
Research Status of Carrier-free Nano Antitumor Drugs:
The Mechanism of Action and Future Trends of Four Carrier-free
Nanomedicines
Dongjun Zuo
a
Northwest University, Xi’an, Shanxi, China
Keywords: Anticancer Drugs, Nano Anticancer Drugs, Self-Assembled Systems.
Abstract: At present, there are four mainstream carrier-free nano anti-tumor drugs. The prodrug self-delivery system
uses self-assembled targeted drugs constructed with small groups and anti-cancer drugs. A pure drug
delivery system that uses two or more pure drugs to construct self-assembled drug-drug conjugates. Based
on the self-delivery system of therapeutic carriers, a self-assembled anticancer drug is constructed using
carriers with auxiliary therapeutic effects. Based on the self-delivery system of non-toxic agents, non-toxic
groups are used to assist anticancer drugs to function. Carrier-free nano anticancer drugs solve the side
effects of traditional nano anticancer drugs that nanocarriers cannot be metabolized by the body, and have
broad research prospects.
1 INTRODUCTION
1
Cancer is the second most harmful disease to human
health. The annual death toll from cancer is second
only to cardiovascular and cerebrovascular diseases.
There are more than 14 million new cancer cases
worldwide each year. At present, humans have made
very impressive research results in cancer. In fact,
all cancers can be cured if they can be detected in
time and treated correctly. The current mainstream
treatment methods include: (1) surgical resection;
(2) use of chemotherapy or other cancer-specific
drugs; (3) use of radiotherapy; (4) immunotherapy;
(5) gene therapy; (6) small molecule targets to
medication (Roy 2016). Chemotherapy is currently a
widely used treatment method. However,
chemotherapy drugs act on the cells of the whole
body and cause serious side effects. Therefore,
precise and efficient drug delivery systems must be
developed. The drug delivery system must solve the
following problems: (1) PK parameters, especially
half-life, biodistribution and maximum drug
concentration; (2) toxic and side effects; (3) target
fixation at the location of the lesion (Vargason
2021).
a
https://orcid.org/0000-0002-6094-8851
2 THE DEVELOPMENT OF
NANO ANTI-TUMOR DRUGS
With the development of nanotechnology, huge
innovations have taken place in drug delivery
systems. Researchers have developed numerous
nano-carrier drug delivery systems based on
nanotechnology; liposomes; nanopolymers;
dendrimers; micelles, etc. They have different
molecular targets, sizes and surface properties.
Nanomaterials as carriers have many advantages: (1)
increase water solubility and increase the
concentration of drugs in the blood; (2) accurately
target organs, tissues or cells to prevent drug toxicity
from accumulating in other organs such as the liver;
(3) can Combine imaging technology to monitor
drug effects in real time. The use of nanomaterials as
drug carriers is undoubtedly an effective solution to
the serious side effects of traditional
chemotherapeutic macromolecular drugs (Li 2017).
434
Zuo, D.
Research Status of Carrier-free Nano Antitumor Drugs: The Mechanism of Action and Future Trends of Four Carr ier-free Nanomedicines.
DOI: 10.5220/0011214100003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 434-439
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

3 ADVANTAGES AND
CONSTRUCTION OF
CARRIER-FREE
NANO-ANTITUMOR DRUGS
However, the shortcomings of nano-carrier drugs are
also obvious and inevitable. Their drug loading is
low, and most of the carrier systems cannot be
metabolized by the body, prone to inflammation at
the lesion site, complex synthesis operations, and
high cost. Therefore, some researchers have
proposed the concept of carrier-free nano anti-tumor
drugs. "Carrier-free nanomedicine" mainly refers to
a system that does not use additional carriers during
the administration process. Some researchers also
named the carrier-free nanomedicine "carrier-free
drug delivery system". According to the different
construction methods, carrier-free nanomedicine is
divided into several types: prodrug self-delivery,
pure drug self-delivery, self-delivery based on
therapeutic carrier, self-delivery system based on
non-toxic agent (Zhang 2018). Carrier-free pure
nanospheres (PND) composed of pure medicinal
active molecules are currently considered to be the
field with the most research potential. Nanoparticles
are composed of two or more drugs, and the
treatment efficiency can be doubled (Zhao 2015).
This article summarizes, analyzes and summarizes
several most significant carrier-free nano anti-tumor
drugs in the research field of carrier-free nano anti-
tumor drugs.
3.1 Prodrug Self-delivery System
The prodrug self-delivery system, that is, the active
drug and the small group are connected through a
cleavable bond that is responsive to the internal
environment of the tumor, and self-assembled to
form a nanostructure to achieve a self-delivery
modality. Based on the assembly principle of
carrier-free nanomedicine, that is, the use of
amphiphilic hydrophobic drugs to assemble into
drug-drug conjugates in water (Wang 2012). The
construction principle of this drug-drug conjugate is
generally to use the hydrophobicity or hydrophilicity
of the precursor for synthesis, and the formed
prodrug self-delivery system can protect the drug
from rapid clearance and inhibit premature burst
release. However, the conditions of prodrug design
and self-assembly, such as pH, concentration, ionic
strength, and kinetics, also affect the eventual
spontaneous delivery. Small molecule activity
modification drugs can be used to form amphiphilic
self-assembled drugs and complete drug self-
delivery. Because traditional nano-carrier drugs use
high-molecular-weight nano-carriers, nano-carrier
drugs usually have very low drug loading levels
(Chen 2015). In the prodrug delivery system, the
drug is added in a quantitative manner, so it has a
higher drug loading. A key feature of nanomedicine
is to release active drugs from the self-delivery
system and induce apoptosis of cancer cells after
intracellular delivery. Since cancer cells have
physical and chemical environments different from
normal cells, such as pH, redox potential, and
special enzymes and proteins, building sensitive
links such as acid, enzyme and redox sensitive bonds
can effectively improve the success rate of drug
release. Since many anti-cancer drugs are
hydrophobic and do not have the amphipathic
characteristics for self-assembly (Sun 2014).
Therefore, the hydrophilic group is indispensable for
the construction of amphiphilic prodrugs. Due to its
excellent biodegradability, biological activity,
adjustable amphiphilicity and sophisticated synthetic
methods, short peptides have become ideal
candidates for the preparation of amphiphilic
prodrugs. It is worth noting that prodrugs composed
of short peptides and active drugs have unique
advantages in terms of self-assembly potential and
drug-carrying ability. At present, some researchers
have designed a method to bind β-sheet peptide to
anti-cancer camptothecin (CPT), which is to self-
assemble this amphiphilic prodrug into a
nanostructure with a drug loading capacity of up to
38%. The formation of nanostructures can
simultaneously protect the hydrolyzable CPT and
the biodegradable linker from the external
environment. After reaching the tumor-related sites,
the nanomedicine breaks the reducible dithiobutyrate
linkage by hydrolysis to release CPT and induce
cancer cell apoptosis. Therefore, in vitro toxicity
studies have found that reduction-sensitive
nanomedicine exhibits greater cytotoxicity than
insensitive maleimide-linked nanomedicine
(Cheetham 2013).
3.2 Pure Drug Self-delivery System
The pure drug self-delivery system is composed of
pure drug self-polymerizing nano-medicine and
carries out intracellular transportation. The self-
delivery system is established based on a single drug
or multiple drugs, and is a true carrier-free system,
which is finally prepared into a nano-level drug in an
aqueous solution. According to the molecular self-
assembly/co-precipitation process, drugs can
Research Status of Carrier-free Nano Antitumor Drugs: The Mechanism of Action and Future Trends of Four Carrier-free Nanomedicines
435
become nano-objects with specific sizes and shapes
through self-aggregation. If all the inert excipients
are removed, the pure drug can form a nanostructure
through self-aggregation, which can reach the
optimal drug loading of 100%, which enhances the
anti-cancer activity of the drug and avoids the
relative toxicity and immunogenicity of the carrier.
In order to achieve this goal, a variety of free anti-
cancer drugs have been used to construct pure nano-
drugs, and great progress has been made in this field.
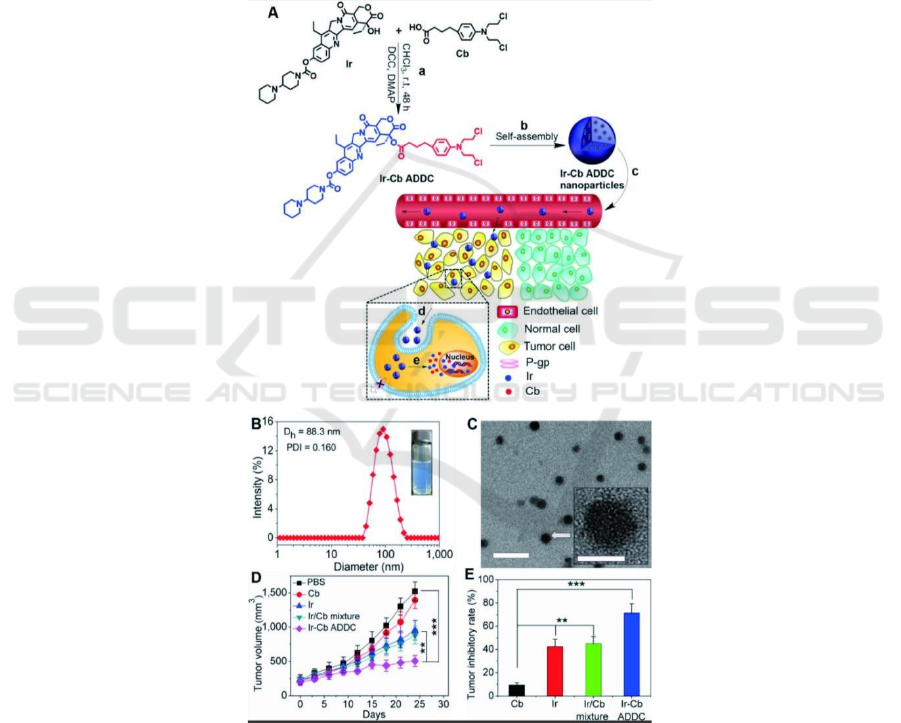
Some researchers have connected water-soluble
irinotecan (Ir) and water-insoluble chlorambucil
(Cb) through ester bonds that are easily hydrolyzed
and broken under acidic conditions, and designed an
amphiphilic drug-drug Conjugate (Huang 2014).
The study found that, compared with the free drug
alone, the Ir-Cb nanoparticles formed by the self-
assembly method of the Ir-Cb copolymer showed a
longer blood circulation half-life and higher tumor
accumulation. After self-delivery in cells, the ester
bonds of Ir-Cb nanoparticles will be hydrolyzed and
broken in the acidic environment of tumor cells, free
Ir and Cb drugs are easily released from Ir-Cb
nanoparticles, thus exerting a synergistic cell
toxicity.
Figure 1: Based on amphiphilic drug-drug conjugated IR-CB nanoparticles and their antitumor activity (Huang 2014).
3.3 Self-delivery System based on
Therapeutic Carrier
The self-delivery system based on the therapeutic
carrier is that both the carrier and the loaded drug
can be used as the therapeutic agent of the
combination therapy. The use of carrier-free
anticancer drugs is to obtain better therapeutic
effects. The idea of the above-mentioned drugs is to
improve the efficacy by reducing the ratio of the
carrier to the drug. Or another way of thinking can
be used, using a therapeutic vector. Such as the self-
assembled micellar nanocomposite of
epigallocatechin gallate (EGCG) derivatives and
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
436

protein drugs (Chung 2014). Among them, EGCG
derivative carriers can also show anti-cancer effects.
EGCG derivatives and anti-cancer proteins produce
stable micellar nanocomplexes through sequential
self-assembly, which show better anti-cancer effects
than free protein drugs in in vitro and in vivo
experiments, realizing the development of EGCG
derivative carriers and drugs Combination therapy.
DOX and EGCG can reach the tumor site at the
same time for treatment of resistance to liver cancer
(Liang 2010). Non-toxic doses of EGCG can
increase the sensitivity of chemotherapy-resistant
liver cancer cells to DOX by inhibiting the activity
of the P-glycoprotein (P-gp) efflux pump, thereby
enhancing the DOX-induced killing effect of liver
cancer cells. In the past few decades, some
compounds containing trace elements have been
proposed as therapeutic agents and therapeutic
nanocarriers, because they have a significant ability
to enhance the immune response of cancer cells and
produce anti-cancer metabolites, which can
effectively Interfere with cell metabolism and induce
cell apoptosis (Liu 2015). An example of this
compound in cancer treatment is a selenium-
containing reagent that regulates ROS in vivo to
induce apoptosis. Self-assembled selenium-
containing nanostructures can be used as self-
delivery therapeutics (Liu 2013). A research team
designed and synthesized an amphiphilic
hyperbranched selenium-containing polymer that
self-assembles into nanomicelles and is
automatically delivered to tumor tissues through the
EPR effect. After triggering the exclusive oxidizing
microenvironment in cancer cells, the nanomicelles
decompose, and the released selenium compounds
can effectively induce cancer cell apoptosis. They
further prepared a hyperbranched selenide
macromolecular anticancer drug, which can not only
achieve self-delivery based on its self-assembled
nanomicelles, but also can be used as a carrier
encapsulating hydrophobic DOX for combination
therapy. In order to reduce the cytotoxicity of
selenium-containing polymer anticancer drugs to
normal cells, the researchers introduced PEGylated
polymers to stabilize macromolecular anticancer
drugs and prevent them from being attacked by
proteins in the blood (Li 2015).
3.4 Self-delivery System based on
Non-toxic Agent
There are also some multifunctional local delivery
systems based on the participation of non-cytotoxic
drugs, which can achieve controlled aggregation
around tumors to induce cell apoptosis. This system
is called a non-toxic agent-based self-delivery
system. The anti-cancer effect of the self-delivery
system based on non-toxic agents is dependent on
non-toxic agents rather than conventional
chemotherapeutic drugs. Conventional
chemotherapeutic drugs, such as DOX and CPT,
inhibit the growth of cancer cells by embedding in
cell DNA and inducing cell death. The role of the
non-toxic unit is to show anti-cancer active cells
through self-aggregation and cause cytotoxicity.
Recently, a research team reported a matrix
metalloproteinase-7 (MMP-7) response precursor,
which transforms into a gel before being taken up by
cancer cells, and further enters the cells to form a
hydrogel (Tanaka 2016). The formation of the
hydrogel causes the pressure inside the cell to
increase, thus initiating cell death. Some researchers
have found that due to the dephosphorylation of D-
peptide derivatives by alkaline phosphatase, the
hydrogel/nanomesh will gather in the gaps of cancer
cells to form a self-assembled structure, and block
communication and mass exchange between cells
(Zhou 2016). Way to remove cancer cells. In
addition to low molecular weight precursors as
therapeutic agents, the use of non-cytotoxic
macromolecules has been extended to another
paradigm for cancer treatment. The mechanism of
macromolecular therapeutics in inducing cell
apoptosis is the special biological recognition
between cell surface receptors and natural or
synthetic binding motifs. Based on this theoretical
basis, a research team has developed a new
therapeutic platform mediated by extracellular
hybridization of two complementary nanoconjugates
to induce apoptosis in B-cell lymphoma cells, which
is further cross-linked B-cell lymphoma
overexpresses the CD20 antigen (Chu 2014). In
summary, these self-delivery systems show great
advantages for cancer treatment, but have less toxic
and side effects on healthy cells/tissues. In addition,
since the efflux effect of the efflux pump in drug-
resistant cells is one of the main mechanisms for the
emergence of MDR, and self-assembled
nanomedicine can bypass the efflux pump of cancer
cells due to its size effect, so nanomedicine is the
same for drug-resistant cancer cells. Can produce
higher curative effect, Due to the unprecedented
drug loading capacity, minimization of systemic
toxicity, flexible preparation strategy and the
nanometer size of passive targeted therapy, the
scientific research of carrier-free nanomedicine has
made great progress in recent years (Kunjachan
2013). However, we still know very little about
Research Status of Carrier-free Nano Antitumor Drugs: The Mechanism of Action and Future Trends of Four Carrier-free Nanomedicines
437

carrier-free nanomedicine. At present, many reports
point out that the physical and chemical properties
of nanomaterials, such as size, shape, surface
properties, are crucial in regulating their cellular
uptake and transport behavior in the body, and may
affect the overall cancer treatment effect (Williford
2015).
4 PREPARATION OF DOX
NANOPARTICLES
First dissolve DOX in DMSO, then add 1 mL of
triethylamine to 10 mL of 1 mg/mL
DOX.HCl/DMSO solution under moderate agitation
at 25°C, and react for about 4 hours After stopping,
the hydrophilic DOX.HCI is converted into
hydrophobic DOX. Three preparation methods of
DOX nanoparticles: (1) DOX 50 (particle size is
about 50 nm): Drop a 3mg/mL DOX/DMSO
solution into 5ml petroleum ether and stir for 5
minutes. (2) DOX100 (particle size about 100 nm):
Drop the DOX/DMSO solution with a concentration
of 3 mg/mL into 5 ml ultrapure water and stir for 5
minutes. (3) Dox 180 (particle size is about 180 nm):
Drop 1 mg/mL DOX/DMSO solution into 5ml
ultrapure water, and stir for five minutes.
5 CONCLUSION
Carrier-free nanomedicine solves the toxicity
problem of nano-carrier drug carrier system.
According to its construction principle, that is, to
complete the self-assembly process through
hydrophobic and hydrophilic groups, various
construction ideas can be developed. It is worth
mentioning that carrier-free nanomedicine provides
different application ideas for some poorly water-
soluble anticancer drugs. By constructing a carrier-
free nanomedicine system, its water solubility can be
greatly improved. The current frontier research on
carrier-free nano-oncology drugs believes that pure
drug carrier-free nano-oncology drugs have the most
research potential and have very broad prospects.
REFERENCES
Cheetham, A. G.; Zhang, P.; Lin, Y. et al. (2013).
Supramolecular nanostructures formed by anticancer
drug assembly. Journal of the American Chemical
Society, 135 (8), 2907-2910.
Chen, W.-H., Luo, G.-F., Lei, Q., et al. (2015). MMP-2
responsive polymeric micelles for cancer-targeted
intracellular drug delivery. Chemical
Communications, 51 (3), 465-468.
Chu, T.-W., Yang, J., Zhang, R., et al. (2014). Cell surface
self-assembly of hybrid nanoconjugates via
oligonucleotide hybridization induces apoptosis. ACS
Nano, 8 (1), 719-730.
Chung, J. E., Tan, S., Gao, S. J., et al. (2014). Self-
assembled micellar nanocomplexes comprising green
tea catechin derivatives and protein drugs for cancer
therapy. Nature Nanotechnology, 9 (11), 907-912.
Huang, P., Wang, D., Su, Y., et al. (2014). Combination of
small molecule prodrug and nanodrug delivery:
amphiphilic drug-drug conjugate for cancer therapy.
Journal of the American Chemical Society , 136 (33),
11748-11756.
Huang P, Wang D, Su Y, et al. (2014). Combination of
small molecule prodrug and nanodrug delivery:
amphiphilic drug-drug conjugate for cancer therapy. J
Am Chem Soc. ;136(33):11748-56. doi:
10.1021/ja505212y. Epub 2014 Aug 7. PMID:
25078892.
Kunjachan, S., Rychlik, B., Storm, G., et al. (2013).
Multidrug resistance: physiological principles and
nanomedical solutions. Advanced Drug Delivery
Reviews, 65 (13-14), 1852-1865.
Li, C., Huang, W., Zhou, L., et al. (2015). PEGylated
poly(diselenide-phosphate) nanogel as efficient self-
delivery nanomedicine for cancer therapy. Polymer
Chemistry, 6 (36), 6498-6508.
Li Z, Tan S, Li S, et al. (2017). Cancer drug delivery in the
nano era: An overview and perspectives (Review).
Oncol Rep. 38(2):611-624. doi:10.3892/or.2017.5718.
Liang, G., Tang, A., Lin, X., et al. (2010). Green tea
catechins augment the antitumor activity of
doxorubicin in an in vivomouse model for
chemoresistant liver cancer. International Journal of
Oncology, 37 (1), 111-123. Sredni, B.,
Immunomodulating tellurium compounds as anti-
cancer agents. Seminars in Cancer Biology 2012, 22
(1), 60-69.
Liu, J., Pang, Y., Zhu, Z., et al. (2013). Therapeutic
nanocarriers with hydrogen peroxide-triggered drug
release for cancer treatment. Biomacromolecules, 14
(5), 1627-1636.
Liu, J., Huang, W., Pang, Y., et al. (2015). Hyperbranched
polyphosphates: synthesis, functionalization and
biomedical applications. Chemical Society Reviews,
44 (12), 3942-3953.
Roy PS, Saikia BJ. (2016). Cancer and cure: A critical
analysis. Indian J Cancer. Jul-Sep;53(3):441-442. doi:
10.4103/0019-509X.200658. PMID: 28244479.
Sun, T., Zhang, Y. S., Pang, B., et al. (2014). Engineered
nanoparticles for drug delivery in cancer therapy.
Angewandte Chemie-International Edition, 53 (46),
12320-12364.
Tanaka, A., Fukuoka, Y., Morimoto, Y., et al. (2016)
Cancer cell death induced by the intracellular self-
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
438

assembly of an enzyme-responsive supramolecular
gelator. Journal of the American Chemical.
Vargason AM, Anselmo AC, Mitragotri S. (2021). The
evolution of commercial drug delivery technologies.
Nat Biomed Eng. doi: 10.1038/s41551-021-00698-w.
Epub ahead of print. PMID: 33795852.
Wang, C., Wang, Z., Zhang, X., (2012). Amphiphilic
building blocks for self-assembly: from amphiphiles to
supra-amphiphiles. Accounts of Chemical Research,
45 (4), 608-618.
Williford, J.-M., Santos, J. L., Shyam, R., et al. (2015).
Shape control in engineering of polymeric
nanoparticles for therapeutic delivery. Biomaterials
Science, 3 (7), 894-907.
Zhang, N., Li, M., Sun, X., et al. (2018). NIR-responsive
cancer cytomembrane-cloaked carrier-free
nanosystems for highly efficient and self-targeted
tumor drug delivery. Biomaterials, 159, 25-36.
Zhao Y, Chen F, Pan Y, et al. (2015). Nanodrug Formed
by Coassembly of Dual Anticancer Drugs to Inhibit
Cancer Cell Drug Resistance. ACS Appl Mater
Interfaces. 7(34):19295-305. doi:
10.1021/acsami.5b05347. Epub 2015 Aug 19. PMID:
26270258; PMCID: PMC4712650.
Zhou, J., Du, X., Yamagata, N., et al. (2016). Enzyme-
instructed self-assembly of small D-peptides as a
multiple-step process for selectively killing cancer
cells. Journal of the American Chemical Society, 138
(11), 3813-3823.
Research Status of Carrier-free Nano Antitumor Drugs: The Mechanism of Action and Future Trends of Four Carrier-free Nanomedicines
439
