
Bioavailability Evaluation of a New Compound Bone Peptide
Formula
Yupeng Song
1a
, Jiakang Mu
1b
, Chunyang Ding
1c
, Jiamin Xue
1d
, Shiqi Li
1e
, Sa Zhou
1f
,
Aqin Wang
2,* g
and Jun Yu
2,* h
1
Key Laboratory of Industrial Fermentation Microbiology of Ministry of Education & Tianjin Key Laboratory of Industrial
Microbiology, College of Biotechnology, Tianjin University of Science and Technology, 300457, Tianjin, China
2
Hangzhou Bibau Biotechnology Co Ltd., 310016, Hangzhou, China
zhousa@tust.edu.cn, haiziaqin@126.com, yujunsz@163.com
Keywords: Bone Peptide, Hydroxyproline, Collagen Peptide, Bioavailability.
Abstract: To investigate the contents of hydroxyproline (Hyp) and Glycine-Proline- Hydroxyproline (Gly-Pro-Hyp) in
the serum of C57/BL mice after the new compound bone peptide formula was gavaged. The bioavailability
and utilization efficiency of the new compound bone peptide formula were evaluated by the contents of above
substances in the serum. Moreover, the area under the drug time curve (AUC) was analyzed and compared to
explore the effective exposure amount of the new bone peptide formula. The results showed that the contents
of Hyp and Gly-Pro-Hyp increased in a dose-dependent manner in the serum. The contents of Hyp and Gly-
Pro-Hyp were the highest after 3 h and remained in 24 h. The results indicated that the utilization rate of bone
peptide samples was significantly higher than that of bone peptide tablet and bone peptide injection. The AUC
of Hyp and Gly-Pro-Hyp in the low-dose bone peptide sample group were significantly higher than that of
clinical dose bone peptide samples. AUC of Hyp and Gly-Pro-Hyp in the low-dose bone peptide sample group
was significantly higher than those in the clinical dose bone peptide injection and bone peptide tablet groups,
indicating that the clinically dose low-dose bone peptide tablet had a good effective exposure to efficacy.
1 INTRODUCTION
Collagen is one of the main macromolecules that
constitutes the extracellular matrix. Bovine bone
peptide is an active peptide obtained by enzymatic
digestion of bovine collagen, which completely opens
the triple helix structure of bovine collagen, and the
peptide chain is degraded into short peptide chains to
obtain a peptide mixture with a molecular mass of
several thousand daltons.
These small molecule peptides are compatible
with living organisms and have better nutritional
functions than proteins and amino acids (Ahn 2019,
a
https://orcid.org/0000-0002-8604-5183
b
https://orcid.org/0000-0001-7093-005X
c
https://orcid.org/0000-0002-5469-711X
d
https://orcid.org/0000-0002-4117-3675
e
https://orcid.org/0000-0001-5979-7171
f
https://orcid.org/0000-0001-8816-6985
g
https://orcid.org/0000-0002-6960-5441
h
https://orcid.org/0000-0003-2402-8000
Bello 2006, Cao 2020, Lee 2019). At the same time,
it has good solubility and stable physical properties.
The small molecular peptides obtained from different
sources and different enzymatic hydrolysis processes
have different biological activities, such as immune
regulation, lowering blood pressure, regulating blood
sugar and blood lipid, anti-aging, anti-oxidation, anti-
cancer, anti-microbial, anti-toxin, increasing bone
density and improving bone toughness (Li, Wu 2018,
Pountos 2016, RosanoBraun 1987). This study
investigated the bioavailability of bovine osteoptin in
mice after administration.
Song, Y., Mu, J., Ding, C., Xue, J., Li, S., Zhou, S., Wang, A. and Yu, J.
Bioavailability Evaluation of a New Compound Bone Peptide Formula.
DOI: 10.5220/0011217300003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 467-470
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
467
Bone peptide is rich in a variety of non-essential
amino acids, such as glycine (Gly), proline (Pro) and
hydroxyproline (Hyp). Hyp is the main component of
collagen tissue, and it is a specific amino acid in
collagen, accounting for about 13% of the total
collagen amino acid (SatoAsai 2020, Wang 2020,
Watanabe 2010). Hyp in blood is a degradation
product of various collagen peptides and is used as a
marker of bone absorption. By measuring the content
of hyp in the serum, the absorption and bioavailability
of bone peptide in mice after sample administration
can be measured (Yazaki 2017). In addition, previous
studies have shown that mice can directly absorb
collagen Gly-Pro-Hyp in the bone peptide sample
polypeptide mixture. Therefore, the measurement of
collagen peptides in mice is another key indicator to
evaluated bone peptide absorption and
bioavailability. In this study, the contents of Hyp and
Gly-Pro-Hyp in mouse plasma were measured after
administration for different times. The bioavailability
of various bone peptide samples was evaluated by
simulating the area model under the curve of drug
time, which provided basic research basis for the
development and application of new bovine bone
peptide products.
2 MATERIALS AND METHODS
2.1 Material
Bone peptide composite powder (Hangzhou
baibeiyou Biotechnology Co., Ltd.); Bone peptide
tablet (Jilin Huakang Pharmaceutical Co., Ltd.
National medicine quasi H20058927); Injection of
compound bone peptide (Nanjing Xinha
Pharmaceutical Co., Ltd. National medicine quasi
H20003533). All samples are stored at 4℃.
2.2 Determination of Serum Index
Preparations of serum: the blood was taken from the
aorta of mouse eyeball and placed in a centrifugal
tube with plug. After standing at room temperature
for 30 min, the blood was centrifuged at 4000 rpm for
10 min. The upper serum was slowly taken out by
pipette and placed in another centrifuge tube with
plug for determination of serum indexes.
2.3 Detection of Hyp in Serum
Healthy C57/BL mice were selected and divided into
6 groups: blank control group, positive
pharmaceutical control group 1 (bone peptide
injection group), positive pharmaceutical control
group 2 (bone peptide group), low (1.6 g/kg), medium
(3.2 g/kg) and high (4.8 g/kg) dose of bone peptide.
After adaptive for a week, 20 mice in each group were
gavaged once. 4 mice were sacrificed at 1, 3, 6, 12,
24 h in in each group and the plasma of each mouse
was obtained. Then the content of hyp in the serum
was determined, and the bioavailability of the
samples was analyzed. The AUC of Hyp was
calculated to compare the effective exposure amount
of drug absorption.
2.4 Detection of Gly-Pro-Hyp
The mice of positive pharmaceutical control groups
and the experimental groups were gavaged and
sacrificed the same as above. The content of Gly-Pro-
Hyp in the serum was determined, and the
bioavailability of the samples was analyzed. The
AUC of Gly-Pro-Hyp was calculated to compare the
effective exposure amount of drug absorption.
3 RESULTS AND ANALYSIS
3.1 Determination of Hyp Content in
the Serum of Mice
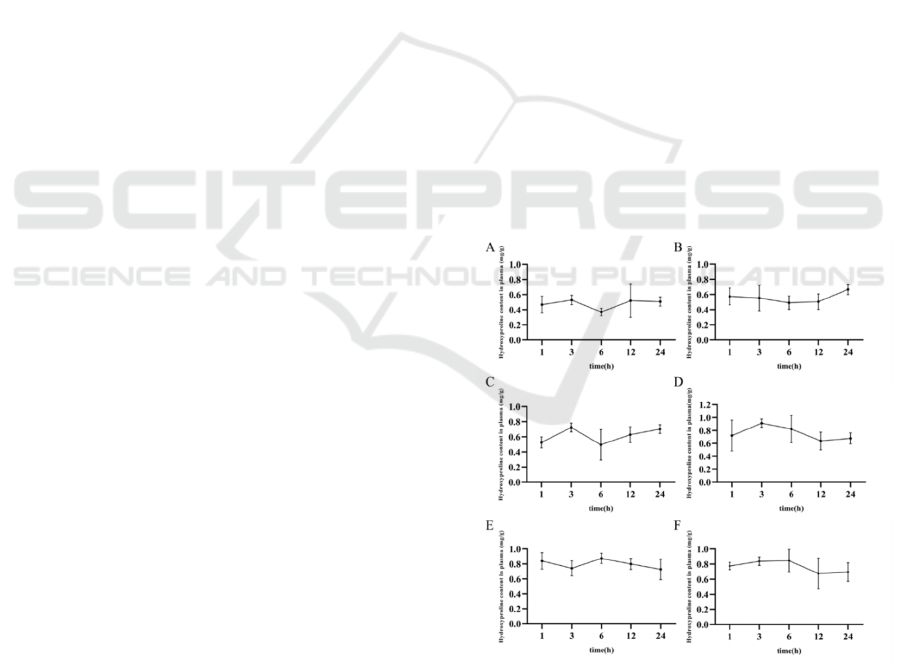
Figure 1: The content of Hyp in serum A: Blank group; B:
Low dose group (1.6 g/kg); C: Medium dose group (3.2
g/kg); D: High dose group (4.8 g/kg); E: Bone peptide tablet
group (576 mg/kg); F: Injection group (90 mg/kg).
The content of Hyp in the serum of the experimental
group can be obtained in Figure 1. The level of Hyp
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
468
was increased in a dose-dependent manner in the
serum. The content of Hyp in the serum of bone
peptide samples was reached the highest level after 3
h, and the Hyp level remained high with the extension
of time, and no significant decrease of Hyp occurred
after 24 h. It indicated that the utilization rate of
compound bone peptide samples was significantly
higher than that of bone peptide tablet and bone
peptide injection.
3.2 Calculation of AUC of Serum Hyp
in Mice
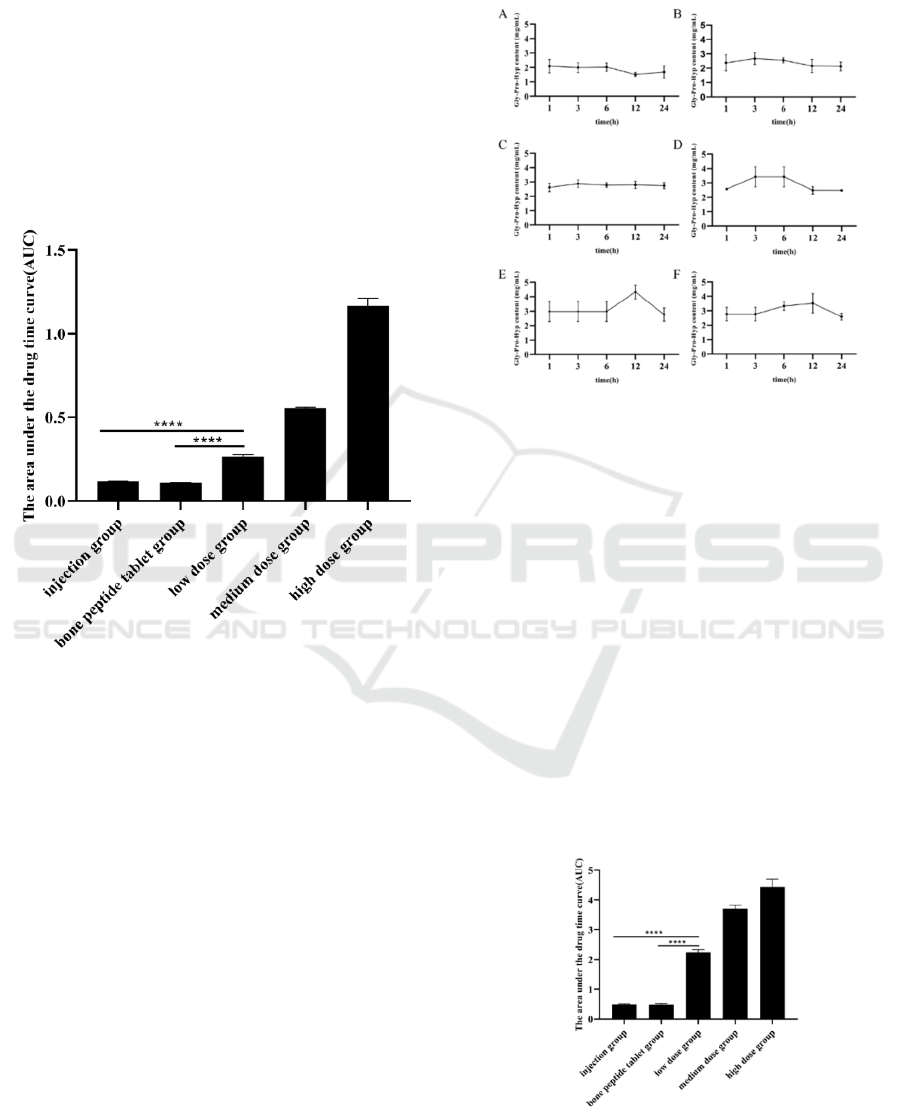
(**** P <0.0001)
Figure 2: The AUC of Hyp in the serum of mice.
The AUC is the area under the curve obtained by
taking the blood concentration after administration as
the ordinate and time as the abscendant. In this study,
the integral method is used to obtain AUC, which
indicate that the drug absorbs in a certain amount of
time after taking a certain dose drug and is
proportional to the amount of drug absorbed by the
organism.
As shown in Figure 2, the AUC of Hyp was
increased in a dose-dependent manner. And the AUC
of low, medium, high-dose bone peptide samples of
Hyp is much higher than the clinical dose bone
peptide injection group and the bone peptide tablet
group. It proved that the advantage of effective
absorption of bone peptide samples. The results
showed that the effective exposure of clinical dosage
- low dose groups was significantly higher than that
of the bone peptide injection and bone peptide tablet.
3.3 Determination of Gly-Pro-Hyp
Content in the Serum of Mice
Figure 3: The content of Gly-Pro-Hyp in serum A: Blank
group; B: Low dose group (1.6 g/kg); C: Medium dose
group (3.2 g/kg); D: High dose group (4.8 g/kg); E: Bone
peptide tablet group (576 mg/kg); F: Injection group (90
mg/kg)
There is a significant increase in the content of Gly-
Pro-Hyp in each experimental group mouse serum.
The level of Gly-Pro-Hyp was increases in a dose-
dependent manner in the serum. And the level of Gly-
Pro-Hyp content is reached the highest after 3 h. The
Gly-Pro-Hyp content was remained high, and no
significant decrease of Gly-Pro-Hyp occurred after
24 h. It indicated that the utilization rate of the bone
peptide samples is significantly higher than the bone
peptide tablet and bone peptide injection.
3.4 Calculation of AUC of Serum
Gly-Pro-Hyp in Mice
(****P<0.0001)
Figure 4: The AUC of Gly-Pro-Hyp in the serum of mice.
Bioavailability Evaluation of a New Compound Bone Peptide Formula
469

Using the same principle of Figure 2, the AUC of
Gly-Pro-Hyp was obtained using the integration
method. As shown in Figure 4, the AUC of Gly-Pro-
Hyp of bone peptide samples increased in a dose-
dependent manner. And the AUC of low, medium
and high-dose bone peptide samples was higher than
the clinical dose bone peptide injection group and
bone peptide tablet group, which proved the
advantage of effective absorption of bone peptide
samples. It indicates that the effective exposure of
clinical dosage - low dose groups was significantly
higher than that of the bone peptide injection and
bone peptide tablet.
4 CONCLUSIONS
In summarize, the results showed that compared with
the blank negative control group, the levels of Hyp
and Gly-Pro-Hyp in the serum of the experimental
groups (positive medicinal bone peptide tablet,
positive medicinal bone peptide injection, low-dose
bone peptide samples and high-dose bone peptide
samples) were significantly increased.
The levels of Hyp and collagen Gly-Pro-Hyp in
mouse serum can reach the highest after at gavaged
for 3 h of the new bone peptide formulation sample,
and the levels of Hyp and collagen Gly-Pro-Hyp was
still remained at high level in 24 h. It indicates that
the utilization rate of mouse bone peptide samples is
significantly higher than that of bone peptide tablet
and bone peptide injection, and the action time of
bone peptide samples is significantly longer than that
of bone peptide tablet and bone peptide injection.
The AUC was obtained by simulating the
pharmacokinetic mathematical model of Hyp and
Gly-Pro-Hyp using the integration method. The
results revealed that the AUC of the new bone peptide
formulation samples increased in a dose-dependent
manner, indicating that the effective exposure of the
mice absorbing the bone peptide samples increased.
And the AUC of Hyp and Gly-Pro-Hyp of low-dose
bone peptide sample group was significantly higher
than those in the clinical dose bone peptide injection
and bone peptide tablet groups. It indicates that the
drug exposure of clinical use dose - low dose bone
peptide samples was significantly higher than that of
bone peptide injection and bone peptide tablet.
REFERENCES
Ahn, C. & J. Je (2019) Bone health-promoting bioactive
peptides. J.food biochemistry, 43, e12529.
Bello, A. E. & S. Oesser (2006) Collagen hydrolysate for
the treatment of osteoarthritis and other joint disorders:
a review of the literature. J.Current medical research
and opinion, 22, 2221-32.
Cao, S., Y. Wang, Y. Hao, W. Zhang & G. Zhou (2020)
Antihypertensive Effects in Vitro and in Vivo of Novel
Angiotensin-Converting Enzyme Inhibitory Peptides
from Bovine Bone Gelatin Hydrolysate. J. Agricultural
and Food Chemistry, 68, 759-768.
Lee, H., H. Jang, D. Ahn, H. Kim, H. Y. Jeon, D. B. Seo, J.
Lee, J. K. Choi & S. Kang (2019) Orally administered
collagen peptide protects against UVB-induced skin
aging through the absorption of dipeptide forms, Gly-
Pro and Pro-Hyp. J. Bioscience, biotechnology, and
biochemistry, 83, 1146-1156.
Li, P. & G. Wu (2018) Roles of dietary glycine, proline, and
hydroxyproline in collagen synthesis and animal
growth. J.Amino acids, 50, 29-38.
Pountos, I., M. Panteli, A. Lampropoulos, E. Jones, G. M.
Calori & P. V. Giannoudis (2016) The role of peptides
in bone healing and regeneration: a systematic review.
J.BMC medicine, 14, 103.
Rosano, C. L., C. B. Braun & C. Hurwitz. 1987. A method
for serum C1q based on its hydroxyproline content.,
398-400..
Sato, K., T. T. Asai & S. Jimi (2020) Collagen-Derived Di-
Peptide, Prolylhydroxyproline (Pro-Hyp): A New Low
Molecular Weight Growth-Initiating Factor for
Specific Fibroblasts Associated With Wound Healing.
J.cell and developmental biology, 8, 548975.
Wang, J., J. Liu & Y. Guo (2020) Cell Growth Stimulation,
Cell Cycle Alternation, and Anti-Apoptosis Effects of
Bovine Bone Collagen Hydrolysates Derived Peptides
on MC3T3-E1 Cells Ex Vivo. J.Molecules, 25.
Watanabe-Kamiyama, M., M. Shimizu, S. Kamiyama, Y.
Taguchi, H. Sone, F. Morimatsu, H. Shirakawa, Y.
Furukawa & M. Komai (2010) Absorption and
effectiveness of orally administered low molecular
weight collagen hydrolysate in rats. J.agricultural and
food chemistry, 58, 835-41.
Yazaki, M., Y. Ito, M. Yamada, S. Goulas, S. Teramoto, M.
Nakaya, S. Ohno & K. Yamaguchi (2017) Oral
Ingestion of Collagen Hydrolysate Leads to the
Transportation of Highly Concentrated Gly-Pro-Hyp
and Its Hydrolyzed Form of Pro-Hyp into the
Bloodstream and Skin. J.agricultural and food
chemistry, 65, 2315-2322.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
470
