
Applications of Metal Organic Frameworks in Drug Delivery and
Therapy
Yubo Li
1,a,
†
, Guanlin Peng
2,b,*,
†
and Yuezhou Yu
3,c,
†
1
Wuhan Britain-China School, Wuhan 430022, China
2
Bi Academy, Chongqing 400010, China
3
Jiangsu Tianyi High School, Wuxi 214171, China
*
Corresponding author
†
These authors contributed equally
Keywords:
Metal Organic Framework, Drug Delivery, Antibody, Therapy.
Abstract:
Metal organic frameworks (MOFs) are organic-inorganic mixtures formed from metal ions and organic
ligands under relatively mild conditions. MOFs are widely used as drug carriers due to their low toxicity, high
drug load, good biocompatibility, and functional diversity. Stimulus-responsive MOFs materials have
attracted extensive attention in the field of drug delivery materials and biological applications. This article
highlights the different types of stimulus-responsive MOFs materials, including pH-Responsive MOFs,
magnetically-responsive MOFs, ion-responsive MOFs, temperature-responsive MOFs, and pressure-
responsive MOFs. MOFs materials are very effective as intermediates for drug transport. In this thesis, we
mainly studied the advantages of MOFs as intermediates. It is stable and can be safely degraded, and it makes
the antibody easier to attach, and have strong plasticity.
1 INTRODUCTION
MOFs are organic-inorganic mixtures formed from
metal ions and organic ligands under relatively mild
conditions (Batten, Champness, Chen, 2013). The
stability of MOF is influenced by many factors,
including the operational environment, metal ions,
organic ligands, coordinate geometry of metal
ligands, hydrophobicity of interstitial surfaces. The
study of MOF stability helps rationalize the influence
of several factors and design stable framework
structures wisely. The relatively volatile coordination
relationships of the skeletal support structure are seen
as the cause of the limited stability of the MOF.
Therefore, the stable structure of the MOF would
need to be highly coordinated. They have been
extensively studied in the basic fields of catalytic
intermediates capture and energy transfer and the
potential practical applications such as gas storage
and separation, heterogeneous catalysis, chemical
sensing, biomedical applications, and proton
conduction (Chughtai, Ahmad, Younus, 2015). Many
early MOFs made from divalent metals, such as Zn2+
or Cu2+, showed extremely high porosity and
showed promise for widespread use, but ultimately
proved unsuitable for harsh conditions due to stability
issues (Eddaoudi, Kim, Rosi, 2002); (Deng, Doonan,
Furukawa, 2010).
Drug delivery systems (DDS) are typical of the
research achievements about new preparations and
dosage forms in modern pharmacy, the crystallization
of modern scientific and technological progress. The
system has made great progress in the theoretical
system, design of new preparation and preparation
process, and application in clinical treatment, mainly
including oral slow and controlled release system,
transdermal drug delivery system, and targeted drug
delivery system. Recent research about this topic has
made a big process because people have already
found an almost perfect carrier for drug delivery,
which is MOFs. MOFs are regarded as the perfect
material because they have many advantages that
other carriers do not have (Neuberger, Schöpf,
Hofmann, 2005).
Compared with other organic porous materials
and inorganic materials, MOFs have the following
characteristics. Firstly, MOF materials are highly
adjustable. MOFs are a hybrid porous material whose
pore surface properties can be adjusted to suit drug
Li, Y., Peng, G. and Yu, Y.
Applications of Metal Organic Frameworks in Drug Delivery and Therapy.
DOI: 10.5220/0011216100003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 455-460
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
455

delivery needs. Secondly, partial MOFs can be
adjusted according to needs so that their toxicity to
the human body is controlled in an acceptable range
to achieve a relatively stable carrier. Because active
functional groups can be inserted into the surface of
MOFs, it is easier to modify the surface to meet the
use requirements. Thirdly, the drug can be loaded by
introducing functional ingredients or changing the
body's flexibility, and then the speed and size of its
release can be controlled. Finally, MOFs can achieve
the purpose of making targeted drugs by introducing
stable special materials. By changing the NMOFs
after the contact surface, MOFs can be transformed
into a method that can make it have the advantages of
nanosensors. For example, the targeting and
bioavailability of drugs can be improved, the stability
of drugs can be increased, the efficacy can be
improved, and the toxic and side reactions can be
reduced. It can also make the drug into the human
body at all levels of the tiny blood vessels, and
pathological tissue cells play a therapeutic role.
MOFs have essentially large surface areas, highly
ordered porosity, and clear structures that give these
materials the ability to load and release different
cargoes, especially therapeutic agents (Ma, Moulton,
2011).
This review will focus on the development of
MOFs in the field of controlled drug release and
effective cancer treatment, including drug
nanocarriers and cancer treatment systems composed
of single MOFs, stimulus-responsive MOFs, and
multifunctional MOFs. This document also covers
basic methods for the application of MOF to biology.
Finally, the development prospects and challenges of
MOF are under discussion.
2 STIMULI-RESPONSIVE MOFs
FOR DRUG DELIVERY
Stimulus-responsive MOFs materials have attracted
extensive attention in the field of drug delivery
materials, especially in the field of biological
applications. As a result, Stimulus-responsive MOFs
have become popular candidate materials for
controlling drug release. Generally, stimulus-
response MOFs can be divided into single stimulus-
response types and multiple stimulus-response types.
Next, we will discuss the main response methods for
these two MOFs (Feng, Wang, Zhang, 2019).
2.1 Single-Stimuli-Responsive MOFs
for Drug Delivery
2.1.1 pH-Responsive MOFs
All porous MOF nanosensors are stimulated by
external stimulation. However, the pH-responsive
MOF is the most widely studied, especially in cancer
treatment, because acidic bonds are particularly
sensitive to the tumor microenvironment and
coordination external doctor many studies have
investigated the pH response of MOF to drug delivery
and cancer therapy (Freeman, Arrott, Watson, 1960).
Recently, Qian's group described an interesting
cationic nanocarrier, ZJU-101 (Zhejiang University,
ZJU) MOF, for delivering the anionic drug diclofenac
sodium. The cation material of the body is in
zirconium and 2,2′-bipyridine-5,5′-dicarboxylate
(BPYDC) ligand. And the high carrying capacity of
diclofenac sodium was 0.546 g/g. The release rate of
diclofenac sodium in inflammatory tissues (pH = 5.4)
was higher than that in normal tissues (pH = 7.4)
because the ion exchange between the anions in PBS
and the drug is more frequent under acidic conditions.
As a result, the coulomb interaction between the
cation ZJU-101 and the anionic drug is weakened. As
a result, diclofenac sodium-sensitive to pH is
expected to be a promising carrier of anti-
inflammatory drugs (Angelos, Khashab, Yang,
2009).
2.1.2 Magnetically-Responsive MOFs
Due to the potential benefits of magnetic response
systems with respect to magnetic separation,
magnetic targeting, magnetic resonance imaging
(MRI) and magnetic hyperthermia, drug delivery is
quite diverse. Therefore, the administration of
magnetic drugs is a unique strategy for improving the
therapeutic effect of concentrating the drug delivery
probe at the tumor site. Since the method was first
proposed by Watson et al. in the 1960s, many MOFs
materials have been found to have good magnetic
properties and can be used for drug separation and
drug delivery (Cavka, Jakobsen, Olsbye, 2008);
(Hergt, Dutz, Müller, 2006); (Jurgons, Seliger,
Hilpert, 2006); (Kumar, Mohammad, 2011). In 2014,
Guan and colleagues reported on a one-step in situ
pyrolysis method for producing γ-Fe
2
O
3
@MOF. In
their work, γ-Fe
2
O
3
@MIL-53 (LA) demonstrated its
potential for controlled magnetic separation and drug
liberation. As anticipated, magnetic nanocomposites
showed a controlled release behavior in 37 salines.
That is, the capsule IBU is fully released after 7 days
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
456
in 3 stages. About 30% of the drug is released rapidly
within the first 3 hours in the first stage. Then 50% of
the drug was released within 2 days. Finally, the
remaining 20 percent of the drug was released within
five days. This confirms that magnetic γ-
Fe
2
O
3
@MIL-53 (AL) is a feasible drug delivery
material (Lee, Hyeon, 2012).
2.1.3 Ion-responsive MOFs
Ion-responsive MOFs open up new drug delivery
pathways. The strong electrostatic interaction
between the drug and the frame enables it to control
the diffusion and release of the drug in the drug
carrier. Therefore, the strong electrostatic interaction
between ionic drugs and ion frames is particularly
interesting because the release of ionic drugs is a
chemically stimulated reaction process that occurs
only in ion exchange. For example, Tamames-Tabar
et al. investigated the cytotoxicity of MOFs
containing Fe, Zn, or Zr central metals on human
cervical cancer cell lines (HeLa) and mouse
macrophages (J774). The cytotoxicity of Fe-MOFs
was less than that of Zn-MOFs and Zr-MOFs.
Although Zn and Fe are trace elements found in the
human body, Zn ions can compete with Fe and Ca
ions to bind ion channels, alter metabolism, and
damage cells. Gao et al. demonstrated Fe-MOFs'
relative biocompatibility by finding that more than
80% of human aortic smooth muscle cells survived
exposure to 200 μg/mL Fe-Mil-53-NH2-FA-5-
FAM/5-FU. Similarly, nh2-MIL-88 (Fe) or NH2-MIL
88(Fe)/Br was incubated at 2000 μg/mL. More than
90% of human primary corneal epithelial cells remain
active, and Fe-MOF exhibits relatively low
cytotoxicity as an imaging agent. The IC50 of MIL-
88A(Fe) on J774 cells was 57±11 μg/mL. Mil-100
(Fe) showed no cytotoxicity to human leukemia cell
line CCRF-CEM and human multiple myeloma cell
line RMI-8226 at 10 mm. By intravenous
administration of 220 mg/kg of Fe-MOFs MIL, the
concentrations of MIL-100 and MIL-88A, MIL-100
and MIL-88B-4CH3 were reduced, supporting the
explanation of MOFs. These things have successfully
become practical iron (Wu, Zhou, Li, 2014).
2.1.4 Temperature-responsive MOFs
In general, temperature-sensitive nanotransporters
are materials that are sensitive to small temperature
variations at a physiological temperature of 37. Sada
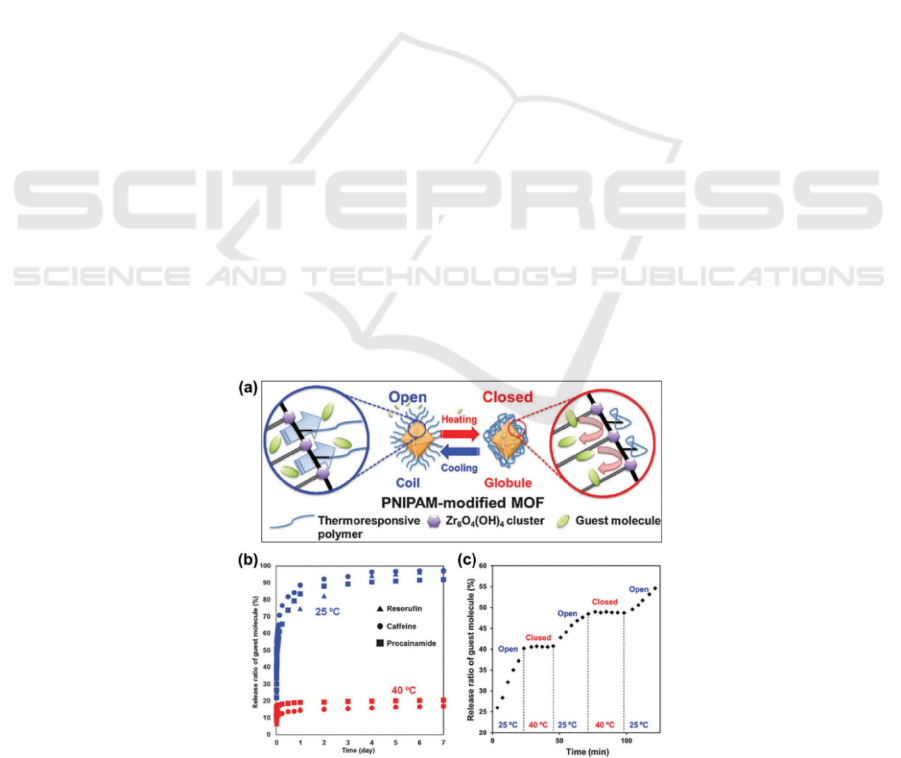
et al. demonstrated a switchable UIO-66 PNIPAM
nanocarrier by immersing UIO-66-PNIPam in a guest
solution and loading L2L m-catechol, caffeine, and
procaine into the nanocarrier (Fig. 1a), and then
evaluated the release behavior at 25 oC or 40 oC (Fig.
1b). As expected, the release increases to 25 and stops
at almost 40 (Fig. 1c), indicating that the controlled
release results in temperature changes. In recent
years, two zinc MOFs were synthesized. The anti-
cancer drug MTX was loaded into both MOFs by
single immersion, and the loading amounts of ZJU-
64 and ZJU-64-CHS were 13.45% and 10.63%,
respectively. ZJU-64 and ZJU-64-CH loaded with
MTX were released at 37 oC for 72 h with the same
amount of release, but at 60 oC for 1.5 h and 6 h,
respectively, indicating that ZJU-64 and ZJU-64-CH
have potential application value as temperature-
sensitive drug carriers (Wu, Yang, 2017); (Nagata,
Kokado, Sada, 2015).
Figure 1: a) The controlled release profiles of UiO-66-PNIPAM. b) Release behavior of drug-loaded UiO-66-PNIPAM in the
water at 25 °C and 40 °C for seven days. c) Temperature-responsive release behavior of UiO-66-PNIPAM resorufin in water
at 572 nm (Lin, Hu, Yu, 2016).
Applications of Metal Organic Frameworks in Drug Delivery and Therapy
457
2.1.5 Pressure-responsive MOFs
To avoid premature drug release before reaching
pathological tissues, several effective, responsive
MOFs have been developed to prolong drug release
time and significantly improve treatment outcomes.
In addition to the stimulus-response MOFs
mentioned above, pressure is also used to control
drug release. For example, Qian and colleagues
documented a Zr-based MOF constructed from
(2E,2E)-3,3-(2-fluoro-1,4-benzene) diacrylic acid (F-
H2PDA) and zirconium clusters with a loading
capacity of 58.80 wt% of high-model drug diclofenac
sodium (DS). Different pressures can adjust the
release kinetics of MOF-loaded DS, and the release
time can be extended to 2-8 days. This demonstrates
the effectiveness of stress control drug release
(Nagata, Kokado, Sada, 2015).
2.2 Multiple-Stimuli-responsive MOFs
for Drug Administration
Because of the intricacy of the human environment,
the capacity to precisely convey drugs in people
utilizing single incitement responsive MOF materials
is restricted. Notwithstanding, multi-boost reaction
MOFs can be utilized as a superior choice to further
develop drug load limit and chemotherapy
proficiency (Jiang, Zhang, Hu, 2016); (Ogoshi,
Kanai, Fujinami, 2008); (Strutt, Zhang, Schneebeli,
2014); (Zhang, Zhao, 2013).
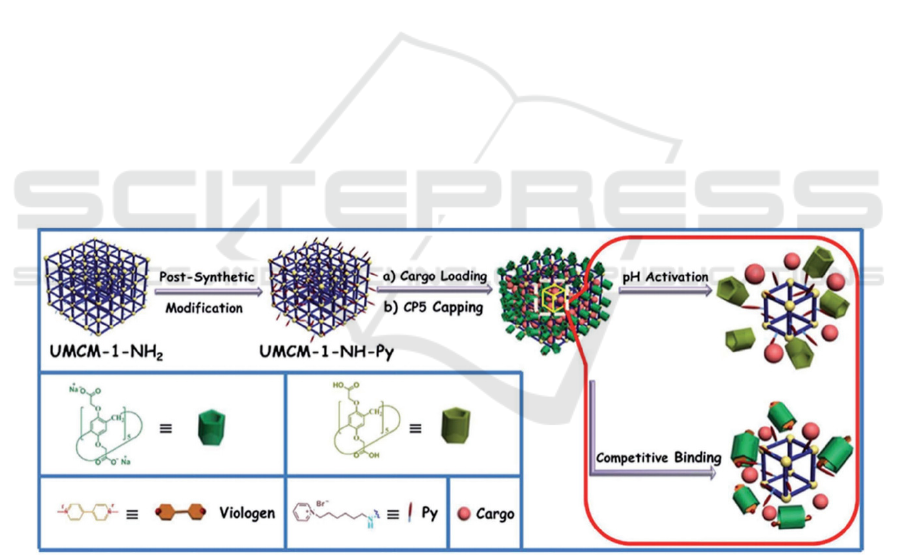
By incorporating pH esteem and additionally
serious restricting response techniques into a solitary
medication nanocervator (Fig. 2), the medication
nanocervator with CPS as the terminal has high
embodiment proficiency, unimportant early delivery,
immaterial cytotoxicity, and optimal biodegradability
and biocompatibility. In the R bunch, MOF
multistimulus delicate nanocervors with CP5 ring as
the guard were additionally examined. For instance,
another CP5-covered UIO-66-NH5-FU nanocarrier
was reported. In this work, UIO-66-NHH is adjusted
by the emphatically charged quaternary ammonium
salt Astals (Q) through the contrarily charged CP5
ring arrangement pseudotaxane goes about as an
energizer with responsive supramolecular gating to
direct medication discharge. Because of the great
partiality among zinc and fluorouracil, zinc * can be
utilized as a cutthroat glue to trigger delivery by
specialists. Furthermore, the expanded temperature
will likewise debilitate the non-covalent bond
cooperation among CP5 and the stem, in this manner
animating medication discharge. Thusly, cp5-gated
MOF-based double boost responsive medication
nanocarriers give new freedoms to treating focal
sensory system sicknesses. (Si, Xin, Li, 2015)
Figure 2: Schematic delineation of double improvements responsive DDS dependent on UMCM-1-NH2 NMOF gated by
pillararenes. Recreated under the details of the CC-BY-NC-3-0 unported license (Doane, Burda, 2012).
3 MOFs FOR ANTIBODY
TRANSPORT
As a biological drug, the antibody is an important part
of immunotherapy and plays an important role in
scientific research, medical diagnosis and disease
treatment. However, many biological drugs,
including antibodies, have disadvantages such as
poor internal stability, easy aggregation and easy
degradation, which greatly reduce the efficacy.
Polymer is one of the most commonly used carriers
to maintain antibody stability, but polymer has certain
immunogenicity and lacks biosafety. Therefore, it is
imperative to develop a simple and efficient biologic
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
458

drug stabilization material. So we found that MOFs
are intermediates for drug transport. The advantages
of MOFs as intermediates are as follows.
3.1 Stability and Security Degradation
Yao Chen of Nankai University, and Shengqian Ma,
of the University of South Florida used MOFs as a
protective layer to prevent antibodies from
accumulating or inactivating in complex
environments in the body. The protected antibody has
good thermal, chemical and mechanical stability and
can stay at 4↔50 °C, 25 °C min-1 when temperature
changes rapidly. More importantly, with the right
stimulus, antibodies can be fully released within 10
seconds. At the same time, MOFs are almost all
degraded, avoiding the immunogenicity and
biosafety problems of residual materials (Tan, Song,
Zhang, 2016).
3.2 Antibody Adhesion
MOFs are widely welcomed as drug carriers in the
biomedical field. MOFs is an ideal drug carrier. On
the one hand, there are many ways to bind drug
molecules to MOFs nanoparticles differently. On the
other hand, the combination of drugs and MOFs
nanoparticles can be adjusted to improve the drug
adsorption rate of MOFs nanoparticles. Abhik et al.
synthesized and used Fe
3
O
4
@MIL-100 to load the
anticancer drug doxorubicin. The study structure
showed that Fe
3
O
4
@MIL-100 could improve the
drug loading rate of adriamycin and achieve the
purpose of drug release. Zhou et al. Ni@MOFs-74
(Ni) were synthesized by the one-pot method, and it
was found that Ni@MOFs-74 (Ni) has high porosity
and strong magnetic properties, which can greatly
improve drug loading rate. The results showed that
Ni@MOFS-74 (Ni) loaded ibuprofen up to 4.1 mg/g.
Lazaro et al. used Zr-MOFs combined with
dichloroacetic acid and 5-fluorouracil to enhance in
vitro cytotoxicity (Tan, Song, Zhang, 2016).
3.3 Highly Modifiability
MOFs has good material modification property. The
remaining uncoordinated carboxyl groups in MOFs
are derived. Under the activation of EDC and SULfo-
NHS, antibody molecules were covalently modified
by peptide bonds, and a kind of antibody
functionalized MOFs material was developed. Then
MOFs materials were grown in situ on ZnO
substrates to construct a cell recognition and capture
platform. The captured cells were observed and
counted by ESEM and confocal fluorescence
microscopy. Several factors affecting the capture of
tumor cells by antibody-functionalized MOFs
materials were studied, including co-incubation time,
cell concentration, material morphology and different
cell lines. EpCAM antibody modified MOFs have a
strong specific capture ability for EPCAM-positive
cell lines, and the capture efficiency is greatly
affected by cell concentration and material
morphology. McF-7 cells are preferentially attached
to the needle-like structure of the material. Europium
complex is superior to antibody-functionalized
ZnMOFs in biocompatibility. In addition, the
cytotoxicity of ZnMOFs depends on the amount of
material used. In contrast, the cytotoxicity of the Eu
complex did not change significantly over the range
of concentrations used in the experiment (Tan, Song,
Zhang, 2016).
4 CONCLUSION
In summary, stimulus-responsive MOF materials can
be divided into single stimulus response and multi-
stimulus response. The single stimulus response
includes pH-Responsive MOFs, magnetically-
responsive MOFs, ion-responsive MOFs,
temperature-responsive MOFs and pressure-
responsive MOFs. Magnetic response system has
great advantages in magnetic separation, magnetic
targeting and magnetic resonance imaging. Specific
advantages are reflected in the accurate release of
drugs, accurate imaging and other aspects. The strong
electrostatic interaction between drug and frame
makes MOF material have many advantages in drug
delivery and release. In addition to stimulus-
responsive MOF materials, pressure can also be used
to control drugs. They stand out in the field of drug
delivery because of their strong drug loading
capability, high microbial capacity and easy
functional properties.
More efficient synthesis for MOF materials is a
very promising research direction. The traditional
synthesis method has a long reaction time, high
reaction temperature, large organic solvent, and
complex reaction equipment, which has become the
bottleneck.
REFERENCES
Angelos S, Khashab N M, Yang Y W, et al. (2009) pH
clock-operated mechanized nanoparticles. Journal of
the American Chemical Society, 131(36): 12912-
Applications of Metal Organic Frameworks in Drug Delivery and Therapy
459

12914.
Batten S R, Champness N R, Chen X -M, et al. (2013)
Terminology of metal-organic frameworks and
coordination polymers (IUPAC Recommendations
2013). Pure Appl. Chem., 85: 1715-1724.
Chughtai A H, Ahmad N, Younus H A, et al. (2015) Metal–
organic frameworks: Versatile heterogeneous catalysts
for efficient catalytic organic transformations. Chem
Soc Rev, 44(19): 6804-6849.
Cavka J H, Jakobsen S, Olsbye U, et al. (2008) A new
zirconium inorganic building brick forming metal
organic frameworks with exceptional stability. Journal
of the American Chemical Society, 130(42): 13850-
13851.
Deng H, Doonan C J, Furukawa H, et al. (2010) Multiple
functional groups of varying ratios in metal-organic
frameworks. Science, 327(5967): 846-850.
Doane T L, Burda C. (2012) The unique role of
nanoparticles in nanomedicine: imaging, drug delivery
and therapy. Chemical Society Reviews, 41(7): 2885-
2911.
Eddaoudi M, Kim J, Rosi N, et al. (2002) Systematic design
of pore size and functionality in isoreticular MOFs and
their application in methane storage. Science,
295(5554): 469-472.
Feng Y, Wang H, Zhang S, et al. (2019)
Antibodies@MOFs: an in vitro protective coating for
preparation and storage of biopharmaceuticals.
Advanced Materials, 31(2): 1805148.
Freeman M W, Arrott A, Watson J H L. (1960) Magnetism
in medicine. Journal of Applied Physics, 31(5): S404-
S405.
Hergt R, Dutz S, Müller R, et al. (2006) Magnetic particle
hyperthermia: nanoparticle magnetism and materials
development for cancer therapy. Journal of Physics:
Condensed Matter, 18(38): S2919.
Jurgons R, Seliger C, Hilpert A, et al. (2006) Drug loaded
magnetic nanoparticles for cancer therapy. Journal of
Physics: Condensed Matter, 18(38): S2893.
Jiang K, Zhang L, Hu Q, et al. (2016) Pressure controlled
drug release in a Zr-cluster-based MOF. Journal of
Materials Chemistry B, 4(39): 6398-6401.
Kumar C S S R, Mohammad F. (2011) Magnetic
nanomaterials for hyperthermia-based therapy and
controlled drug delivery. Advanced drug delivery
reviews, 63(9): 789-808.
Lee N, Hyeon T. (2012) Designed synthesis of uniformly
sized iron oxide nanoparticles for efficient magnetic
resonance imaging contrast agents. Chemical Society
Reviews, 41(7): 2575-2589.
Lin W, Hu Q, Yu J, et al. (2016) Low cytotoxic metal-
organic frameworks as temperature-responsive drug
carriers. ChemPlusChem, 81(8): 804.
Ma Z, Moulton B. (2011) Recent advances of discrete
coordination complexes and coordination polymers in
drug delivery. Coordination Chemistry Reviews,
255(15-16): 1623-1641.
Neuberger T, Schöpf B, Hofmann H, et al. (2005)
Superparamagnetic nanoparticles for biomedical
applications: possibilities and limitations of a new drug
delivery system. Journal of Magnetism and Magnetic
materials, 293(1): 483-496.
Nagata S, Kokado K, Sada K. (2015) Metal-organic
framework tethering PNIPAM for ON-OFF controlled
release in solution. Chemical Communications, 51(41):
8614-8617.
Nagata S, Kokado K, Sada K. (2015) Metal-organic
framework tethering PNIPAM for ON0OFF controlled
release in solution. Chemical Communications, 51(41):
8614-8617.
Ogoshi T, Kanai S, Fujinami S, et al. (2008) para-Bridged
symmetrical pillar [5] arenes: their Lewis acid
catalyzed synthesis and host–guest property. Journal of
the American Chemical Society, 130(15): 5022-5023.
Strutt N L, Zhang H, Schneebeli S T, et al. (2014)
Functionalizing pillar [n] arenes. Accounts of chemical
research, 47(8): 2631-2642.
Si W, Xin P, Li Z T, et al. (2015) Tubular unimolecular
transmembrane channels: construction strategy and
transport activities. Accounts of chemical research,
48(6): 1612-1619.
Tan L L, Song N, Zhang S X A, et al. (2016) Ca
2+
, pH and
thermo triple-responsive mechanized Zr-based MOFs
for on-command drug release in bone diseases. Journal
of Materials Chemistry B, 4(1): 135-140.
Wu Y, Zhou M, Li S, et al. (2014) Magnetic metal-organic
frameworks: γ‐Fe2O3@MOFs via confined in situ
pyrolysis method for drug delivery. Small, 10(14):
2927-2936.
Wu M X, Yang Y W. (2017) Metal-organic framework
(MOF)‐based drug/cargo delivery and cancer therapy.
Advanced Materials, 29(23): 1606134.
Zhang H, Zhao Y. (2013) Pillararene‐based assemblies:
Design principle, preparation and applications.
Chemistry-A European Journal, 19(50): 16862-16879.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
460
