
Gold Nanoparticles for Cancer Photothermal Therapy
Rui Cao
a
School of Life Science and Technology, Huazhong University of Science and Technology, Wuhan, China
Keywords: Gold Nanoparticles, Photothermal Therapy, Cancer Therapy, Nanostructure.
Abstract: In recent years, there has been a great deal of interest in the new therapy of cancer treatment. Photothermal
therapy is one of the new ways to inhibit tumor formation. Many studies have confirmed gold nanoparticles
can absorb light at specific wavelengths, especially near-infrared light, through their unique optical
properties called localized surface plasmon resonance (LSPR) to achieve photothermal treatment of tumor
cells. Moreover, a large number of experiments have shown that gold nanoparticles with different structures
have different absorption spectra and LSPR peak position, which make them have different photothermal
efficiencies. In addition, gold nanoparticles modified by different functionalized compounds have better
biocompatibility and targeting ability, which greatly broadens the scope of its application in tumor
photothermal therapy and improves the therapeutic effect. In this paper, we conclude recent progress in gold
nanoparticles for cancer photothermal therapy. First, we introduce the optical properties of gold
nanoparticles and the principle of photothermal conversion. Then, the influence of different nanostructures
and functional modifications on the effect of photothermal treatment is discussed. Finally, we briefly
describe several common types of multifunctional gold nanoparticles, and introduce their basic principles
and functions. Due to the large amount of experimental data in relevant aspects, this paper mainly discusses
the research progress of gold nanoparticles in the field of photothermal therapy after 2015.
1 INTRODUCTION
1
Many studies have confirmed that gold nanoparticles
have unique optical properties. One of the most vital
optical properties of gold nanoparticles is the
collective coherent oscillation of their free
conduction band electrons, or the localized surface
plasmon resonance (LSPR) (Austin 2014). LSPR is
the coherent oscillation of the nanostructure’s
conduction band free electrons in resonance with the
incident electromagnetic field. Since the incident
light causes a high degree of polarization of the free
conduction band electrons, when gold nanoparticles
are placed in an external field, a displacement of
negative and positive charges will occur, that is, a
net charge difference is generated at the boundaries
of the nanoparticles (Ghosh 2007). Gold
nanoparticles can absorb/scatter incident light, and
photon confinement causes strong electromagnetic
fields and various optical phenomena on the metal
surface (Abadeer, 2016). This interaction strongly
depends on the composition, size, geometry,
a
https://orcid.org/0000-0001-9373-000X
dielectric environment and particle-particle
separation distance of nanoparticles (Petryayeva,
2011). So, the LSPR peak of the gold nanoparticles
can be changed by preparing nanoparticles with
different structures.
It is precisely because of the characteristics of
LSPR that gold nanoparticles can absorb light at a
specific resonance frequency, and the energy
absorbed can also be attenuated in the form of
radiation (such as optical scattering) and non-
radiation. Photothermal therapy uses the non-
radiative attenuation of energy, and uses the LSPR
effect to cause coherent oscillations of electrons in
the crystal lattice. In a very short time (femtosecond
scale), the electron pulses collide with the gold
crystal lattice, causing the electrons to generate
extremely high temperatures. Then, heat is
transferred to the outer surface of the gold
nanoparticle through the interaction between the
electron and the electron, the electron and the
phonon, and the phonon and the phonon, so that the
gold nanoparticle can heat the surrounding medium
(Austin, 2014).
Since tumors are more sensitive to temperature, a
short period of high temperature can effectively kill
Cao, R.
Gold Nanoparticles for Cancer Photothermal Therapy.
DOI: 10.5220/0011217400003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 471-477
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
471
cancer cells around the gold nanoparticles and
induce their apoptosis. Also, by changing the
structure of gold nanoparticles, the LSPR can be
excited at near-infrared light, and near-infrared light
has a stronger penetration of physiological
structures, which can better activate the
nanoparticles in the body.
Gold nanoparticles can also be functionalized
with surface modification to improve their targeting
ability and specificity, so that they can be better
enriched at the tumor site, thereby improving the
effect of photothermal treatment.
Therefore, this review focuses on the impact of
the structure and functionalization of gold
nanoparticles on the effect of photothermal therapy.
By summarizing the photothermal conversion
efficiency of gold nanoparticles of various
structures, we try to find the most advantageous
structure, hoping to provide structural suggestions
for the future research of gold nanoparticle
photothermal therapy.
2 CHARACTERIZATION OF
GOLD NANOPARTICLE
A large number of studies have shown that in
addition to increasing the heat output by changing
the incident light power, the shape, size and surface
characteristics of the gold nanoparticles can also be
optimized to adjust the LSPR peak and photothermal
conversion efficiency (Singh, 2018). Considering the
penetration depth and safety of light in physiological
tissues, the LSPR peak of gold nanoparticles used in
most photothermal treatments is in the first (650-850
nm) or second (950-1350 nm) near-infrared window
(Riley, 2017). Therefore, the structure of the
nanoparticles needs to be adjusted to maximize the
absorption of laser light in this window.
Studies have shown that small size (<8nm) gold
nanoparticle is little cytotoxicity, which can be
filtered in the kidneys, whereas larger nanoparticles
size (> 10nm) likely to remain in the body cannot be
discharged, which aggregates in the liver and
kidneys, causing damage to cells (Vines, 2019).
However, small-sized gold nanoparticles are easily
excreted and are not easy to aggregate in tumor cells,
and oversized nanoparticles are not easy to pass
through the blood vessel wall into tumor cells. Both
of these will affect the killing effect of photothermal
therapy on tumor cells. Therefore, it is necessary to
control the size of gold nanoparticles to find the best
effect.
At present, common gold nanoparticle shapes
include gold nanospheres, gold nanorods, gold
nanoshells, gold nanostars, and gold nanocages.
These shapes have different light absorption cross-
section and LSPR peaks, so as to have a different
wavelength of light absorption and photothermal
conversion efficiency. In addition, the different
shapes mean that the surface properties of these gold
nanoparticles are different, which affects their ability
to adsorb to tumor cells or the difficulty of being
swallowed by them. These can be changed by
improving the structure of nanoparticles, thereby
ultimately increasing their enrichment at the tumor
site, reducing damage to the surrounding normal
tissues, and effectively improving the efficiency of
photothermal conversion (Guo, 2017).
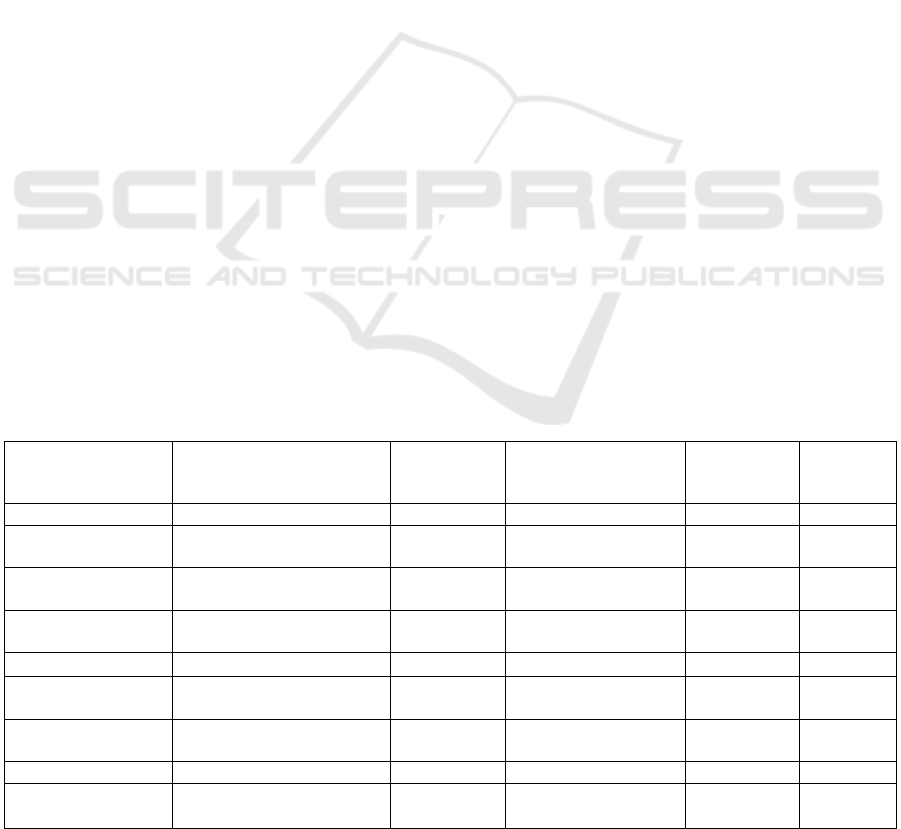
Table 1: Photothermal properties of some gold nanoparticles with different shapes.
Gold nanoparticle
shape
Size(nm)
LSPR peak
position(nm)
Laser
Photothermal
conversion
efficiency
References
nanorods
Au-TEMPO NRs 39.2(aspect ratio 3.85) 785 808nm,1.13 W/cm2 - (Xia 2018)
AuNR-MEND 68±18.3 788 750~900nm,1.0W/cm2 -
(Paraiso
2017)
Bi2S3-Au NRs 271±19.5 - 808nm,0.75W/cm2 51.06%
(Cheng
2018)
AuNR-Glu 134.4(±6.2)×23.9(±1.8) 1070 1064nm,1.0W/cm2 43.12% (Li 2018)
GNRS-HA-FA-DOX 70.9±1.4 779 808nm,2.0W/cm2 - (Xu 2017)
GNR-HA-
ALA/Cy7.5-HER2
55.1(±1.7)×14.1(±1.1) 800 808nm,2.0W/cm2 - (Xu 2019)
nanoshells
Tf-GNRS 205.8(±13.1)×112.0(±4.8) 808 808nm,8.0W/cm2 17.70%
(Zhao
2017)
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
472
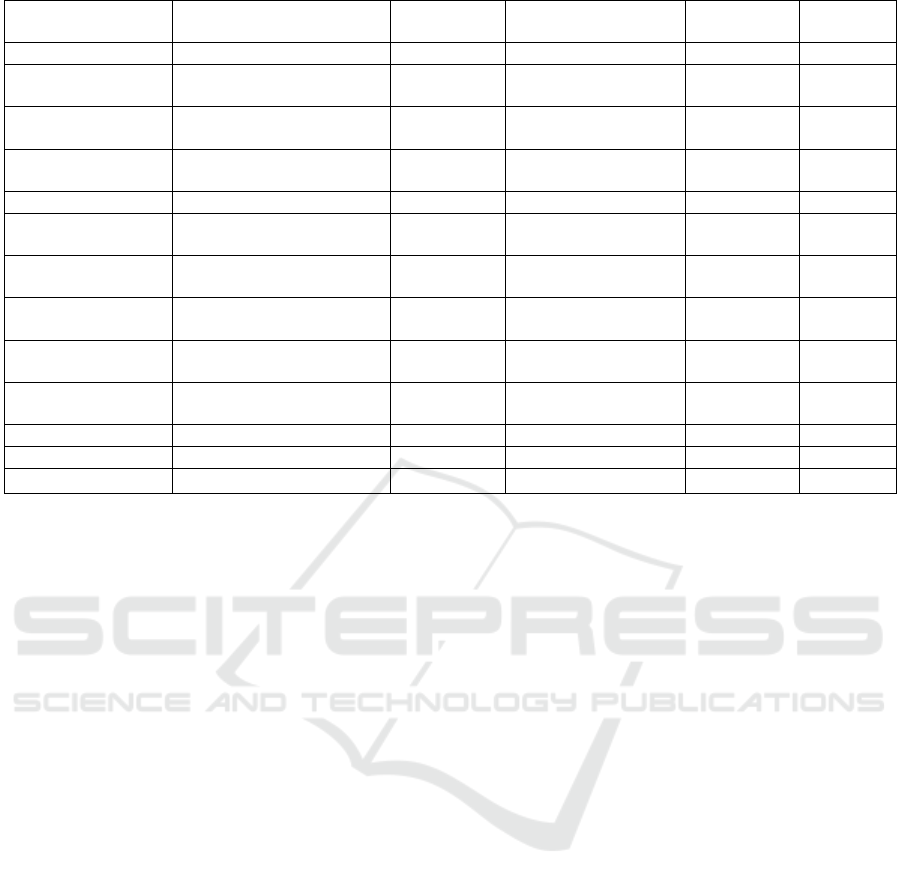
ICG−Au@BSA−Gd 151.1 ~850 808nm,1.5W/cm2 21.77% (You 2017)
nanocages
CM-EM-
GNCS@DOX
~60 790 808nm,0.5W/cm2 - (Sun 2020)
GSNCs 35±3 532 808nm,1.0W/cm2 - (Qin 2019)
EpCam–RP AuNs 69.7 750 808nm,2.5W/cm2 - (Zhu 2018)
nanospheres
GSH-AuNPs 3 515 800nm,2.5W/cm2 90%
(Barram
2021)
AuNP@Mo4Zol2Mn 47.6±7.8 528 680nm,1.7W/cm2 59±5%
(Tomane
2021)
dAuNPs 20.5±1.9 700~900 808nm,1.0W/cm2 78.80%
(Cheng
2017)
BSA-AuNPs ~4 540 800nm,0.5W/cm2 -
(Jawad
2018)
LACP 101.2±5.6 - 514nm,24mW/cm2 -
(Wang
2018)
nanostars
AuNSs@PDA-PEG 114 806 808nm,0~2.0W/cm2 - (Li 2019)
rGADA 51.3 - 808nm,0.1W/cm2 66.30% (Jia 2020)
There have been many studies on the influence
of the structure and size of gold nanoparticles on the
efficiency of photothermal conversion. The structure
and photothermal conversion efficiency of them are
shown in the table, and there is a big difference
between them. (Table 1) It is noted that the LSPR
peak positions of the gold nanoparticles with the
same shape are almost the same. Most of them will
have a certain blue shift or red shift due to the
difference in functional modification and size.
However, a few of them have different structures
due to special synthesis or processing methods,
which makes them have a big difference in optical
properties from other nanoparticles of the same type
of structure. The positions of the LSPR peaks of the
gold nanoparticles prepared into different shapes are
quite different, since the different surface
characteristics caused by their shapes leads to the
differences of the electromagnetic wave wavelengths
which cause surface ion resonance. However, the
photothermal conversion efficiency is not
necessarily related to the difference in these
structures or surface modifications. Since these
efficiencies are not measured in a physiological
environment, the photothermal conversion efficiency
of gold nanoparticles in vivo may be affected by
many factors and changes. It is noted that many
reports indicate that gold nanoparticles may
aggregate in a physiological environment, and
changing the intensity of laser irradiation and the
concentration of the gold nanoparticle solution will
also affect the photothermal conversion, which
makes it impossible to compare the efficiencies
measured in different experiments. In addition, some
materials used for functionalization or surface
modification of gold nanoparticles will also affect
their light/heat conductivity, thereby affecting the
efficiency of photothermal conversion. For example,
gold nanorods functionalized based on hyaluronic
acid are modified differently to have different
photothermal treatment effects. Xu. W et al (Xu,
2017). In vitro experiments, the total apoptotic rate
of MCF-7 cells treated with hyaluronic acid
functionalized gold nanorods modified by folic acid
(GNRS-HA-FA-DOX,66.00%) was much higher
than that of those that were not modified by folic
acid (GNRS-HA-DOX,37.17%). Therefore, it is
impossible to conclude which shape or
functionalization of gold nanoparticles has the best
photothermal conversion efficiency. In view of this,
it is considered that it is useful to calculate the
photothermal conversion efficiency when improving
the photothermal treatment effect of a certain gold
nanoparticle, which can reflect its heating efficiency
in the body and its killing effect on cancer cells to a
certain extent.
Although the properties of gold nanoparticles
with the same structure will be very different, they
also have many commonalities. For example, due to
its unique shape, gold nanorods have been a popular
structural research direction since they were first
synthesized. As a slender anisotropic shape, it has
two LSPR peak positions, including transverse
plasmon resonance (TSPR) on the short axis (mostly
Gold Nanoparticles for Cancer Photothermal Therapy
473

in the visible region) and longitudinal plasmon
resonance (LSPR) on the long axis (mostly in the
near-infrared region) (Elahi 2018). In addition, the
longitudinal absorption peak is affected by the
aspect ratio, which shows that the peak position
redshifts as the aspect ratio increases (Brolossy
2008). Thus, because of its unique optical and
physicochemical properties, the gold nanorods are
widely used in photothermal therapy. As one of the
first studied structures, gold nanospheres have
gained popularity due to their small size and ease of
synthesis. The position of the LSPR peak of gold
nanospheres is mainly affected by its size. The size
increases from 1 to 100 nm, and the relative
absorption peak of the size is 500 to 550 nm (Elahi
2018). Due to its small size, it can be coupled or
modified with many ligands, effectively improving
its photothermal performance.
3 MULTIFUNCTIONAL GOLD
NANOPARTICLES
In many studies, gold nanoparticles for photothermal
therapy are not single functional. Many of them will
be combined with other popular anti-cancer
detection methods or treatment methods, including
photodynamic therapy, tumor imaging, biosensing
and drug delivery.
The common multifunctional combination of
gold nanoparticles is photodynamic therapy and
photothermal therapy, both of which use light
absorption to kill tumor cells. Effective fluorescence
quenching and local surface plasmon resonance
(LSPR) absorption, easy to combine with
mercaptans, disulfides and amines, gold binding
facilitates intracellular penetration is also the reason
why gold nanoparticles can be used for
photodynamic therapy. The difference is that
photodynamic therapy is a biochemical action
induced by a photochemical reaction, which uses
light, photosensitizers, and oxygen from tissues.
During the process, the photosensitizer needs to be
injected into the tumor site. Because the half-life of
the photosensitizer administered systemically or
locally is different in each tissue, the concentration
of the photosensitizer in the tumor tissue is
significantly higher than that in the normal tissue
after a period of time. Selective retention, under the
action of excitation light of a specific wavelength,
and in the presence of molecular oxygen, singlet
oxygen and other reactive oxygen species (ROS) are
produced, leading to tumor cell necrosis and
apoptosis. Secondly, PDT can also destroy the
capillaries in tumor tissues, causing ischemia and
hypoxia, leading to cell death. Finally, PDT can
induce a variety of immune cells to rapidly infiltrate
the tumor, activate the complement system, and
promote the production and release of a variety of
cytokines/chemical factors, and finally initiate the
body's immune response to kill the tumor (Singh,
2018, Elahi, 2018). Unlike PTT, which does not rely
on oxygen, PDT is completely dependent on the
availability of tissue oxygen. Therefore,
photodynamic therapy does not affect the
photothermal treatment effect of gold nanoparticles
itself. For example, based on the new photosensitizer
BPS, combined with the plasma photothermal agent
Au nanoparticles and the targeting agent Fe3O4
nanoparticles, the multifunctional
BPS@Au@Fe3O4 was successfully prepared
through a simple, gentle and reproducible method.
The final prepared composite material has a wide
light absorption band and photodegradable
properties. Yang, D et al (Yang, 2017).
Experimental results show that BPS@Au@Fe3O4
nanoparticles have a high degree of biocompatibility,
and low-power near-infrared laser-mediated
synergistic photothermal and photodynamic therapy
shows excellent tumor suppression effects. At the
same time, the combination of photodynamic effect,
photothermal effect and magnetic resonance imaging
is helpful for comprehensive and integrated
treatment of tumor sites and improve the effect of
cancer treatment.
The most common combination is to use gold
nanoparticles as a carrier, not only for photothermal
therapy, but also as a carrier for other drugs or
ingredients, to play a role in drug delivery. The
coupling of gold nanoparticles and drug molecules
plays an important role in the treatment of
intracellular diseases. Their unique physiological
properties can promote the delivery of drugs into
cells, thereby improving the efficacy of drugs.
Antibiotics or other drug molecules can be directly
coupled to gold nanoparticles through ionic or
covalent bonds or physical absorption. At the same
time, because AuNPs have unique optical, physical
and chemical properties, biocompatibility, functional
flexibility, adjustable monomolecular membrane,
controllable dispersibility, high drug loading density
surface area, stability and non-toxicity, etc. Make it
an effective nanocarrier in the drug delivery system
(DDSS). These effective nanocarriers can transfer
various drugs, such as peptides, proteins, plasmid
DNA (PDNAs), small interfering RNAs (SiRNAs)
and chemotherapeutic drugs. Gold nanoparticle
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
474

carriers can be used to control drug delivery and
release methods, such as the use of external stimuli
(such as light) or internal stimuli. Therefore, it can
effectively combine chemotherapy and photothermal
therapy, while using physical and biochemical
methods to kill tumors (Elahi, 2018, Kong, 2017).
For example, the gold nanocage wrapped in red
blood cell membrane is used as a carrier to load the
anti-tumor drug paclitaxel (PTX) for targeted
photothermal and chemotherapy for cancer. Zhu, D
et al (Zhu, 2018). The results show that EpCam-
RPAuNs nanoparticles have better targeting ability
to 4T1 cells than unmodified nanoparticles. The high
temperature generated by AUNS under 808 nm near-
infrared radiation has a dual effect. First, the
increase in temperature promotes the release of PTX
by destroying red blood cell vesicles. Second, as a
photothermal treatment method, it directly uses
hyperthermia to kill cancer cells. In addition,
overheated AuNs will cause a large number of
cancer cell deaths, which will greatly reduce the
viability of 4T1 cells. The combination of the two
results in better tumor treatment efficiency. For
another example, a cancer cell-erythrocyte hybrid
coated doxorubicin (DOX) gold-loaded nanocage
(CM-EM-GNCs@DOX) is used for the combined
treatment of breast cancer with
photothermal/radiotherapy/chemotherapy. Sun, M et
al (Sun 2020). CM-EM-GNCs@DOX has good
photothermal conversion effect and near-infrared
response drug release behavior. Compared with
naked GNCs, CM-EM-GNCs@DOX has high
homology targeting to MCF-7 cells, and because it
retains the characteristics of cancer cell membranes
and red blood cell membranes, it has a good immune
escape ability. Under near-infrared irradiation, CM-
EM-GNCs@DOX exhibits a high photothermal
effect, which not only breaks CM-EM-GNCs@DOX,
releases DOX for precise and controllable
chemotherapy, and is enhanced by photothermal
therapy Received chemotherapy/radiotherapy.
In addition to the above two common
multifunctional gold nanoparticles combined with
photothermal therapy, some gold nanoparticles are
designed with both sensing and imaging in mind,
integrating tumor inspection and treatment, thereby
improving cancer treatment efficiency. Due to the
LSPR effect, gold nanoparticles show strongly
enhanced radiation properties (ie, light absorption,
scattering, and fluorescence), making them a
potential multi-modal imaging agent. The currently
commonly used iodinated aromatic compound
contrast agents have high water solubility and low
toxicity, but the blood circulation time is very short,
and they will be excreted through the kidneys soon.
Compared with ordinary reagents, gold
nanoparticles can stay in the blood vessel for a
longer period of time while taking into account the
non-toxic and harmless safety. Therefore, it can be
used as a contrast agent for tumor imaging at the
same time as photothermal therapy (Elahi, 2018,
Dreaden, 2012). The rich chemical and physical
properties of gold nanoparticles make them also
useful for biosensing. Sensors for different purposes
take advantage of the different characteristics of
AuNPs. For example, fluorescence-based sensors
utilize the fluorescence quenching properties of
AuNPs, surface plasmon resonance sensors based on
the optical properties of AuNPs, and biological
barcode analysis based on strong binding affinity to
thiols and visible color changes due to aggregation
of gold nanoparticles (Elahi, 2018). These sensors
that use the properties of gold nanoparticles can be
combined with photothermal therapy to perform
treatment while detecting.
4 CONCLUSION AND
PERSPECTIVES
In conclusion, we summarized the basic principles
of the photothermal properties of gold nanoparticles,
and analyzed the latest research on nanoparticles of
various structures to find out how the structure and
functionalization affect the photothermal conversion
efficiency, such as changing the effective light
absorption area, aspect ratio or size. Besides, we
have confirmed the commonalities of nanoparticles
with the same structure, which allows us to use the
advantages of gold nanoparticles with different
structures to solve different photothermal treatment
needs.
In recent years, the technology of synthesis and
functional modification of gold nanoparticles has
developed rapidly, leading to the production of
many gold nanoparticles with unique properties and
different structures. And because of the antibacterial,
anti-oxidant and easy modification properties of
gold itself, taking advantage of these characteristics
and functionalizing nanoparticles can make it more
widely used in the field of photothermal therapy. In
future research work, more attention should be paid
to the targeting properties of gold nanoparticles so
that they can be more actively targeted to specific
sites. Moreover, it is necessary to continue to
improve the structure or surface modification to
reduce its cytotoxicity and avoid its excessive
Gold Nanoparticles for Cancer Photothermal Therapy
475

discharge from the body or long-term retention in
the body. Last but not least, it is also vital to study
gold nanoparticles that combine photothermal
therapy with other tumor treatment methods, such as
drug delivery or optical imaging, so as to achieve a
comprehensive cancer treatment that integrates
diagnosis and treatment.
REFERENCES
Abadeer, N. S., & Murphy, C. J. (2016). Recent progress
in cancer thermal therapy using gold nanoparticles.
Journal of Physical Chemistry. C, 120(9), 4691–4716.
https://doi.org/10.1021/acs.jpcc.5b11232
Austin, L. A., Mackey, M. A., Dreaden, E. C. et al. (2014).
The optical, photothermal, and facile surface chemical
properties of gold and silver nanoparticles in
biodiagnostics, therapy, and drug delivery. Archives of
Toxicology, 88(7), 1391–1417.
https://doi.org/10.1007/s00204-014-1245-3
Barram, L. F. A. (2021). Laser enhancement of cancer cell
destruction by photothermal therapy conjugated
glutathione (GSH)-coated small–sized gold
nanoparticles. Lasers in Medical Science, 36(2), 325–
337. https://doi.org/10.1007/s10103-020-03033-y
Brolossy, T. A., Abdallah, T., Mohamed, M. et al. (2008).
Shape and size dependence of the surface plasmon
resonance of gold nanoparticles studied by
photoacoustic technique. The European Physical
Journal. ST, Special Topics, 153(1), 361–364.
https://doi.org/10.1140/epjst/e2008-00462-0
Cheng, X., Sun, R., Yin, L. et al. (2017). Light-Triggered
assembly of gold nanoparticles for photothermal
therapy and photoacoustic imaging of tumors in vivo.
Advanced Materials (Weinheim), 29(6), 1604894-n/a.
https://doi.org/10.1002/adma.201604894
Cheng, Y., Chang, Y., Feng, Y. et al. (2018). Deep-Level
defect enhanced photothermal performance of bismuth
Sulfide–Gold heterojunction nanorods for
photothermal therapy of cancer guided by computed
tomography imaging. Angewandte Chemie
(International Ed.), 57(1), 246–251.
https://doi.org/10.1002/anie.201710399
Dreaden, E. C., Alkilany, A. M., Huang, X. et al. (2012).
The golden age: Gold nanoparticles for biomedicine.
Chemical Society Reviews, 41(7), 2740–2779.
https://doi.org/10.1039/c1cs15237h
Elahi, N., Kamali, M., & Baghersad, M. H. (2018). Recent
biomedical applications of gold nanoparticles: A
review. Talanta (Oxford), 184, 537–556.
https://doi.org/10.1016/j.talanta.2018.02.088
Ghosh, S. K., & Pal, T. (2007). Interparticle coupling
effect on the surface plasmon resonance of gold
nanoparticles: from theory to applications. Chemical
Reviews, 107(11), 4797–4862.
https://doi.org/10.1021/cr0680282
Guo, J., Rahme, K., He, Y. et al. (2017). Gold
nanoparticles enlighten the future of cancer
theranostics. International Journal of Nanomedicine,
12, 6131–6152. https://doi.org/10.2147/IJN.S140772
Jawad, S. M. H., Taha, A. et al. (2018). Synthesis and
characterization of small-sized gold nanoparticles
coated by bovine serum albumin (BSA) for cancer
photothermal therapy. Photodiagnosis and
Photodynamic Therapy, 21, 201–210.
https://doi.org/10.1016/j.pdpdt.2017.12.004
Jia, X., Xu, W. et al. (2020). Functionalized
Graphene@Gold Nanostar/Lipid for pancreatic cancer
gene and photothermal synergistic therapy under
Photoacoustic/Photothermal imaging Dual–Modal
guidance. Small (Weinheim an Der Bergstrasse,
Germany), 16(39), e2003707-n/a.
https://doi.org/10.1002/smll.202003707
Kong, F., Zhang, J., Li, R. et al. (2017). Unique roles of
gold nanoparticles in drug delivery, targeting and
imaging applications. Molecules (Basel, Switzerland),
22(9), 1445.
https://doi.org/10.3390/molecules22091445
Li, X., Zhou, J., Dong, X. et al. (2018). In vitro and in
vivo photothermal cancer therapeutic effects of gold
nanorods modified with mushroom β-Glucan. Journal
of Agricultural and Food Chemistry, 66(16), 4091–
4098. https://doi.org/10.1021/acs.jafc.8b00292
Li, Y., Wang, X., Yang, D. et al. (2019). Polydopamine-
coated gold nanostars for near-infrared cancer
photothermal therapy by multiple pathways. Journal of
Materials Science, 54(18), 12036–12048.
https://doi.org/10.1007/s10853-019-03774-4
Paraiso, W. K. D., Tanaka, H., Sato, Y. et al. (2017).
Preparation of envelope-type lipid nanoparticles
containing gold nanorods for photothermal cancer
therapy. Colloids and Surfaces, B, Biointerfaces, 160,
715–723.
https://doi.org/10.1016/j.colsurfb.2017.10.027
Petryayeva, E., & Krull, U. J. (2011). Localized surface
plasmon resonance: Nanostructures, bioassays and
biosensin—A review. Analytica Chimica Acta,
706(1), 8–24.
https://doi.org/10.1016/j.aca.2011.08.020
Qin, Z., Du, T., Zheng, Y. et al. (2019). Glutathione
induced transformation of partially hollow Gold-Silver
nanocages for cancer diagnosis and photothermal
therapy. Small (Weinheim an Der Bergstrasse,
Germany), 15(35), e1902755-n/a.
https://doi.org/10.1002/smll.201902755
Riley, R. S., & Day, E. S. (2017). Gold nanoparticle-
mediated photothermal therapy: Applications and
opportunities for multimodal cancer treatment. Wiley
Interdisciplinary Reviews. Nanomedicine and
Nanobiotechnology, 9(4), n/a.
https://doi.org/10.1002/wnan.1449
Singh, P., Pandit, S., Mokkapati, V. et al. (2018). Gold
nanoparticles in diagnostics and therapeutics for
human cancer. International Journal of Molecular
Sciences, 19(7), 1979.
https://doi.org/10.3390/ijms19071979
Sun, M., Duan, Y., Ma, Y. et al. (2020). Cancer cell-
erythrocyte hybrid membrane coated gold nanocages
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
476

for near infrared light-activated photothermal/radio/
chemotherapy of breast cancer. International Journal
of Nanomedicine, 15, 6749–6760.
https://doi.org/10.2147/IJN.S266405
Tomane, S., Wilhelm, C., Boujday, S. et al. (2021).
Gold/Polyoxometalate Core/Shell nanoparticles for
combined Chemotherapy-Photothermal cancer
therapy. ACS Applied Nano Materials, 4(3), 2339–
2344. https://doi.org/10.1021/acsanm.0c03187
Vines, J. B., Yoon, J., Ryu, N. et al. (2019). Gold
nanoparticles for photothermal cancer therapy.
Frontiers in Chemistry, 7, 167–167.
https://doi.org/10.3389/fchem.2019.00167
Wang, P., Zhang, L., Zheng, W. et al. (2018). Thermo-
triggered release of CRISPR-Cas9 system by
Lipid‐Encapsulated gold nanoparticles for tumor
therapy. Angewandte Chemie (International Ed.),
57(6), 1491–1496.
https://doi.org/10.1002/anie.201708689
Xia, L., Zhang, C., Li, M. et al. (2018). Nitroxide-radicals-
modified gold nanorods for in vivo CT/MRI-guided
photothermal cancer therapy. International Journal of
Nanomedicine, 13, 7123–7134.
https://doi.org/10.2147/IJN.S171804
Xu, W., Qian, J., Hou, G. et al. (2017). Hyaluronic acid-
functionalized gold nanorods with pH/NIR dual-
responsive drug release for synergetic targeted
photothermal chemotherapy of breast cancer. ACS
Applied Materials & Interfaces, 9(42), 36533–36547.
https://doi.org/10.1021/acsami.7b08700
Xu, W., Qian, J., Hou, G. et al. (2019). A dual-targeted
hyaluronic acid-gold nanorod platform with triple-
stimuli responsiveness for photodynamic/photothermal
therapy of breast cancer. Acta Biomaterialia, 83, 400–
413. https://doi.org/10.1016/j.actbio.2018.11.026
Yang, D., Yang, G., Yang, P. et al. (2017). Assembly of
au plasmonic photothermal agent and iron oxide
nanoparticles on ultrathin black phosphorus for
targeted photothermal and photodynamic cancer
therapy. Advanced Functional Materials, 27(18),
1700371-n/a. https://doi.org/10.1002/adfm.201700371
You, Q., Sun, Q., Yu, M. et al. (2017). BSA-Bioinspired
gadolinium hybrid-functionalized hollow gold
nanoshells for NIRF/PA/CT/MR quadmodal
diagnostic imaging-guided
Photothermal/Photodynamic cancer therapy. ACS
Applied Materials & Interfaces, 9(46), 40017–40030.
https://doi.org/10.1021/acsami.7b11926
Zhao, R., Han, X., Li, Y. et al. (2017). Photothermal effect
enhanced cascade-targeting strategy for improved
pancreatic cancer therapy by gold
Nanoshell@Mesoporous silica nanorod. ACS Nano,
11(8), 8103–8113.
https://doi.org/10.1021/acsnano.7b02918
Zhu, D., Xie, W., Xiao, Y. et al. (2018). Erythrocyte
membrane-coated gold nanocages for targeted
photothermal and chemical cancer therapy.
Nanotechnology, 29(8), 084002–084002.
https://doi.org/10.1088/1361-6528/aa9ca1
Gold Nanoparticles for Cancer Photothermal Therapy
477
