University.
Liu, L.Y. (2011). Sodium caseinate-polysaccharide
interactions at the oil-water interface: Effect on
emulsion stability. D. South China University of
Technology.
Li, W. (2009). Effect of pH and NaCl Concentration on the
Stability of Surfactant-Free foam films. J.
Langmuir.25(1), 294-297.
Li, X. (2020). Engineered interface for explaining foaming
characteristics of egg white protein systems and
regulation mechanisms. D. Jiangnan University.
Murray, B.S. DickinsonI, E. Gransard, C. et al. (2006).
Effect of thickeners on the coalescence of protein-
stabilized air bubbles undergoing a pressure drop. J.
Food Hydrocoll. 20,114-123.
Murray, B.S & Ettelaie, R. (2004). Foam stability: proteins
and nanoparticles. J. Curr Opin Colloid In. 9(5), 314-
320.
Murray. B.S. (2007). Stabilization of bubbles and foams. J.
Curr Opin Colloid In. 12(5), 232-241.
Narsimhan, G. & Nin, X. (2018). Role of Proteins on
Formation, Drainage, and Stability of Liquid Food
Foams. J. Annu Rev Food Sci T. 9, 45-63.
Niño-Medina, G. Muy-Rangel, D. (2019). Dietary Fiber
from Chickpea (Cicer arietinum) and Soybean (Glycine
max) Husk Byproducts as Baking Additives:
Functional and Nutritional Properties. J. Molecules.
24(5), 991-1000.
Perez, O.E. Sanchez, C.C. Pilosof, A. M. (2006). Dynamics
of adsorption of hydroxypropyl methylcellulose at the
air–water interface. J. Food Hydrocoll. 22(3), 872-878.
Renate, A. Martien, A, et al.(2005). Use of polysaccharides
to control protein adsorption to the air–water interface.
J. Food Hydrocoll. 20(6), 872-878.
Sadahira, M.S. Rodrigues, M.I. Akhtar, M.(2018).
Influence of pH on foaming and rheological properties
of aerated high sugar system with egg white protein and
hydroxypropyllmethylcellulose. J. LWT-Food Sci
Technol. 89, 350-357.
Schmidt, I. Novales, B. Boue, F.(2010). Foaming properties
of protein/pectin electrostatic complexes and foam
Research Progress of Air-water Interface Stability in Food Foam System
105

structure at nanoscale.J. J Colloid Interf Sci. 2010,
345(2), 316-324.
Shankaran, P.I.& Chinnaswamy, A. (2019). Instant coffee
foam: An investigation on factors controlling
foamability, foam drainage, coalescence, and
disproportionation. J. J Food Process Eng. 42(6), 1-12.
Sheng, L. Tang, G.Y. Wang, Q. (2020). Molecular
characteristics and foaming properties of ovalbumin-
pullulan conjugates through the Maillard reaction. J.
Food Hydrocoll. 100, 105384-105392.
Teng, H.N. Wang, F. Sun, M.J. (2005). Influence of Salts
on Aqueous Two-phase System of Cationic and
Anionic Surfactant Aqueous Mixture. J. Acta Chim
Sinica. (17), 1570-1574+1548.
Wang, M.M. Guo, D.H. (2007). Foamability of foaming
agent and effecting factors. Advances. J. In Fine
Petrochemicals. (12), 40-44+47.
Wu, G. (2017). Effect of inorganic salts on foam stability
of surfactant and its composite systems. D. China
University of Petroleum (East China).
Xiao, X. (2013). Study on the relationship between beer
surface tension and foam durability. D. Gansu
Agricultural University.
Yu, D. Huang, X. Deng, M. (2010). Effects of Inorganic
and Organic Salts on Aggregation Behavior of Cationic
Gemini Surfactants. J. J Phys Chem B. 114(46), 14955-
64.
Yu, J. (2016). Research on citrus pectin-soy protein isolate
electrostatic interaction, characteristic of interfacial
adsorption and emulsifying stability. D. Huazhong
Agricultural University.
Zhan, F.C. Hu, J.G. He, C. (2020).Complexation between
sodium caseinate and gallic acid: Effects on foam
properties and interfacial properties of foam. J. Food
Hydrocoll. 99, 105365.
Zhan, F.C. Li, J. Shi, M.Q. (2019). Foaming properties,
linear and nonlinear surface dilatational rheology of
sodium caseinate, tannin acid and octenyl succinate
starch ternary complex. J. J Agr Food Chem. 67(8),
2340–2349.
Zhan, F.C. Li, J. Wang, Y.T. (2018). Bulk, foam and
interfacial properties of tannic acid/sodium caseinate
nanocomplexes. J. J Agr Food Chem. 6832-6839.
Zhang, H.H. (2012). The effect of bovine serum albumin on
the foam properties of tweens surfactants. D. Shandong
University.
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
106
