
Artemisinin Derivatives: Anti-cancer Effects and Mechanisms
Yuwei He
1,† a
, Chang Liu
2,† b
and Linkun Zhang
3,† c
1
Department of pharmacoanalysis, Heilongjiang University of Chinese Medicine, Harbin, China
2
Department of chemistry, University of Waterloo, Waterloo, Canada
3
Department of biotechnology, Henan Normal University, Zhengzhou, China
†
These authors contributted equally
Keywords: Artemisinin Derivatives, Apoptosis, Oncosis, Ferroptosis, SM1044.
Abstract: Abstract-Artemisinin derivatives have been studied as anti-malaria drugs in many aspects, while were found
anti-cancer affects recently. However, the mechanisms of their anti-cancer effect remain unclear. Research
has showed that Artemisinin derivatives may affect the cell cycle of cancer cells through blocking G1 to S
phase, and subsequently reduce cell proliferation. Artemisinin derivatives may also cause oncosis and cancer
cell apoptosis by promoting Ferroptosis. A new water-soluble derivative, SM1044, was found its anti-cancer
ability through inducing apoptosis and blocking cell cycle. Future research is required to better understand
the potential mechanisms as well as to expand their clinical applications.
1 INTRODUCTION
Cancer is a serious hazard to human health and is
listed as one of the most important public health
problems in the world, which needs to be solved
urgently (Yang, 2020). The cancer epidemiology data
released by Bray et al. indicated a great burden caused
on society (Bray, 2018). There were 1,806,590 new
cancer cases and 606,520 deaths in 2020 in the United
States. It was estimated that there would be 28.4
million cancer cases worldwide in 2040 (Liu, 2021).
Artemisinin has been known as an effective anti-
malaria drug. This active ingredient is isolated from
the Chinese herbal medicine Artemisinin annua.
Artemisinin and its derivatives can usually be
extracted from plant species at the same time as a
mixture. They are sesquiterpene lactones with peroxy
bridges, which have strong anti-malarial effects.
Through the structural modification of artemisinin
scientists designed and synthesized new derivatives
of artemisinin. Obtained dihydroartemisinin (DHA)
and new derivatives with different substituents
introduced at each point of dihydroartemisinin.
Through research in recent years, artemisinin and
its derivatives have been confirmed to have anti-
a
https://orcid.org/0000-0003-4439-0429
b
https://orcid.org/0000-0002-0763-9670
c
https://orcid.org/0000-0002-1643-5127
cancer effects, though the mechanisms of their anti-
cancer effects remain unclear. Current researchers
found that the possible mechanism may include the
following aspects: (1) blocking the growth of cancer
cells by inhibiting the cell cycle, such as ART
inducing apoptosis of human retinoblastoma cells
(Yang, 2019); (2) causing cell death through oncosis;
(3) reacting with a huge number of ferrous ions in
cells which are cancerous. Importantly, during the
oncosis and ferroptosis in cancer cells, the oxidative
stress was caused by artemisinin derivatives through
producing of reactive oxygen species, which leads to
the expansion of intracellular organelles, the disorder
of the antioxidant mechanism in cancer cells, as well
as other factors which caused cell death. Though the
mechanisms of oncosis and Ferroptos remain unclear.
The anti-cancer mechanism of the newly synthesized
artemisinin derivative SM1044 and its advantages
over the anti-cancer mechanism of traditional
artemisinin derivatives is a new direction to
preparation and innovation of anticancer drugs.
He, Y., Liu, C. and Zhang, L.
Artemisinin Derivatives: Anti-cancer Effects and Mechanisms.
DOI: 10.5220/0011211500003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 385-390
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
385
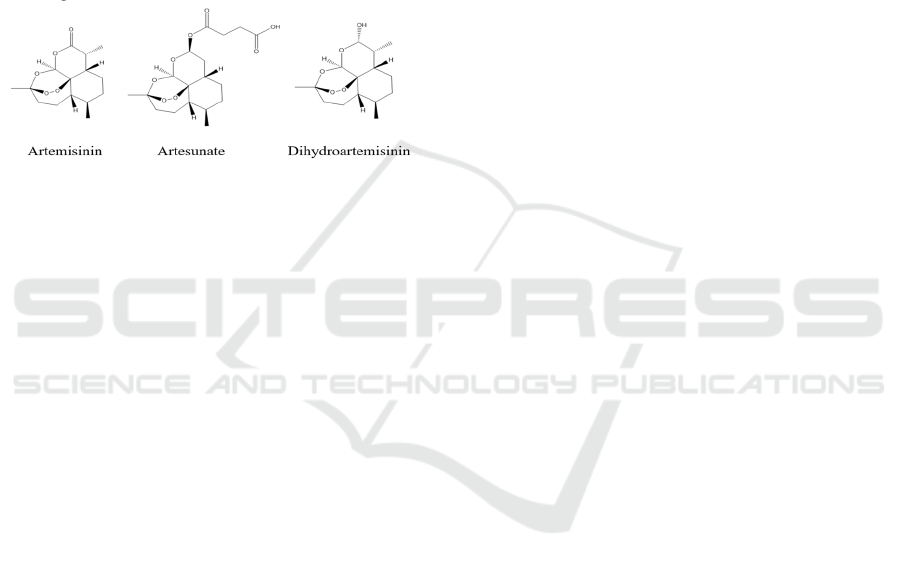
2 THE MECHANISM OF
ARTEMISININ DERIVATIVES
2.1 Properties of Artemisinin and Its
Derivatives
Artemisinin is a sesquiterpene lactone. There are
several names. Artemisinin, Arteannuin,
Artemisinine, Qinghaosu. It is chemically knows as
(1R,4S,5R,8S,9R,12S,13R)-1,5,9-trimethyl-
11,14,15,16-
tetraoxatetracyclo[10.3.1.04,13.08,13]hexadecan-10-
one (Fig.1).
Figure 1: Chemical structure diagram.
Artemisinin is colorless needle crystal and tastes
bitter, soluble in organic solvent. Though by
modifying the structure of the Artemisin such as
doubling hydrogen of artemisinin, in order to improve
the double hydrogen artemisinin molecules
chemically unstable hemiacetal structure, obtained
derivatives showed better antimalarial activity.
Further structure modification could get the
artemether, artemisia ether, artesunate (Fig.1) and
other dissolved solution and better compound
(Mercer, 2007).
The common feature of artemisinin compounds is
that they all have a peroxide bridge structure in their
components. Studies have shown that the peroxide
bridge is an essential structure for artemisinin to exert
its pharmacological activity, and its pharmacological
activity is significantly reduced after the loss of the
peroxide bridge structure (Mercer, 2007).
2.2 Affecting the Cell Cycle by
Blocking G1 to Enter S
Blocking proliferation of uncontrolled cell growth is
one of the important ways to inhibit tumorigenesis. In
the normal cells, cyclin will express special proteins
at specific periods and activate CDK to drive cells to
complete the cell cycle and proliferation. There are
two important stages: G1 to S and G2 to M. This
review focuses on the G1 to S, which rely on the
CyclinD1 combine with the CDK4 (Li, 2018).
CyclinD1 regulates the cell proliferation cycle by
binding and activating to CDK4 protein, which
promotes cell proliferation. Then the complexes
promote the phosphorylation of Rb protein,
promoting the cell cycle from G1 phase to S phase,
and finally accelerating the cell cycle, which
improves the cell proliferation. Researchers found
that high expression of CyclinD1 protein would
accelerated the cell cycle and rapid abnormal
proliferation (Vermeulen, 2003). Eventually, when
cancer cells were inhibited, the expression of
CyclinD1 and CDK4 would be significantly reduced
(Li, 2018). As a result, cell cycle would be negatively
regulated, and cell proliferation would be inhibited.
P16 plays a key role in regulating cell cycle
through preventing the CyclinD1 combining with the
CDK4 (Ding, 2019). In normal cells, the balance of
P16 and cyclinD1 maintains a stable state, which
keeps the cells in a relatively stable cell cycle. One
possible mechanism of conversion from normal cells
to cancer cells is losing control of the cell cycle
(Lutful Kabir, 2013).
DHA, as another derivative apart from
Artesunate, showed an inhibition effect on the
proliferation of cancer cells by blocking the process
from G1 to S phase, which reduced the DNA
synthesis and replication of cells in vitro (Gao, 2020,
Caglar, 2020) (Tab.1). Through determination of
intracellular protein levels, it was found that cyclinD1
and P16 play an important role in the regulation of
cancer cell cycle. With the increase of DHA
concentration, cyclinD1 protein expression was
gradually down-regulated, while P16 protein up-
regulated (Caglar 2020). DHA reduced the combining
of cyclinD1 to CDK4 and up-regulated P16 protein,
thus achieving cell cycle arrested from G1 to S phase
and inducing apoptosis of cancer cells. Importantly,
normal cells also have a complete cell cycle which
may also be one of the targets of anti-cancer drugs
together with cancer cells. Researchers found that
compared with traditional anticancer drugs, DHA has
the advantage of specific selection for cancer cells
(Caglar, 2020). Future research may focus on
explanation of the specific expression of DHA in
cancer cells. If the cancer specificity of DHA can be
combined with molecular targeting technology to find
the specific antigen sites of cancer cells, it might be
able to achieve a breakthrough in the synthesis of
anticancer drugs.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
386

Table 1: Effect to different cell models within Artemisinin derivatives.
Cell line Dru
g
Effect Ref.
BGC-823 DHA
Inhibit cell growth.
Induction of apoptosis.
Make cell c
y
cle sta
y
in G1.
(Gao 2020)
Panc-1 ART
Mitochondria with decreased matrix
density and swelling of
cristae, cells severely damaged.
(Du 2010)
BJeLR and DRD siGPX4 Cell death (Yang 2014)
BJeH and BJeHLT siGPX4 No cell death (Yang 2014)
HL60 SM1044
Inhibited the proliferation, promoted the
apoptosis of HL60 cells
(Yu 2013)
Kasumi-1 SM1044
Change cell cycle apoptosis-related
protein to inhibit the proliferation of
Kasumi-1 cells
(Liu 2011)
SU-DHL-4
(DLBCL)
SM1044
Induced he apoptosis of SU-DHL-4cells
in a dose-dependent manner up-regulated
the expression of caspase 3 nd PARP
fragments
(Yu 2014)
2.3 Oncosis
The effect of artemisinin derivatives on cancer cells
can lead to cell oncosis (Weerasinghe, 2012). Oncosis
originated in the Greek word “onkos” which means
swelling. Oncosis and apoptosis have obvious
morphological differences. Apoptosis is a process in
which cells actively die through gene regulation. The
main manifestations of apoptosis are cell atrophy and
nuclear fragmentation. Oncosis is passive cell death
caused by some factors such as the physical and
chemical properties of drugs or other chemical
reagents or the environment (Leppo, 2003). The main
manifestations of oncosis are the expansion of cells
and organelles, the increase of cell membrane
permeability and the dissolution of cell nuclei
(Majno,
1995).
It was exerted that artemisinin derivatives caused
mitochondrial dysfunction when acting on cancer
cells, leading to the expansion of the intracellular
mitochondrial plasma reticulum (Du, 2010). In
addition, the cells undergoing oncosis did not appear
to undergo apoptosis after the Hoechst dye entering
the cells.
The mechanisms of oncosis induced by
artemisinin derivatives remain unclear. Du et al.
found that on the death of pancreatic cancer cells
induced by artesunate, artemisinin derivatives
induced changes in mitochondrial membrane
potential (MMP, or Δψm) and reactive oxygen
species mediated cell death in pancreatic cancer cells
(Du, 2010) (Tab.1).
The change of mitochondrial membrane potential
is one of the most important signs of cell
physiological state (Guan, 2018). Through the
detection of mitochondrial membrane potential, it can
be roughly inferred whether cell homeostasis is
disrupted, etc. (Xiao, 2020). Thus, the change of
MMP may be a manifestation of cell oncosis. The
excessive production of ROS (reaction oxygen
species) leads to oxidative stress and damage to
intracellular molecules and organelles.
Overall, oncosis, as a different pathway of cell
death from apoptosis, could be induced by partial
derivatives of artemisinin, which might be a new and
effective anti-cancer approach for cancer cells with
defective apoptotic pathways. Futher research is
required to better understand the mechanisms of
oncosis induced by artemisinin derivatives and
potential of artemisin derivatives as anti-cancer
drugs.
2.4 Ferroptosis
Ferroptosis is an iron-dependent way of inducing
apoptosis by attacking the cell's antioxidant system,
which was named by Dr. Brent R. Stockwell (Dixon,
2012). Different from other types of apoptosis such as
necroptosis, autophagic cell death and apoptosis,
normal apoptosis inhibitors are ineffective against
ferroptosis, but iron chelator can inhibit the
production of ferroptosis (Dixon, 2012). These facts
indicated that ferroptosis refers to an iron-dependent,
non-apoptotic cell death (Imai, 2017). Here are some
of the mechanisms of ferroptosis.
Artemisinin Derivatives: Anti-cancer Effects and Mechanisms
387

Cancer cells are usually over proliferating with
high metabolization level and oxygen consumption,
which requires more haemoglobin. In turn, with
overcrowded haemoglobin, excessive Fe2+ can
produce ferroptosis in cells through Fenton reaction.
The Fenton reaction means that Fe2+ reacts with the
peroxy group (the -OOH) in DHA to produce OH•,
which belongs to Reactive oxygen species (ROS).
Normal doses of ROS are metabolized by cells
through a series of reactions (such as peroxide can be
degraded by CAT into water and oxygen) while
excess ROS can overwhelm the antioxidant system in
cells, leading to cell death.
GSH plays a crucial role in the antioxidant system
of the body through specifically combining with ROS
and eliminating the toxicity of ROS to cells. The
expression of GSH is regulated by SLC7A11/XcT,
GR and GPX4 (Yang, 2014) (Tab.1). SLC7A11/XcT
can transport Cystine into cells to provide raw
material for GSH synthesis. GR provides hydrogen
for converting GSSG to GSH. GPX4 not only
eliminates peroxide, but also converts excess GSH
into GSSG for storage. Although there are so many
mechanisms in the cell to promote GSH production,
the rate of any chemical reaction has an upper limit.
When the ROS production rate exceeds the GSH
clearance rate, the cell would be enriched in ROS and
ROS will attack the cell, leading to cell death.
It was found that intracellular ROS levels of
SMMC⁃7721 and Huh⁃7, of which the genomic
characteristics are very sensitive to the number of
passages and detecting gene expression is a
convenient and inexpensive way, increased 2.6 times
and 2.1 times respectively at DHA 35 μmol/L, and
lipid peroxides increased 2.3 times and 1.7 times(Li
2019). In the SMMC⁃7721 cells GSH decreased by
59% and GPX4 decreased by 81.3% at DHA 35
μmol/L (Li, 2019).
These findings suggested that the inhibitory effect
of DHA on the proliferation and anticancer
mechanism of Hepatocellular Carcinoma (HCC) cells
may not only be limited to cell apoptosis, but also iron
death plays an important role in the anti-HCC
mechanism of DHA. However, the interaction
between iron death and apoptosis in inhibiting
hepatocellular carcinoma cell activity and its
mechanisms require to be further studied.
Figure 2: Mechanisms of anti-cancer effect of DHA.
2.5 SM1044
In recent years, artemisinin and its derivatives have
been making great contributions to drug research.
Fat-soluble drugs have been proved to be very
inefficient in drug absorption in experiments, and
their efficacy is usually not fully utilized. Among the
many artemisin derivatives, SM1044 is a newly
developed water-soluble artemisinin derivative. It is
superior to fat-soluble drugs in blocking cell growth
cycle, inducing cell apoptosis and other therapeutic
regimens.
Another study of the effects of artemisinin
derivative SM1044 is on acute myeloid leukemia cell
line HL60 to analyse the anti-cancer effects of
SM1044 on AML and related mechanisms. It was
found that SM1044 inhibited the proliferation of
HL60 cells; promoted the apoptosis of HL60 cells
with an increase of the percentage of apoptosis
population with a dose-dependent manner. There are
two main pathways of apoptosis: external pathway
and internal pathway respectively. The external
pathway is the activation of death receptors by
extracellular signals, which causes the self-activation
of apoptosis promoter caspase -8. The internal
pathway is that when it is activated, mitochondrial
transmembrane potential is lost, then releasing
cytochrome C to stimulate the self-activation of the
apoptotic promoter cystein-9. However, after the
activated caspase 8 and caspase 9 react each other and
stimulate the production of caspase 3, which in turn
degrades important proteins including PARP leading
to apoptosis. Therefore, apoptosis may occur when
both cysteinase-8 and cysteinase-9 are activated and
mitochondrial transmembrane potential is lost after
the action of SM1044 (Yu, 2013). Hence, the effect
of SM1044 on the expression of apoptosis-related
proteins was similar as that of SM1044 on kasumi-1
cells and effect of SM1044 on mitochondrial
transmembrane potential of HL60 cells was the same
as that of SM1044 on transmembrane expression of
SU-DHL-4 cells. Therefore, the in vitro studies
showed an anti-AML effect of the SM1044, though
in vivo studies and potentially clinical studies are
required to better understanding the mechanisms and
its therapeutic effects (Yu, 2013) (Tab.1).
Kasumi-1 cells were used as the model to test the
effect of SM1044, and the results showed that
SM1044 could inhibit the proliferation of Kasumi-1
cells through alter in cell cycle and apoptosis-related
proteins. The main possible mechanism is that with
an increasing concentration of SM1044 (μ24=0.92,
μ48 =0.98, μ72 =0.97, p˂0.05) an increasing portion
of Kasumi-1 cells were blocked in the G0/G1 phase,
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
388

which in turn inhibited the proliferation of Kasumi-1
cells with a time- and dose-dependent manner,
stopped the cell growth, and thus induced cell
apoptosis. Second, L Caspase inhibitor was added in
the experiment of co-incubation with different
concentrations of SM1044 and cells for 24 hours, and
it was found that Caspase was the main mediating
mode leading to cell apoptosis. Finally, examination
the expression of apoptosis-related proteins showed
that SM1044 could induce apoptosis by activating
apoptosis-related proteins, including associated
protein Caspase 3, PARP and the fusion protein
AML1-ETO (Liu, 2011) (Tab.1).
NHL is a hematologic malignancy that causes
more than 60,000 new cases in the United States each
year, with Diffuse Large B-cell lymphoma (DLBCL)
being the most common, so the development of new
drugs to combat this disease has become a priority
(Cultrera, 2012). In 2006, artemisinin-based ACTs
were found to have a 95% cure rate against
plasmodium falciparum malaria (Njuguna, 2012). In
the study of DLBCL cell line SU-DHL-4 treated with
SM1044, it was found that SM1044 induced the
apoptosis of SU-DHL-4 cells in a dose-dependent
manner, with a possible mechanism through up-
regulated the expression of caspase 3 and PARP
fragments which were related to apoptosis. It was
known that artemisinin may directly act on membrane
structures such as the endoplasmic reticulum (ER)
which plays an important role in the endogenous
apoptosis pathway. Therefore, future studies may
focus on endoplasmic reticulum stress and the
mechanism of SM1044 inducing SU-DHL-4 cell
apoptosis. Thus, according to the recent study,
SM1044 can induce the expression of ER stress-
related genes and proteins in SU-DHL-4 cells.
Artemisinin exerted their anti-malaria effect by
targeting the calcium dependent ATPase PfATP6 in
plasmodium/sarcoplasmic reticulum, thus greatly
increasing the calcium ion level in parasites and
leading to the death of plasmodium parasites (Yu,
2014). The role of calcium ion SM1044 in inducing
endoplasmic reticulum stress and apoptosis of SU-
DHL-4 cells was determined. Hence, SM1044
induced apoptosis of SU-DHL-4 cells may be a
complex process involving multiple mechanisms.
Due to the greater safety of SM1044 and the strong
water solubility of other artemisinin derivatives,
SM1044 might be a promising new anti-cancer drug
(Yu, 2014) (Tab.1).
Until now, most studies have concluded that the
mechanism of artemisinin-induced apoptosis is
similar to its antimalarial mechanism, that is, the
reaction between the internal peroxide group and iron
ions produces free radicals or electron-philic
intermediates, which increases the intracellular
reactive oxygen species level and then activates the
upstream apoptotic signalling pathway (Mercer,
2007). Follow-up work may continue to investigate
the mechanisms of anti-cancer effects of SM1044 as
well as its potential clinical application.
3 CONCLUSIONS
In conclusion, the results of different studies and
experiments indicate that uncontrolled cell growth is
known to cause cancer and many complications in
vivo and inducing apoptosis is the primary treatment
for blocking the overgrowth of cancer cells. Firstly,
in terms of the stages of cell division, the G1 to S
stages play a key role in regulating proteins that block
cancer cell growth which SM1044 could induce
Kasumi-1 cell cycle and block Kasumi-1 cells at
G0/G1 phase, and at the same time, S phase cells
decreased significantly. Secondly, through
antimalarial mechanisms, the Fenton reaction
between the peroxide bridge and iron destroys cancer
cells and blocks cell growth. Finally, among
artemisinin derivatives, water-soluble derivatives are
more efficient in absorption and function than fat-
soluble derivatives.
REFERENCES
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre,
L. A., Jemal, A. (2018) Global cancer statistics 2018:
Globocan estimates of incidence and mortality
worldwide for 36 cancers in 185 countries. CA Cancer
J Clin, 68(6),394-424.
Caglar, H. O., Biray Avci, C. (2020) Alterations of cell
cycle genes in cancer: Unmasking the role of cancer
stem cells. Mol Biol Rep, 47(4),3065-3076.
Cultrera, J.L, Dalia, S.M. (2012) Diffuse large B-cell
lymphoma: current strategies and future directions.
Cancer Control, 19(3),204-213.
Ding, C., Wei, R., Rodríguez, R. A., Del Mar Requena
Mullor, M. (2019) LncRNA PCAT-1 plays an
oncogenic role in epithelial ovarian cancer by
modulating cyclinD1/CDK4 expression. International
journal of clinical and experimental pathology, 12(6),
2148-2156.
Dixon, S.J., K.M. Lemberg, M.R. Lamprecht, R. Skouta,
E.M. Zaitsev, C.E. Gleason, D.N. Patel, A.J. Bauer,
A.M. Cantley, W.S. Yang, B. Morrison, 3rd, et al.
(2012) Ferroptosis: An iron-dependent form of
nonapoptotic cell death. Cell, 149(5),1060-72.
Du, J.H., Zhang, H.D., Ma, Z.J., Ji, K.M. (2010) Artesunate
induces oncosis-like cell death in vitro and has
Artemisinin Derivatives: Anti-cancer Effects and Mechanisms
389

antitumor activity against pancreatic cancer xenografts
in vivo. Cancer Chemother Pharmacol, 65(5),895-902.
Gao C., M.J. Ma, Z.G. He, Y.H. Feng, and B.Y. Xie. (2020)
Effects of dihydroartemisinin on the expression of
cyclin D1 P16 protein in gastric cancer cell line PGC-
823 in vitro. Chinese Journal of Medicine and Clinical
Medicine, 20(04),508-511.
Guan, R., Y. Chen, L. Zeng, T.W. Rees, C. Jin, J. Huang,
Z.S. Chen, L. Ji, and H. Chao. (2018) Oncosis-inducing
cyclometalated iridium(iii) complexes. Chem Sci,
9(23),5183-5190.
Imai, H., M. Matsuoka, T. Kumagai, T. Sakamoto,
Koumura, T.. (2017) Lipid peroxidation-dependent cell
death regulated by gpx4 and ferroptosis. Curr Top
Microbiol Immunol, 403,143-170.
Leppo J. (2003) Imaging cell injury and death. Curr Cardiol
Rep. Jan;5(1):40-4.
Li, S., Y.M. Ma, P.S. Zheng, and P. Zhang. (2018) Gdf15
promotes the proliferation of cervical cancer cells by
phosphorylating akt1 and erk1/2 through the receptor
erbb2. J Exp Clin Cancer Res, 37(1),80.
Li, Y.C., Y. Zhou, X. Wang, X. Wang, S.F. Chen, Y. Wang,
and J. Du. (2019) Dihydroartemisinin inhibits
hepatocarcinoma cell growth by inducing iron death.
Chinese Journal of Biochemistry and Molecular Biol,
35(12),1361-1366.
Liu, J.J, Fei, A.M., Nie, R.M.,Wang, J., Li, Y., Wang, Z.Y.,
Mi, J.Q. (2011) Apoptosis of Kasumi-1 cells induced
by artemisinin derivative SM1044 and its mechanism.
Journal of Experimental Hematology, 19(3),607-611.
Liu, Z.C., Li, Z.X., Zhang, Y., Zhou, T., Zhang, J.Y., You,
W.Y., Pan, K.F., Li, W.Q. (2021) Interpretation on the
report of Global Cancer Statistics 2020. Journal of
Multidisciplinary Cancer Management (Electronic
Version), 7(02),1-14.
Lutful Kabir, F. M., Agarwal, P., Deinnocentes, P., Zaman,
J., Bird, A. C., Bird, R. C. (2013) Novel frameshift
mutation in the p16/ink4a tumor suppressor gene in
canine breast cancer alters expression from the
p16/ink4a/p14arf locus. J Cell Biochem, 114(1),56-66.
Majno G, Joris I. (1995) Apoptosis, oncosis, and necrosis.
An overview of cell death. Am J Pathol.146(1),3-15.
Mercer, A. E., Maggs, J. L., Sun, X. M., Cohen, G. M.,
Chadwick, J., O'Neill, P. M., Park, B. K. (2007)
Evidence for the involvement of carbon-centered
radicals in the induction of apoptotic cell death by
artemisinin compounds. J Biol Chem, 282(13),9372-
9382.
Njuguna, N.M., Ongarora, D.S., Chibale, K. (2012)
Artemisinin derivatives: a patent review (2006-
present). Expert Opin Ther Pat, 22(10), 1179-1203.
Vermeulen, K., Van Bockstaele, D. R., Berneman, Z. N
(2003) The cell cycle: a review of regulation,
deregulation and therapeutic targets in cancer. Cell
proliferation, 36(3),131-149.
Weerasinghe, P. Buja, L.M. (2012) Oncosis: An important
non-apoptotic mode of cell death. Exp Mol Pathol,
93(3),302-8.
Xiao, C., Chen, S.J., Wei, D.H., Wang, C.Y. (2020)
Detection method of mitochondrial function. Chemistry
of Life. 40(02),222-229.
Yang, J., He, Y., Li, Y., Zhang, X., Wong, Y. K., Shen, S.,
Zhong, T., Zhang, J., Liu, Q., Wang, J. (2020)
Advances in the research on the targets of anti-malaria
actions of artemisinin. Pharmacol Ther, 216,107697.
Yang, W.S., R. SriRamaratnam, M.E. Welsch, K. Shimada,
R. Skouta, V.S. Viswanathan, J.H. Cheah, P.A.
Clemons, A.F. Shamji, C.B. Clish, L.M. Brown, et al.
(2014) Regulation of ferroptotic cancer cell death by
gpx4. Cell, 156(1-2),317-331.
Yang, Y., N. Wu, Y. Wu, H. Chen, J. Qiu, X. Qian, J. Zeng,
K. Chiu, Q. Gao, and J. Zhuang. (2019) Artesunate
induces mitochondria-mediated apoptosis of human
retinoblastoma cells by upregulating kruppel-like factor
6. Cell Death Dis, 10(11),862.
Yu, P., Song, Z., Zhao, L., Wang, Y., Wang, J., Li, Y., Mi,
J. (2013) Effects of artemisinin derivative SM1044 on
acute myeloid leukemia cell line HL60. Journal of
Internal Medicine Concepts & Practice, 8(3),187-191.
Yu, P., Song, Z., Zhao, L., Wang, Y., Wang, J., Li, Y., Mi,
J., (2014) Study on the mechanism of artemisinin
derivative SM1044 inducing apoptosis of diffuse large
B-cell lymphoma cell line SU-DHL-4. Journal of
Internal Medicine Concepts & Practice, 9(4), 274-279.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
390
