
The Cause and Therapy of Myasthenia Gravis
Yucong Geng
1,*,†
, Ruocen Song
2,†
and Jiaqi Liu
3,†
1
University of California, Berkeley, U.S.A.
2
University of Southern California, Los Angeles, U.S.A.
3
The High School Affiliated to Renmin University of China, Beijing, China
2
ruocenso@usc.edu,
3
3038740976@qq.com
†
These authors contributed equally
Keywords: Myasthenia Gravis, Main Cause, Treatment Method, Rituximab.
Abstract: Myasthenia Gravis is essential for people to have a clearer understanding of the cause as well as the potential
treatments since this disease can happen to every person at any age and at any time. Deep research and data
collection have shown the main causes of physiological problems and gene regulation and the three most
effective types of treatments- thymectomy, blood-derived, and medications. Some clinical research data
results indicate the effectiveness of using the medication, rituximab, by using placebo studies. It turns out that
rituximab helps people lower the rate of doing blood-derived treatments. The three treatments, especially the
medication and blood-derived treatments should be used together to remain the muscle contraction of the
Myasthenia Gravis patient. With all the information collected, it is crucial for people to raise attention to
myasthenia gravis and look forward to the innovation of new treatments.
1 INTRODUCTION
The most common form of Myasthenia Gravis (MG)
is a chronic autoimmune neuromuscular disorder
characterized by fluctuating weakness of the
voluntary muscle groups. In recent days, six
categories are Ocular MG, early-onset MG, late-
onset MG, MG with thymoma, MG with anti-muscle-
specific tyrosine kinase antibodies and MG with no
defined antibodies (Gilhus 2009, Gilhus, Nacu,
Andersen, & Owe 2015).
There is no known cure for MG, but there are
many effective treatments that can make managing
life with MG easier, the symptoms can be relieved
after resting or controlled by medications. There is a
need for attention for this disease because it can
happen to anyone of any race and gender, especially
for young women, whose ages are between 20 and 30,
and men, whose ages are 50 and older. In the US, it
is estimated that 20 in 100,000 have MG;
approximately 36,000 to 60,000 cases. However, as
myasthenia gravis often remains underdiagnosed, the
prevalence is most likely higher.
The general cause of MG is the dysfunction of
nerve-muscle junction transmission. MG is
considered an autoimmune disease, which is a
neuromuscular transmission disorder related by
acetylcholine receptor (AChR), the muscle Specific
Kinase (MuSK) and the low-density lipoprotein
receptor-related protein (LRP4). The researchers
found that the HLA locus, the locus for cytotoxic T-
lymphocyte-associated protein 4 (CTLA4), PTPN22,
IL-1β, IL-10, TNF-α and IFN-γ are related to
MGound (Berrih-Aknin, & Le Panse 2013).
There are currently a lot of treatments, and most
of the medications and therapies are based on the
cause of the disease. Three main treatments are
considered more effective. Firstly, thymectomy is the
only surgical treatment that completely removes the
thymus gland, causing the MG. Secondary, blood-
derived treatments include plasmapheresis, the
replacement of good antibodies with abnormal
antibodies in the blood. Last but not least, various
medications such as anticholinesterase
immunosuppressive medicines are trying to make the
antibodies not bind to the acetylcholine receptors or
make the acetylcholine stay in the junction longer, so
they have more chance to bind to the receptors. Also,
there is an emphasis on some traditional Chinese
medications that can help relieve the symptoms and
minimize the side effects of immunosuppressive
medications (table 2) (Giraud, Vandiedonck, &
92
Geng, Y., Song, R. and Liu, J.
The Cause and Therapy of Myasthenia Gravis.
DOI: 10.5220/0011231600003438
In Proceedings of the 1st International Conference on Health Big Data and Intelligent Healthcare (ICHIH 2022), pages 92-99
ISBN: 978-989-758-596-8
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

Garchon 2008). This article introduces what MG is
and main causes and the recent treatment of the MG
.
2 BACKGROUND
INFORMATION: WHAT IS MG,
PATIENT DISTRIBUTION
Myasthenia gravis (MG) is a disease caused by an
autoimmune disorder related by antibodies of the
neuromuscular junction, resulting in Visual
problems, skeletal muscle weakness and fatigability
of body function; however, the cause of myasthenia
gravis and the cure are unknown (Meriggioli, &
Sanders 2009). In clinical diagnosis, MG may be
classified by different factors, such as the different
parts of the muscles, the age of MG onset (Guptill,
Soni, & Meriggioli 2016), and the nature of thymic
pathology.
According to the data, table 1 shows that
geographical variations affect the different
incidences of MG (Dresser, Wlodarski, Rezania, &
Soliven 2021). Different epidemiological studies
estimate the incidence rate of MG with a range
between 1.7 and 21.3 per million people each year
(Bettini, Chaves, Cristiano, Pagotto, Perez, Giunta, &
Rugiero 2017). In Poland, the incidence rate is only
2.36 per million in 2019, which is lower than in other
countries (Sobieszczuk, Napiórkowski, Szczudlik, &
Kostera-Pruszczyk 2021). In Norway, the data shows
the average incidence rate of 1.6 per million each year
with a stable incidence rate over the last 25 years, and
the prevalence rate is 3.6–13.8 per million people
(Popperud, Boldingh, Brunborg, Faiz, Heldal,
Maniaol, Müller, Rasmussen, Oymar, & Kerty 2016).
In Sweden, the incidence of MG is 2.9 per million
people and the prevalence is 36.1 per million people
in 2016, but the incidence rate is increasing each year
(Westerberg, & Punga 2020). In addition, in North
American and Asian areas, the annual incidence rate
is not too high compared to other countries. In China,
MG's estimated annual incidence rate is between 1.55
to 3.66 per million people each year, and the
estimated prevalence of MG is 2.19–11.07 per
million people based on insurance records (Fang, Li,
Mo, Wang, Qiu, Ou, Lin, Huang, Feng, He, Wang,
Xu, Wang, Ran, & Liu 2020). In Canada, the
incidence rate is 2.1–2.6 per million, and the
prevalence rate ranges from 25.4 to 27.3 per million
in 2013 (Breiner, Widdifield, Katzberg, Barnett, Bril,
& Tu 2015). In South Africa, an annual incidence rate
is 8.5 per million (Mombaur, Lesosky, Liebenberg,
Vreede, & Heckmann 2015). However, the incidence
of MG is 38.8 cases per million in the Argentina area
(Dresser, Wlodarski, Rezania, & Soliven 2021). But
the data in table 1 may have some issues to cause the
different results in geographical regions.
Table 1: The recent increasing rate of MG in different regions.
Re
g
ion Countr
y
Rate
(p
er million each
y
ear
)
Europe
Polan
d
2.36
Norwa
y
1.6
Sweden 2.9
Asia China 1.55-3.66
North American Canada 2.1
–
2.6
South Africa Argentina 38.8
3 CAUSES OF DISEASE
3.1 Antibodies
Myasthenia gravis (MG) is an autoimmune disorder
caused by antibodies against acetylcholine receptors
(AChR) or other structural proteins of the
neuromuscular junction. This diminishes cholinergic
transmission, thus leading to exercise-induced
fatigue and sometimes manifest muscle weakness,
including the bulbar and ocular musculature
(Müllges, & Stoll 2019).
These autoantibodies bind to the nicotinic
acetylcholine receptor (AchR) itself, or muscle-
specific tyrosine kinase (MuSK), lipoprotein
receptor-related protein 4 (LRP4) and agrin involved
The Cause and Therapy of Myasthenia Gravis
93

in clustering of AchRs within the postsynaptic
membrane and structural maintenance of the
neuromuscular synapse. This results in the
disturbance of neuromuscular transmission and thus
the clinical manifestation of the disease (Melzer,
Ruck, Fuhr, Gold, Hohlfeld, Marx, Melms,
Tackenberg, Schalke, Schneider-Gold, Zimprich,
Meuth, & Wiendl 2016).
In the US there are about 18,000 people with MG.
Myasthenia gravis crisis (MGC) is defined as any
MG exacerbation necessitating mechanical
ventilation. Most patients presenting with MGC have
an identifiable risk factor. The diagnosis of MGC
should be suspected in all patients with respiratory
failure, particularly those with unclear etiology.
Acute management of MGC requires supportive
general and ventilatory therapy and institution of
measures to improve the neuromuscular blockade
(Bershad, Feen, & Suarez 2008).
3.2 The Thymus Gland
The thymus gland is a small gland located in the
upper chest beneath the sternum. It helps control the
immune system, and when it malfunctions, it may
cause MG. Many people with MG have a large or
overactive thymus gland. Some even develop tumors
on their thymus gland. These tumors are called
thymomas. These tumors can be harmless, but also
can turn into cancer or cause lasting health issues.
Research has shown that in most cases, MG
patients have increased numbers of cells in the
thymus, and about 10-15 percent of affected
individuals have thymomas (tumors) in the thymus.
Researchers suggest that the thymus of an MG patient
may trigger or maintain the production of the
antibodies that block the transmission of nerve
signals. Thus, the malfunction of the thymus gland
may cause MG, and the treatment was directed at the
thymus gland.
3.3 Human Leukocyte Antigen (HLA)
The HLA gene contains several genetic loci (HLA-A,
B, C and D) (COMPSTON, VINCENT, NEWSOM-
DAVIS, & BATCHELOR 1980). It has a relationship
with a large number of diseases, such as
hemochromatosis. According to the bulk clinical
experiments, the major histocompatibility complex
has been identified to be associated with autoimmune
MG with thymus hyperplasia, and the major
histocompatibility complex plays an important role in
MG as the first and the most important factor (Giraud,
Vandiedonck, & Garchon 2008). In addition, MG and
thymus hyperplasia are associated with HLA, and
HLA-DR3 and HLA-DR7 show opposite influences
on MG patients (Giraud, Beaurain, Yamamoto,
Eymard, Tranchant, Gajdos, & Garchon 2001).
In previous studies, the relationship between the
disease and different ethnic groups was identified
(Giraud, Vandiedonck, & Garchon 2008).
Myasthenia gravis (MG) in European Caucasoids has
found that they are associated with HLA-B8 and
HLA-DR3. The significant increase in HLA-A1,
HLA-B8 and HLA-DR5 in American blacks with
generalised adult-onset myasthenia gravis or ocular
myasthenia gravis (OMG) (Christiansen, Pollack,
Garlepp, & Dawkins 1984). MG in China shows
differences with the patients in Caucasians. Patients,
who are in the first 20 years of life, restricted ocular
myasthenia in them has a relationship with absence
or low titres of acetylcholine receptor antibody and
HLA-DR9; however, restricted ocular myasthenia in
the patients, whose age is older than 20 years old, has
high titres of acetylcholine receptor antibody
(Hawkins, Yu, Wong, Woo, Ip, & Dawkins 1989). In
another study, OMG in Chinese is associated with
HLA-BW46 (Hawkins, Ip, Lam, Ma, Wy, Yeung, &
Dawkins 1986). In conclusion, compared to
European Caucasoids, MG in Chinese is at an earlier
age at onset, more ocular forms, and less clinically
severe illness. HLA-DR9 and HLA-Bw46 (Chen,
Chiu, & Hseih 1993) have strongly impacted them.
In other Asian countries, Japanese in the childhood
with MG are associated with HLA-DR9 and HLA-
DRw13 (or DQw1 and DQw3), which act
synergistically in the disease; however, no significant
association was shown in Japanese with adult-onset
MG, and the risk of MG decreases with the age of
onset. Moreover, no patients had HLA-B8 and HLA-
DR3 which are related to European Caucasoids. So
MG in Japanese individuals differs from European
Caucasoids with MG (Matsuki, Juji, Tokunaga,
Takamizawa, Maeda, Soda, Nomura, & Segawa
1990). Thus, according to the previous studies,
patients in China are similar to those in Japan because
both of them are associated with HLA-DR9.
3.4 Other Genes
Cytotoxic T-lymphocyte-associated protein
4(CTLA4), Protein tyrosine phosphatase, non-
receptor type 22(PTPN22), meanwhile, Fc fragment
of IgG, low-affinity IIIb(FCGR3B) are not specific to
MG, which also encode proteins related to lymphoid
cell activation and other autoimmune diseases
(Giraud, Vandiedonck, & Garchon 2008). CTLA4
plays an important role in downregulating to control
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
94

the cellular and the humoral responses by controlling
responses of activated T cells (Huang, Liu, Norén,
Xia, Trifunovic, Pirskanen, & Lefvert 1998). It is
estimated to protect patients from MG in thymoma
patients against several autoimmune diseases;
however, it also increases the risk to lead to
paraneoplastic MG in thymoma patients (Chuang,
Ströbel, Gold, Nix, Schalke, Kiefer, Opitz, Klinker,
Müller-Hermelink, & Marx 2005). PTPN22 reduces
T-cell activation and generation of interleukin-2, and
it has been associated with various autoimmune
diseases. Hungarian and German MG patients are
highly overrepresented of the common autoimmune
polymorphism PTPN22 1858C/T (Greve, Hoffmann,
Illes, Rozsa, Berger, Weissert, & Melms 2009).
Moreover, PTPN22 is associated with early-onset
MG and thymoma-associated MG (Chuang, Ströbel,
Belharazem, Rieckmann, Toyka, Nix, Schalke, Gold,
Kiefer, Klinker, Opitz, Inoue, Kuo, Müller-
Hermelink, & Marx 2009). The Fcgamma receptors
include FcgammaRIIa, FcgammaRIIIa, and
FcgammaRIIIb, and they have different abilities for
IgG binding and phagocytosis. Thymoma MG
patients have a high expression on the
FcgammaRIIA-H/H genotype, so FcgammaRIIa-
R/R131 genotype is a biomarker for susceptibility to
MG (Raknes, Skeie, Gilhus, Aadland, & Vedeler
1998). In summary, different associations with MG
are highly diverse and in order to better define their
relationship, future studies need to focus on different
controls, such as the region, age and gender of
patients, thymus pathology, subtypes of MG.
4 TREATMENT
4.1 Thymectomy
Thymectomy is considered the first treatment that
people have found, as well as the only surgical
treatment that is found to be effective. However,
there are still a lot of controversies and risks about it.
Thymus anomalies can occur in the majority of MG
patients with MG and AChr antibodies. It is observed
that 60% -70% have abnormalities of the gland,
including hyperplasia and 10%–15% tend to develop
thymoma. These findings lead to the development of
thymectomy, but this procedure heavily depends on
the age of the patient, the severity of the disease, the
presence of AChr antibodies or MuSK antibodies,
and so on (Romi 2011). Until today, those researchers
have settled down the age limit of thymectomy
surgery for patients under 65 years old. It is because
the elderly cannot respond well due to “high thymic
involution incidence,” and the side effects may
overturn the benefits. Other than the age limit,
thymectomy is best processed with mild and
moderate MG. The remission of the mild MG patient
is much higher through studies from 1985 to 2014
and especially effective when it is performed 6 to 12
months after the first symptoms occurred (Mao, Hu,
Lu, & Hackett, 2015). In addition, MG patients with
the absence of AChR are recommended if they do not
respond well to IS therapy that thymectomy can
minimize the effects of IS therapy (Mao, Hu, Lu, &
Hackett 2015). After the surgical procedure, it was
found that patients in the surgical group had
approximately twice the rate of remission and
improvement as those in the control group. Within a
few months after surgery, about 60% to 80% of
patients were in remission (U.S. Department of
Health and Human Services.). However, there are
also risks for patients who have thymic tumors.
Patients with thymoma should have surgery to
remove the tumor, but it will not guarantee an
improvement in MG, and further treatments need to
be based on patients. If thymectomy is performed
aggressively, these patients did not respond well to
the thymectomy and were generally more seriously
ill (Mao, Hu, Lu, & Hackett 2015).
There is always a desire for the best procedure of
thymectomy. Some common procedures are
transsternal thymectomy, transcervical thymectomy,
and it turns out that minimally invasive techniques,
which use some tiny incision in the chest, have
become increasingly popular due to their low
procedural morbidity and mortality, short hospital
stay, optimal cosmesis, minor surgical access trauma,
better preservation of pulmonary function (Marulli,
& Rea 2015). Especially for young patients,
clinicians want to ensure that the young patients can
have a regular and comfortable life after the surgery,
especially for children with generalized AChR
antibody-positive MG, “possibility of a congenital
myasthenic syndrome or other neuromuscular
condition should be entertained” and these should be
exclaimed before the thymectomy (Sanders 2016).
4.2 Blood-derived Treatments
As time goes, blood-derived treatments,
plasmapheresis, becomes accessible as people get
more knowledge that the antibodies in the blood are
blocking the receptors in the neuro junction.
Plasmapheresis (PLEX) is simply replacing the
plasma in MG patients with healthy plasma. And
later, intravenous immunoglobulin (IVIg), the
injection of healthy antibodies to temporarily change
The Cause and Therapy of Myasthenia Gravis
95

the immune system's working (U.S. Department of
Health and Human Services.). Unlike thymectomy
that has a chance to remission the disease, PLEX and
IVIg can only keep the symptoms away within six
days to six weeks depending on the half-life of AChr
Ab, as a result, patients have to do the procedure
repeatedly. It is recommended to serve MG patients
with life-threatening problems. Other than that,
people often use them as an add-on to medication to
enhance the best effect of restoring muscle
contraction. However, there are also some
unavoidable side effects. As the clinical studies show,
10%~15% of patients produce toxic symptoms due to
plasma exchange therapy to enter more citron acid,
which causes low blood calcium. Some symptoms
include numbness around the mouth and lip, nausea,
vomiting, cardiac arrhythmia, which can be
alleviated by using supplemental calcium. The other
side effects are Hypovolemic or hypotension that can
occur during plasma separation because more blood
volume is required for cardiopulmonary bypass. In
mild cases, tachycardia, sweating, nausea, tinnitus,
and other symptoms may occur. In severe cases,
seizures of syncope or heart or cerebral infarction
may occur. It is required that the blood sampling
speed should not be too fast, and the colloidal
substances should be properly supplemented to
correct the adverse reactions (Sedef Iskit 2018). In
short, both PLEX and IVIg also do need a lot of
caution with the consultation of patients’ conditions.
4.3 Medications, the Rising of
Rituximab
With more interest as well as a deeper understanding
of the cause and process of MG, clinicians start to
figure out drugs that can make the acetylcholine stay
long during the junction so it has a better chance to
bind to the receptor or try to remove the bad
antibodies. Anticholinesterase medications, which
slow down the breakdown of acetylcholine, and
immunosuppressive drugs that suppress the
production of antibodies are some conventional
medications that can treat MG. For children MG
patients. It is shown that steroid medications can have
some severe side effects, such as the probability of
infection, growth failure. Considering all the factors,
it illustrates that immunosuppressive drugs,
corticosteroids, should be used as a long-term
treatment to minimize side effects. The more novel
medications have shown for better effectiveness,
including monoclonal antibodies, attack the process
when the bad antibodies bind to the acetylcholine
receptors (U.S. Department of Health and Human
Services.) and B cell depletion, such as, rituximab
(RTX), which was developed in the 2000s for cancer
and other autoimmune disorders and is a murine-
human chimeric anti-CD20 glycoprotein monoclonal
antibody (Menon, Barnett, & Bril 2020). The
clinicians gave a placebo test to the MG patients, it
turned out that the MMT score has been stable for
patients who took rituximab and the frequency of
doing the blood-derived treatments had been lowered
(Anderson, Phan, Johnston, & Siddiqi 2016) as
shown in Figure 1. Traditional Chinese medicine
treatment of myasthenia gravis is getting more and
more attention. MG is considered in the category of
"impotence". According to the theory of traditional
Chinese medicine, the addition of traditional Chinese
medicine in the treatment can reduce the side effects
caused by immunosuppressants, play an escort role in
the treatment of myasthenia gravis, and rebuild the
effect of the autoimmune function. Other than that,
Chinese therapy, acupuncture, also can be add-on
treatments to delay and reduce the symptoms of MG.
Table 2: Summary of Three Treatments.
Thera
py
Pur
p
ose Outcome
Thymectomy Removal of thymoma or hyperplastic 60 to 80% are in remission.
Intravenous immunoglobulin (IVIg) Inject the healthy antibodies to alter the
operations of the immune system.
Only last within six days to sie
weeks
Plasmapheresis (PLEX) Plasma exchange to get the health antibodies. Only last within six days to sie
weeks
Immunosuppressive Reduce the production of abnormal
antibodies
Corticosteroid,
cyclophosphamide
Monoclonal antibody Targets the process by which acetylcholine
antibodies injure the neuromuscular junction
Rituximab
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
96
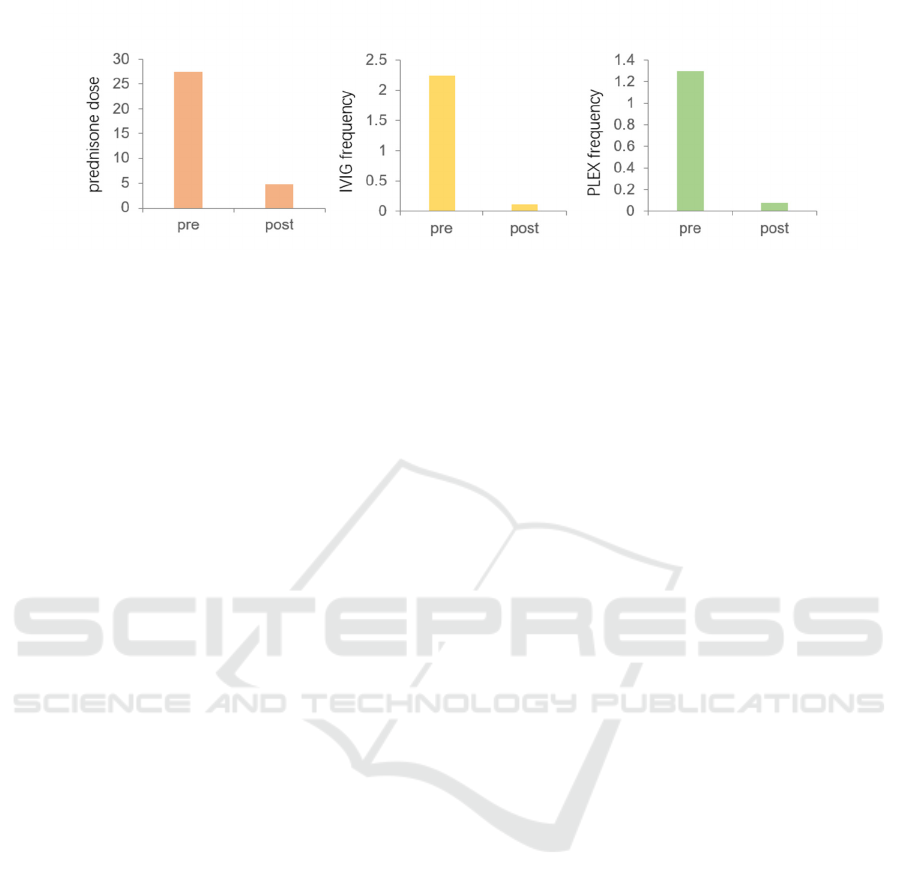
The left, intermediate and right graphs represent prednisone dose, IVIG frequency, PLEX frequency, respectively.
Figure 1: Rituximab Effectiveness (compare premedication with post medication) (Anderson, Phan, Johnston, & Siddiqi
2016).
5 CONCLUSIONS
To sum up, Antibodies to the acetylcholine receptor,
the muscle-specific tyrosine kinase, and the
lipoprotein receptor protein 4, characterize disease
subtypes with distinct clinical traits and immune-
pathogenic mechanisms. Also, experiments found
that MG and thymus hyperplasia are associated with
HLA, and HLA-DR3 and HLA-DR7 show the
opposite influence on MG patients. Besides, CTLA4,
PTPN22, and FCGR3B are not specific to MG, which
also encode proteins related to lymphoid cell
activation and other autoimmune diseases. In order to
solve this disease, there are many kinds of treatment,
like Thymectomy, Intravenous, immunoglobulin,
plasmapheresis, immunosuppressive and
Monoclonal antibodies, which both are effective
treatments that can make managing life with MG
easier. However, some issues need to be addressed to
find better treatment in future research. For example,
it is unclear how HLA or other genes control MG. In
the future, a huge amount of clinical research is
needed to find out the other causes or treatments of
MG. In this article, understanding the disease
mechanism, the cause and the treatment method of
MG will provide a brief summary to future
researchers and conduct further research.
REFERENCES
Anderson, D., Phan, C., Johnston, W. S., & Siddiqi, Z. A.
(2016). Rituximab in refractory myasthenia gravis: a
prospective, open‐label study with long‐term follow‐
up. Annals of Clinical and Translational Neurology,
3(7), 552–555.
Berrih-Aknin, S., & Le Panse, R. (2013). Myasthenia
gravis: A comprehensive review of immune
dysregulation and etiological mechanisms. Journal of
Autoimmunity, 52, 90–100.
Bershad, E. M., Feen, E. S., & Suarez, J. I. (2008).
Myasthenia gravis crisis. Southern medical journal,
101(1), 63–69.
Bettini, M., Chaves, M., Cristiano, E., Pagotto, V., Perez,
L., Giunta, D., & Rugiero, M. (2017). Incidence of
Autoimmune Myasthenia Gravis in a Health
Maintenance Organization in Buenos Aires, Argentina.
Neuroepidemiology, 48(3-4), 119–123.
Breiner, A., Widdifield, J., Katzberg, H. D., Barnett, C.,
Bril, V., & Tu, K. (2015). Epidemiology of myasthenia
gravis in Ontario, Canada. Neuromuscular Disorders:
NMD, 26(1), 41–46.
Chen, W. H., Chiu, H. C., & Hseih, R. P. (1993).
Association of HLA-Bw46DR9 combination with
juvenile myasthenia gravis in Chinese. Journal of
neurology, neurosurgery, and psychiatry, 56(4), 382–
385.
Christiansen, F. T., Pollack, M. S., Garlepp, M. J., &
Dawkins, R. L. (1984). Myasthenia gravis and HLA
antigens in American blacks and other races. Journal of
Neuroimmunology, 7(C), 121–129.
Chuang, W. Y., Ströbel, P., Belharazem, D., Rieckmann,
P., Toyka, K. V., Nix, W., Schalke, B., Gold, R.,
Kiefer, R., Klinker, E., Opitz, A., Inoue, M., Kuo, T.
T., Müller-Hermelink, H. K., & Marx, A. (2009). The
PTPN22 gain-of-function+1858T (+) genotypes
correlate with low IL-2 expression in thymomas and
predispose to myasthenia gravis. Genes and immunity,
10(8), 667–672.
Chuang, W. Y., Ströbel, P., Gold, R., Nix, W., Schalke, B.,
Kiefer, R., Opitz, A., Klinker, E., Müller-Hermelink,
H. K., & Marx, A. (2005). A CTLA4 high genotype is
associated with myasthenia gravis in thymoma
patients. Annals of neurology, 58(4), 644–648.
COMPSTON, D. A. S., VINCENT, A., NEWSOM-
DAVIS, J., & BATCHELOR, J. R. (1980).
CLINICAL, PATHOLOGICAL, HLA ANTIGEN
AND IMMUNOLOGICAL EVIDENCE FOR
DISEASE HETEROGENEITY IN MYASTHENIA
GRAVIS. Brain (London, England: 1878), 103(3),
The Cause and Therapy of Myasthenia Gravis
97

579–601.
Dresser, L., Wlodarski, R., Rezania, K., & Soliven, B.
(2021). Myasthenia Gravis: Epidemiology,
Pathophysiology and Clinical Manifestations. Journal
of clinical medicine, 10(11), 2235.
Fang, W., Li, Y., Mo, R., Wang, J., Qiu, L., Ou, C., Lin, Z.,
Huang, Z., Feng, H., He, X., Wang, W., Xu, P., Wang,
L., Ran, H., & Liu, W. (2020). Hospital and healthcare
insurance system record–based epidemiological study
of myasthenia gravis in southern and northern China.
Neurological Sciences, 41(5), 1211–1223.
Gilhus, N. E. (2009). Autoimmune myasthenia gravis.
Expert Review of Neurotherapeutics, 9(3), 351–358.
Gilhus, N. E., Nacu, A., Andersen, J. B., & Owe, J. F.
(2015). Myasthenia gravis and risks for comorbidity.
European Journal of Neurology, 22(1), 17–23.
Giraud, M., Beaurain, G., Yamamoto, A. M., Eymard, B.,
Tranchant, C., Gajdos, P., & Garchon, H. J. (2001).
Linkage of HLA to myasthenia gravis and genetic
heterogeneity depending on anti-titin antibodies.
Neurology, 57(9), 1555–1560.
Giraud, M., Vandiedonck, C., & Garchon, H.-J. (2008).
Genetic Factors in Autoimmune Myasthenia Gravis.
Annals of the New York Academy of Sciences,
1132(1), 180–192.
Giraud, M., Vandiedonck, C., & Garchon, H.-J. (2008).
Genetic Factors in Autoimmune Myasthenia Gravis.
Annals of the New York Academy of Sciences,
1132(1), 180–192.
Greve, B., Hoffmann, P., Illes, Z., Rozsa, C., Berger, K.,
Weissert, R., & Melms, A. (2009). The autoimmunity-
related polymorphism PTPN22 1858C/T is associated
with anti-titin antibody-positive myasthenia gravis.
Human immunology, 70(7), 540–542.
Guptill, J. T., Soni, M., & Meriggioli, M. N. (2016).
Current Treatment, Emerging Translational Therapies,
and New Therapeutic Targets for Autoimmune
Myasthenia Gravis. Neurotherapeutics, 13(1), 118–
131.
Hawkins, B. R., Ip, M. S., Lam, K. S., Ma, J. T., Wy, C. L.,
Yeung, R. T., & Dawkins, R. L. (1986). HLA antigens
and acetylcholine receptor antibody in the
subclassification of myasthenia gravis in Hong Kong
Chinese. Journal of neurology, neurosurgery, and
psychiatry, 49(3), 316–319.
Hawkins, B. R., Yu, Y. L., Wong, V., Woo, E., Ip, M. S.,
& Dawkins, R. L. (1989). Possible evidence for a
variant of myasthenia gravis based on HLA and
acetylcholine receptor antibody in Chinese patients.
The Quarterly journal of medicine, 70(263), 235–241.
Huang, D., Liu, L., Norén, K., Xia, S. Q., Trifunovic, J.,
Pirskanen, R., & Lefvert, A. K. (1998). Genetic
association of Ctla-4 to myasthenia gravis with
thymoma. Journal of neuroimmunology, 88(1-2), 192–
198.
Mao, Z., Hu, X., Lu, Z., & Hackett, M. L. (2015).
Prognostic factors of remission in myasthenia gravis
after thymectomy. European Journal of Cardio-
Thoracic Surgery, 48(1), 18–24.
Marulli, G., & Rea, F. (2015). Myasthenia gravis and
thymectomy: many doubts and few certainties.
European Journal of Cardio-Thoracic Surgery, 48(1),
46–47.
Matsuki, K., Juji, T., Tokunaga, K., Takamizawa, M.,
Maeda, H., Soda, M., Nomura, Y., & Segawa, M.
(1990). HLA antigens in Japanese patients with
myasthenia gravis. The Journal of clinical
investigation, 86(2), 392–399.
Melzer, N., Ruck, T., Fuhr, P., Gold, R., Hohlfeld, R.,
Marx, A., Melms, A., Tackenberg, B., Schalke, B.,
Schneider-Gold, C., Zimprich, F., Meuth, S. G., &
Wiendl, H. (2016). Clinical features, pathogenesis, and
treatment of myasthenia gravis: a supplement to the
Guidelines of the German Neurological Society.
Journal of neurology, 263(8), 1473–1494.
Menon, D., Barnett, C., & Bril, V. (2020). Novel
Treatments in Myasthenia Gravis. Frontiers in
Neurology, 11, 538–538.
Meriggioli, M. N., & Sanders, D. B. (2009). Autoimmune
myasthenia gravis: emerging clinical and biological
heterogeneity. Lancet Neurology, 8(5), 475–490.
Mombaur, B., Lesosky, M. R., Liebenberg, L., Vreede, H.,
& Heckmann, J. M. (2015). Incidence of acetylcholine
receptor-antibody-positive myasthenia gravis in South
Africa. Muscle & Nerve, 51(4), 533–537.
Müllges, W., & Stoll, G. (2019). Myasthenia gravis
[Myasthenia gravis]. Der Nervenarzt, 90(10), 1055–
1066.
Popperud, T., Boldingh, M., Brunborg, C., Faiz, K., Heldal,
A., Maniaol, A., Müller, K., Rasmussen, M., Oymar,
K., & Kerty, E. (2016). Juvenile myasthenia gravis in
Norway: A nationwide epidemiological study.
European Journal of Paediatric Neurology, 21(2), 312–
317.
Raknes, G., Skeie, G. O., Gilhus, N. E., Aadland, S., &
Vedeler, C. (1998). FcgammaRIIA and FcgammaRIIIB
polymorphisms in myasthenia gravis. Journal of
neuroimmunology, 81(1-2), 173–176.
Romi, F. (2011). Thymoma in Myasthenia Gravis: From
Diagnosis to Treatment. Autoimmune Diseases,
2011(1), 474512–474515.
Sanders, D. B., Wolfe, G. I., Benatar, M., Evoli, A., Gilhus,
N. E., Illa, I., Kuntz, N., Massey, J. M., Melms, A.,
Murai, H., Nicolle, M., Palace, J., Richman, D. P.,
Verschuuren, J., & Narayanaswami, P. (2016).
International consensus guidance for management of
myasthenia gravis: Executive summary. Neurology,
87(4), 419–425.
Sedef Iskit, P. D. (2018, April 18). Plasmapheresis.
Myasthenia Gravis News.
https://myastheniagravisnews.com/role-
plasmapheresis-myasthenia-gravis/.
Sobieszczuk, E., Napiórkowski, L., Szczudlik, P., &
Kostera-Pruszczyk, A. (2021). Myasthenia Gravis in
Poland: National Healthcare Database Epidemiological
Study. Neuroepidemiology, 55(1), 62–69.
U.S. Department of Health and Human Services. (n.d.).
Myasthenia gravis fact sheet. National Institute of
Neurological Disorders and Stroke.
https://www.ninds.nih.gov/Disorders/Patient-
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
98

Caregiver-Education/Fact-Sheets/Myasthenia-Gavis-
Fact-Sheet#4.
Westerberg, E., & Punga, A. R. (2020). Epidemiology of
Myasthenia Gravis in Sweden 2006–2016. Brain and
Behavior, 10(11), e01819–n/a.
The Cause and Therapy of Myasthenia Gravis
99
