
Research and Development of Xenotransplantation using Animals
Kerong Pang
1,† a
, Tianzheng Wan
2,† b
and Xiang Yuan
3,† c
1
Qingdao, Shandong, China
2
College of Life Sciences, Northwest A&F University, Yangling, Shaanxi, China
3
Lifescience Gateway, McMaster University, Guangdong, Shenzhen, China
†
These authors contributed equally
Keywords: Xenotransplantation, Immune Rejection, Immunosuppression.
Abstract: Xenotransplantation is the transplantation of a tissue or organ from one species into another. It is expected to
be used in the clinical treatment of end-organ failure in the future. In recent years, xenotransplantation is being
widely studied because of its great potential. Because the homotransplantation donors are mainly derived from
human origin and are in clinical shortage in quantity, while the xenotransplantation donors are from pigs,
there is no apparent difference in organ quality between human and pig sources. xenotransplantation donors
also have adequate quantity. This paper first introduces the general situation of xenotransplantation. Then, it
discusses the technology and process of xenotransplantation in detail. What is more, this study also provides
the prospects of xenotransplantation. It is hoped this article can provide new ideas to the research in
xenotransplantation.
1 INTRODUCTION
Xenotransplantation is a new technique in the field of
medicine. Xenotransplantation refers to the
transplantation of tissue from one species into another.
For example, from pigs to people. Different from
homotransplantation, there are great differences
between different species, which makes the
possibility of successful transplantation very small.
Animal organs are used as substitutes to reduce the
shortage of organs. This technology is in great
demand. Xenotransplantation is the only effective
way to treat end-stage organs.
However, the shortage of organs continues to
increase. The organs needed for transplantation
mainly come from donors and criminals. Every year,
more than 1 million people in the world need organ
transplantation because of end-stage organ problems
or accidents, but only 10% of them receive organ
transplantation. Many people don’t get treatment.
Compared with human organ transplantation,
xenotransplantation has enough donor sources and
can be mass-produced, so its price should be lower
a
https://orcid.org/0000-0002-3261-749X
b
https://orcid.org/0000-0002-8235-0885
c
https://orcid.org/0000-0002-7853-4817
than human organ transplantation (in some countries,
the price of heart transplantation is about 450,000
yuan). The research of xenotransplantation began in
1667. In recent years, there are more and more
researches on xenotransplantation. For example, 75%
of heart valve surgery patients choose biological
valves, i.e. pig valves or bovine pericardium, as tissue
materials. The survival rate was over 90%. If the
technology works. This will be great progress in the
field of medicine, accelerating human civilization and
prolonging human life.
There are abounding technology to achieve the
goal that is applying xenotransplantation in daily life.
The traditional technique implemented the
transformation of heterologous organs in patients by
a humanized modification for gene knockout of donor
organs from transgenic animals and using
immunosuppressants to promote transplantation.
With the development of gene editing, CRISPR/Cas
technology was also applied in xenotransplantation,
which offers changes based on repairing broken DNA
strands. Even though such technology is successfully
used in xenotransplantation, some barriers also exist
during application. For example, animals without
Pang, K., Wan, T. and Yuan, X.
Research and Development of Xenotransplantation using Animals.
DOI: 10.5220/0011216500003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 461-466
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
461

correct gene editing cannot be converted into mature
organ donors. Before implementing
xenotransplantation, the heterologous organ required
the inhibition of cellular immune responses,
modifying the major histocompatibility complex,
eliminating the cross-species transmission risk of
virus from donor animals, and so on.
This article systematically introduces the general
situation of xenotransplantation in this paper,
including its development history, technical route,
and the test results in recent years. Especially narrates
the difficulties of current xenotransplantation
technology. In addition, this paper also reasonably
analyzes the relevant literature and predicts its bright
future.
2 BASIC INTRODUCTION OF
XENOTRANSPLANTATION
We strongly encourage authors to use this document
for the preparation of the camera-ready. Please follow
the instructions closely in order to make the volume
look as uniform as possible (Moore, Lopes, 1999).
Please remember that all the papers must be in
English and without orthographic errors.
Do not add any text to the headers (do not set
running heads) and footers, not even page numbers,
because text will be added electronically.
For a best viewing experience the used font must
be Times New Roman, on a Macintosh use the font
named times, except on special occasions, such as
program code (Section 2.3.7).
2.1 Concept of Xenotransplantation
Xenotransplantation can provide an unlimited source
of donors to humans through animals. Therefore, It is
the dream of humans for many years. More and more
people are researching xenotransplantation. In theory,
it is the best choice to choose the closest primate (such
as a monkey) as the source of graft. But the primate
number is very rare. As a result, pigs, which are
similar to human organs in size and function, become
the best choice for researchers. In theory, for
example, pig islets could be transplanted into humans.
Because pig insulin works for humans. And pigs can
reproduce rapidly, which makes it easy for them to be
genetically modified.
The biggest problem of xenotransplantation is the
rejection of transplanted organs. This is mainly due to
the common rejection of cells and immune cells in
organs. Lead to adverse reactions. It can even kill
people. At the same time, there is a potential risk of
xenotransplantation, that is, the risk of infection
source transmission. For example, viruses in pigs
spread from pigs to people. To solve these problems,
scientists need further research.
2.2 The Status of Xenotransplantation
The effects of xenotransplantation on organs have
been improved (Cowan 2017). It improves the
research efficiency of xenotransplantation.
Xenotransplantation is the best way to treat organ
failure. Therefore, there is a huge demand for this
technology. Many people worldwide are waiting for
the success of xenotransplantation and integration
into people's lives. However, there are many concerns
about this technology. Scientists have proved that
xenotransplantation is a feasible alternative to
homotransplantation. However, the current situation
of this technology is not mature.
However, with the continuous research of
scientists, people have found a large number of
obstacles to xenotransplantation, and designed many
potential solutions. Scientists have made models of
xenogeneic corneal transplantation. An anterior
lamellar keratoplasty model of non-human primates
in pigs was established. This model can predict the
effect of transgenic pigs on xenogeneic corneal
transplantation (Vabres 2020). At the same time, the
experimental model of transplanting pig liver into
baboon has also been completed (Navarro-Alvarez
2020). The experiment of islet transplantation from
human to rodent has also been completed (Iuamoto
2017). These experiments and models have made a
great step forward in human xenotransplantation.
2.3 Results of Xenotransplantation and
Its Effect on People
In recent years, there is a growing interest in
xenotransplantation. The effect of
xenotransplantation on many organs has been
significantly improved. It improves the research
efficiency of xenotransplantation. The PERV virus
has subsided. However, the prevention of xenograft
rejection is still a great challenge (Cowan 2017).
In 2016, Swiss researchers made a breakthrough
in this field. With the administrative and security
parts of clinical preliminaries entering the program
and using polygenic pigs. The endurance pace of pig
xenotransplantation in non-human primate models
has been altogether improved (Yung 2017). To make
xenotransplantation a clinical reality, it is necessary
to work on the resistant procedure. The most
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
462
interesting possibility soon is the utilization of
homozygotes α-Organs of galactose quality knockout
pigs.
Variety of organic pathways in the all-out range
of xenograft dismissal (Hoerbelt 2004). With the
continuous advancement of human progress and the
efforts of scientists, there will be more and more
breakthroughs in xenotransplantation. Then the
solution and the technology are officially recognized
and put into use. So that the choice of patients whose
organs have reached the end of their life is no longer
limited to allogeneic transplantation.
Xenotransplantation will become the best choice for
them.
3 BARRIERS OF
XENOTRANSPLANTATION
AND THE TECHNOLOGY TO
BREAKTHROUGH
3.1 Obstacles and Strategies to
Overcome in Xenotransplantation
When human organ donor is in a shortage, it is well
known that the application of xenotransplantation can
acquire more benefit than homotransplantation
technology. However, before a huge amount of organ
supplementation from animals, some obstacles need
to be overcome, for example, the immunological
rejection of heterologous organ in the human body
prevents the goal to utilize animals as an organ donor
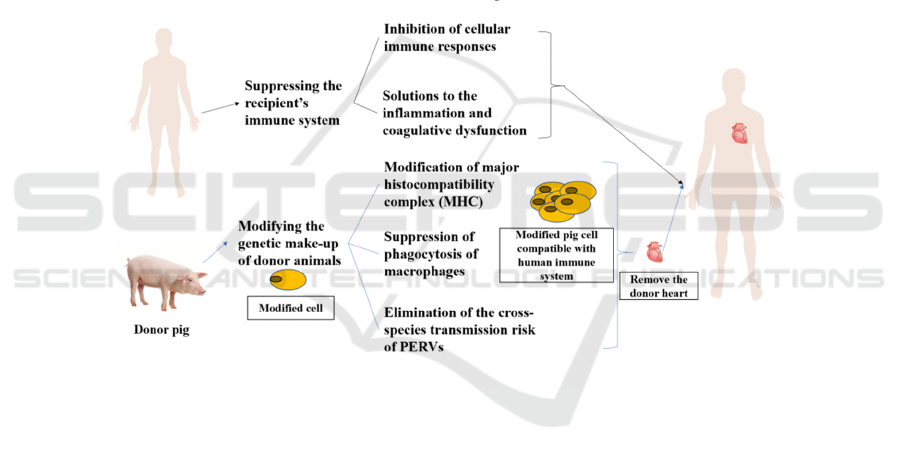
(figure 1).
Figure 1: The main obstacle in xenotransplantation lies in how to eliminate the immune rejection after the operation. For
patients, immunosuppressant adjuvant therapy is needed to reduce cellular immune response and optimize treatment for
transplant-induced inflammation and coagulopathy. For donor pigs, editing and modification at the gene level should be
carried out in advance, which mainly includes the modification of MHC, the inhibition of macrophage function, and the
elimination of endogenous viruses that can be transmitted across species.
In recent years, the development of genetic
technology and research of immunosuppression
medications had prepared the basics for the effective
regulation of these flaws. As an example, the porcine
gene had been optimized to enable human
immunoreaction decline, also, it decreased liveness
rates of porcine endogenous retroviruses (PERVs),
prevented coagulation disorders and other reactions.
Meanwhile, researchers need to ensure the organ is
functioning normally and living for a long period
after xenotransplantation, for instance, operate
corresponding immunosuppression strategy to
transfer the pancreas from transgenic pig to human
and make certain the organ working, as usual, to
return blood sugar and insulin level to normal. Deeper
research is necessary, applying the
immunosuppression strategy still has a limitation for
the health of the patient. Moreover, another strategy,
immunotherapy, can build a mouse model (gene and
tissue of mice are substituted by human genes and
cells), simulating the growing environment of
xenograft organs and tissue under the human immune
system. This model is one of the latest advances in
immunosuppression strategies.
Research and Development of Xenotransplantation using Animals
463

3.2 Suppressive Regulation of Immune
Response
Many experiments had been done in the study of
xenotransplantation, especially in the control of the
immune response. For example, the application of
TEVMP with good mechanical and physiological
characteristics as a biological artificial artery in pigs
to simulate the physiological blood flow system of the
human body, and the study of xenograft rejection in
blood vessels (Kim 2021). In addition, many
transplantation experiments have been performed to
challenge xenoimmunological rejection, such as the
implantation of human cancer cells in humanized
mice (which have a human immune system). The
mice were also tested for tumor treatment results
which have replaced the original human patients (Jin
2021).
As a deeper study, CD47 is a ligand for
macrophage inhibitory receptors, if introducing
human inhibitory regulators of macrophage into the
pig, it could effectively inhibit the rejection reaction
of macrophage in the human body after
xenotransplantation. By the technique of chromatin
transfer, researchers successfully obtained ideal
hCD47 Knock-in Pigs, after two rounds of clone.
Moreover, to test the results' effectiveness, the
researchers transplanted porcine progenitor cells into
mice and found that several alleles in the mice were
able to bind with hCD47. This result showed that the
expression of hCD47 had a significant protective
effect on the transplantation and persistence of
porcine cells in this model, possibly by regulating
phagocytosis of macrophages (Tena 2014).
3.3 Second Sectionxenotransplantation
Experiment Practice
Interestingly, there have also been practices involving
xenografts, for instance, using pig islet grafts as
substitutes for allografts. Researchers evaluated
clinically available drug immunosuppression
regimens, such as belimumab for maintenance and
adalimumab for control of inflammatory responses.
The experimental subjects were non-human primates.
After transplantation of pig islets, blood glucose was
normal, and there were no organ failures and no
serious adverse reactions. The survival days of the
transplanted organs in all three recipients were more
than 100 days (Kim 2021).
To ensure long-term insulin independence, in
another paper, researchers used several strategies.
The implantation of embryonic porcine pancreas
tissue into diabetic animals is a key aspect of
xenotransplantation, to be more precise, the location
of the islet implantation is also critical. In previous
experiments, it was placed in the liver, where low
oxygen and inflammatory reactions could hinder the
survival of islets. However, when implanted into the
renal capsule, the islet was well protected, but the site
was prone to ischemic injury. Finally, when the islet
was transplanted into the gastric submucosal-space,
its direct contact with the blood flow is delayed, but
the arterial blood can continue to maintain. As a
result, transporting oxygen and nutrients can enable
the graft to survive. Another advantage is that
drainage of venous blood allows insulin to work
directly in the liver (Marigliano 2011).
4 REDUCE IMMUNE
REJECTION AND
INFLAMMATION
There are still many problems to be overcome in
xenogeneic heart transplantation. Such problems
mainly focus on postoperative immune rejection,
inflammatory reaction, and infection. At the same
time, there are also some ethical and religious issues
(Garcia 2021). At present, various research teams
have proved several possible methods to reduce
immune rejection and inflammation.
4.1 Gene Editing Pig
First of all, it is necessary to cultivate donor pigs with
gene editing in routine xenotransplantation
experiments, knock out or modify some genes that
can cause immune rejection, and porcine cell virus
genes, to reduce the potential risk of immune
rejection (Tomasi 2021). Due to the different genes of
patients, there will be slight differences between gene
editing pigs and different recipients. These
differences may cause a serious inflammatory
reaction after transplantation (Reichart 2021). Li et al.
designed a new method to quickly detect the
xenogeneic immune response of humans to pigs,
which can express special endothelial cell markers,
verify the stimulation strength of inflammatory
cytokines, and detect xenogeneic immune response
by the culture and transformation of immortalized
primary porcine living derived cell (Li 2021).
In addition, how to improve the production
efficiency of gene editing pigs is also one of the hot
issues. Cho et al. introduce the human endothelial
protein C receptor (hEPCR) and human
thrombomodulin (hTM) genes into porcine neonatal
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
464

ear fibroblasts. It can be used as donor cells for
reclining to increase production efficiency. The
cloned fetal kidney cells also have the same function
(Cho 2022).
The survival time of organs after the operation can
be improved, and immune rejection can be reduced
by improving the preservation method of the donor's
heart before operation (Goerlich 2021). Corbin et al.
Found that compared with traditional preservation
methods, low-temperature standing preservation was
carried out on the ice, and used XVIVO© Heart
solution (XHS) based cardioplegia can improve the
survival rate and function of grafts.
4.2 Proinflammatory Factor and
Cardiomyocyte
There are two additional ways to reduce the
occurrence of immune rejection and inflammation
after surgery. One is to continuously apply anti-CD40
antibodies and other immunosuppressants and control
the expression of pro-inflammatory factor hTNF
(Hara 2021, Mohiuddin 2016). When human blood
contacts pig hearts, it induces the activation and
proliferation of cytotoxic T cells and NK cells. hTNF
is one of the important factors in the process of
activation and proliferation.
The other is by inhibiting the harmful
proliferation of donor heart cells (Längin 2018).
Research shows excessive proliferation and
hypertrophy of cardiomyocytes will lead to
multifocal myocardial necroses and thrombosis and
cause secondary liver failure. Matthias et al. tested
with baboons. They weaned the recipient baboons at
an early stage, applied hypertension treatment, and
took additional tacrolimus, which finally reduced the
excessive growth of the heart.
5 CONCLUSIONS
In summary, this paper analyzes the basic
introduction of xenotransplantation, the current
situation of xenotransplantation, the results of
xenotransplantation, and its impact on human beings.
Some effective strategies for suppressing the immune
system can enhance the survival rate and durability of
transplanted organs. However, some methods still
need a deeper study that researchers have not
eliminated the threat of inflammatory reaction and
virus infection carried by allogeneic organs
completely. The next research will focus on Gene
editing pigs. Those donor pigs in use today can
survive as long as six months with just a few genes
knocked out, and it is not hard to imagine that these
xenoorgans could survive even longer when more
genes are modified. Researchers can also find more
suitable immunosuppressants with better effects and
fewer side effects. In the next study, they can
gradually start clinical research under international
recognition and supervision.
REFERENCES
Cho, J., Kim, G., Qamar, A. Y., Fang, X., Roy, P. K.,
Tanga, B. M., Bang, S., Kim, J. K., Galli, C., Perota, A.,
Kim, Y. T., Che, J. H., & Park, C. G. (2022). Improved
efficiencies in the generation of multigene-modified
pigs by recloning and using sows as the recipient.
Zygote (Cambridge, England), 30(1), 103–110.
https://doi.org/10.1017/S0967199421000423
Cowan, P. J., & Tector, A. J. (2017). The Resurgence of
Xenotransplantation. American journal of
transplantation: official journal of the American
Society of Transplantation and the American Society of
Transplant Surgeons, 17(10), 2531–2536.
https://doi.org/10.1111/ajt.14311
Garcia, L. R., Brito, F. S., Felicio, M. L., Garzesi, A. M.,
Tardivo, M. T., Polegato, B. F., Minicucci, M. F., &
Zornoff, L. (2021). Clinical trials in cardiac
xenotransplantation: Are we ready to overcome
barriers?. Journal of cardiac surgery, 36(10), 3796–
3801. https://doi.org/10.1111/jocs.15747
Goerlich, C. E., Griffith, B., Singh, A. K., Abdullah, M.,
Singireddy, S., Kolesnik, I., Lewis, B., Sentz, F.,
Tatarov, I., Hershfeld, A., Zhang, T., Strauss, E.,
Odonkor, P., Williams, B., Tabatabai, A., Bhutta, A.,
Ayares, D., Kaczorowski, D. J., & Mohiuddin, M. M.
(2021). Blood Cardioplegia Induction, Perfusion
Storage and Graft Dysfunction in Cardiac
Xenotransplantation. Frontiers in immunology, 12,
667093. https://doi.org/10.3389/fimmu.2021.667093
Hara, H., Iwase, H., Nguyen, H., Miyagawa, Y., Kuravi, K.,
Foote, J. B., Eyestone, W., Phelps, C., Ayares, D., &
Cooper, D. (2021). Stable expression of the human
thrombomodulin transgene in pig endothelial cells is
associated with a reduction in the inflammatory
response. Cytokine, 148, 155580.
https://doi.org/10.1016/j.cyto.2021.155580
Hoerbelt, R., & Madsen, J. C. (2004). Feasibility of xeno-
transplantation. The Surgical clinics of North America,
84(1), 289–307. https://doi.org/10.1016/S0039-
6109(03)00208-1
Iuamoto, L. R., Franco, A. S., Suguita, F. Y., Essu, F. F.,
Oliveira, L. T., Kato, J. M., Torsani, M. B., Meyer, A.,
Andraus, W., Chaib, E., & D'Albuquerque, L. (2017).
Human islet xenotransplantation in rodents: A literature
review of experimental model trends. Clinics (Sao
Paulo, Brazil), 72(4), 238–243.
https://doi.org/10.6061/clinics/2017(04)08
Jin, K. T., Du, W. L., Lan, H. R., Liu, Y. Y., Mao, C. S.,
Du, J. L., & Mou, X. Z. (2021). Development of
Research and Development of Xenotransplantation using Animals
465

humanized mouse with patient-derived xenografts for
cancer immunotherapy studies: A comprehensive
review. Cancer science, 112(7), 2592–2606.
https://doi.org/10.1111/cas.14934
Kim, J. M., Hong, S. H., Shin, J. S., Min, B. H., Kim, H. J.,
Chung, H., Kim, J., Bang, Y. J., Seo, S., Hwang, E. S.,
Kang, H. J., Ha, J., & Park, C. G. (2021). Long-term
control of diabetes in a nonhuman primate by two
separate transplantations of porcine adult islets under
immunosuppression. American journal of
transplantation: official journal of the American
Society of Transplantation and the American Society of
Transplant Surgeons, 21(11), 3561–3572.
https://doi.org/10.1111/ajt.16704
Kim, T. H., Yan, J. J., Jang, J. Y., Lee, G. M., Lee, S. K.,
Kim, B. S., Chung, J. J., Kim, S. H., Jung, Y., & Yang,
J. (2021). Tissue-engineered vascular
microphysiological platform to study immune
modulation of xenograft rejection. Science advances,
7(22), eabg2237.
https://doi.org/10.1126/sciadv.abg2237
Längin, M., Mayr, T., Reichart, B., Michel, S., Buchholz,
S., Guethoff, S., Dashkevich, A., Baehr, A., Egerer, S.,
Bauer, A., Mihalj, M., Panelli, A., Issl, L., Ying, J.,
Fresch, A. K., Buttgereit, I., Mokelke, M., Radan, J.,
Werner, F., Lutzmann, I., … Abicht, J. M. (2018).
Consistent success in life-supporting porcine cardiac
xenotransplantation. Nature, 564(7736), 430–433.
https://doi.org/10.1038/s41586-018-0765-z
Li, P., Walsh, J. R., Lopez, K., Isidan, A., Zhang, W., Chen,
A. M., Goggins, W. C., Higgins, N. G., Liu, J.,
Brutkiewicz, R. R., Smith, L. J., Hara, H., Cooper, D.,
& Ekser, B. (2021). Genetic engineering of porcine
endothelial cell lines for evaluation of human-to-pig
xenoreactive immune responses. Scientific reports,
11(1), 13131. https://doi.org/10.1038/s41598-021-
92543-y
Marigliano, M., Bertera, S., Grupillo, M., Trucco, M., &
Bottino, R. (2011). Pig-to-nonhuman primates
pancreatic islet xenotransplantation: an overview.
Current diabetes reports, 11(5), 402–412.
https://doi.org/10.1007/s11892-011-0213-z
Mohiuddin, M. M., Singh, A. K., Corcoran, P. C., Thomas
Iii, M. L., Clark, T., Lewis, B. G., Hoyt, R. F., Eckhaus,
M., Pierson Iii, R. N., Belli, A. J., Wolf, E., Klymiuk,
N., Phelps, C., Reimann, K. A., Ayares, D., & Horvath,
K. A. (2016). Chimeric 2C10R4 anti-CD40 antibody
therapy is critical for long-term survival of
GTKO.hCD46.hTBM pig-to-primate cardiac
xenograft. Nature communications, 7, 11138.
https://doi.org/10.1038/ncomms11138
Navarro-Alvarez, N., & Vagefi, P. A. (2020). Liver
Xenotransplantation in a Nonhuman Primate Model.
Methods in molecular biology (Clifton, N.J.), 2110,
197–211. https://doi.org/10.1007/978-1-0716-0255-
3_13
Puga Yung, G. L., Rieben, R., Bühler, L., Schuurman, H.
J., & Seebach, J. (2017). Xenotransplantation: Where
do we stand in 2016?. Swiss medical weekly, 147,
w14403. https://doi.org/10.4414/smw.2017.14403
Reichart, B., Längin, M., Denner, J., Schwinzer, R., Cowan,
P. J., & Wolf, E. (2021). Pathways to Clinical Cardiac
Xenotransplantation. Transplantation, 105(9), 1930–
1943. https://doi.org/10.1097/TP.0000000000003588
Tena, A., Kurtz, J., Leonard, D. A., Dobrinsky, J. R.,
Terlouw, S. L., Mtango, N., Verstegen, J., Germana, S.,
Mallard, C., Arn, J. S., Sachs, D. H., & Hawley, R. J.
(2014). Transgenic expression of human CD47
markedly increases engraftment in a murine model of
pig-to-human hematopoietic cell transplantation.
American journal of transplantation: official journal of
the American Society of Transplantation and the
American Society of Transplant Surgeons, 14(12),
2713–2722. https://doi.org/10.1111/ajt.12918
Tomasi, R., Tariq, M., Hübner, M., Strauss, G., Längin, M.,
Zeuzem-Lampert, C., Vandewiele, S., Kreth, S., &
Abicht, J. M. (2021). T-Cell Response in a Cardiac
Xenotransplant Model. Experimental and clinical
transplantation: official journal of the Middle East
Society for Organ Transplantation, 19(7), 708–716.
https://doi.org/10.6002/ect.2020.0359Vabres, B.,
Vanhove, B., & Blancho, G. (2020). Corneal
Xenotransplantation: Anterior Lamellar Keratoplasty.
Methods in molecular biology (Clifton, N.J.), 2110,
245–251. https://doi.org/10.1007/978-1-0716-0255-
3_16
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
466
