
PiRNAs Involved in the Memory Formation of Fear-conditioning
Tests Migrating from the Brain to the Germline
Lin Li
Bromsgrove School, Birmingham, U.K.
Keywords: Pirna, Odor Fear-Conditioning, Olfactory, Glomerulus, Learning Memory.
Abstract: Small piRNAs regulate and manipulate gene expressions and are important for forming memories. These
piRNAs are thought to be germline-specific and can help offspring inherit memories from their parents. In
this paper, we conducted odor fear-conditioning tests to identify a piRNA that increased in abundance and is
involved in the memory formation of the fear-conditioning test to determine how the offspring can inherit
memory. A mutant piRNA is created using a virus vector and introduced into the mice brains to see if it can
migrate from the brain to the germline. If the the mutant piRNA is found in the sperm cells, then we know
that the piRNAs can migrate from the brain to the sperm cells and thus inherit the memory of the odor used
in the odor fear conditioning test.
1 INTRODUCTION
In a recent study, Moore et al. reported that C.elegans
worms, upon exposure to PA14 (Moore, 2019), can
transmit avoidance memory to their offspring for
several generations. This transgenerational memory
can provide their offspring with advantageous
mechanisms to increase their chances of survival.
Besides, the study has helped us think of a possible
way how memories can be transferred from parent
animals to their offspring i.e., via piRNAs.
piRNAs are a class of 26-28 nucleotide small non-
coding RNAs and are associated with piwi proteins to
regulate gene expression and form memories
(Rajasethupathy, 2012). In Kandel’s study, it was
identified that piRNAs increased in amount in the
neurons of the Aplysia adult brain and thus amplified
its sensitivity to serotonin by inhibiting the
transcription of CREB2. Furthermore, the offspring
of the Aplysia were tested to see if the neurons can
also display long-term potentiation (LTP) from
serotonin stimulus, and was found that the offspring
will also display LTP. However, it is still unclear how
this transgenerational memory is achieved.
In this paper, we are going to create a mutant
piRNA to the piRNA that is involved in the memory
formation of odor-fear conditioning in the olfactory
bulb and see if it can be detected in the germline. If it
is detected and the progeny has the memory, then we
can suggest that transgenerational memory is
achieved via piRNAs.
We hypothesize that if the specific piRNA
associated with the fear-conditioning neuronal circuit
changes in abundance in the olfactory bulb, the sperm
cells would be able to get these piRNAs that migrated
from the brain region and thus inherit the memory of
the odor used in the odor fear conditioning.
2 MATERIALS AND METHODS
2.1 Odor-Fear Conditioning Tests
All mice used in the experiments are M71-GFP mice
strains. These mice are transgenic and have
fluorescent neurons when M71 receptors sense
acetophenone in the olfactory bulb. In this way, we
can observe changes and take out these specific
neurons more easily. The adult mice were kept in
standard cages with a 12-hour light/dark cycle with
free access to food and water.
The experiment utilized 2 different odorants to see
if the mice can discriminate between them: the first
odorant consisted of 10% acetophenone and the
control odorant consisted of 10% propanol in
propylene glycol.
Before the fear-conditioning tests, the mice were
given 3 weeks to habituate to the startle chambers to
ensure a strong odor-shock association. They
Li, L.
PiRNAs Involved in the Memory Formation of Fear-conditioning Tests Migrating from the Brain to the Germline.
DOI: 10.5220/0011208000003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 361-365
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
361
received 2 training sessions per week and each
training session consisted of 5 trials of 10 s odor
conditioned stimulus followed by a 0.24 s, 0.4 mA
electric footshock. The trials were separated by a 120
s time gap (Jones, 2008).
When the solenoid switch is closed, clean air can
flow freely with no difference in airflow. Backflow
of the air is prevented by several one-way valves
(yellows arrows). To remove the odor an exhaust
hose (green arrow) is utilized. The electric shock is
generated by a machine-driven animal shocker and
delivered through electric bars installed in the cage
floor. During the behavioral fear-conditioning
testing, the startle is recorded by a 105dB white noise
outburst, and the activity and startle amplitude are
measured by a piezoelectronic device underneath the
floor of the cage. (Jones, 2008)
The first experiment consisted of 6 mice with an
acetophenone stimulus + electric footshock. The
second experiment consisted of 6 control mice and
was given 10 s of acetophenone stimulus only. This
control experiment was carried out to see if any
changes in the neurons and glomeruli sizes were
found without given footshocks. The third
experiment also consisted of 6 control mice and was
given 10 s of propanol odor + electric footshock to
see if whether other odors can also be detected by the
M71 receptors.
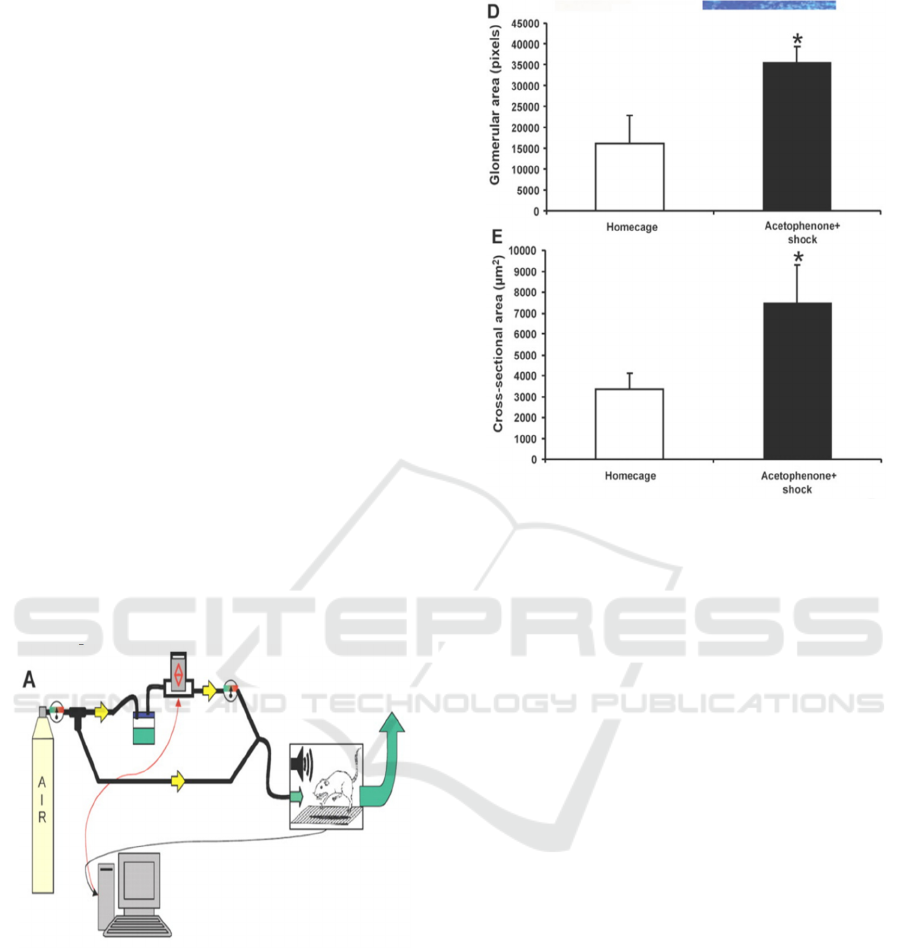
Figure 1: The flow of the air through the odorant jar and
into the startle chamber is controlled using a SR-Lab
Response Software which uses a solenoid switch (red
arrows) to control the flow of compressed air.
Figure 2: The glomerular surface area and cross-sectional
area were larger in the acetophenone + shock trained group
than in the homecage group (p < 0/05) (Jones, 2008).
2.2
Sequencing and Identification of
piRNA
First, the glomeruli with acetophenone-sensitive
neurons are isolated. These should be easy to identify
as the neurons with the M71 receptors will fluoresce
in the presence of acetophenone. After obtaining the
RNA clusters a reverse transcriptase enzyme is used
to form cDNAs of the RNAs. These cDNAs are then
transferred into an Illumina high-throughput
sequencing machine to be sequenced. Following
having sequenced the cDNAs, we can identify the 26-
28 nucleotide long piRNAs.
This experiment needs to be done before and after
the fear-conditioning test. The second experimental
group of the fear-conditioning test (the group of mice
with exposure to acetophenone only) can be used to
find the initial amount of piRNA in the brain and the
first experimental group of the fear conditioning test
can be used to find the piRNA that is involved in the
formation of acetophenone memory. By using RNA
sequencing, the machine will be able to show which
piRNA has increased in an amount most, thus we can
find which piRNA was associated with the test. The
piRNA identified with the most significant increase
in abundance is then called piRNA-X.
As shown in figure 3 the glomelum should have
an increased M71 axon density and glomerular size
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
362
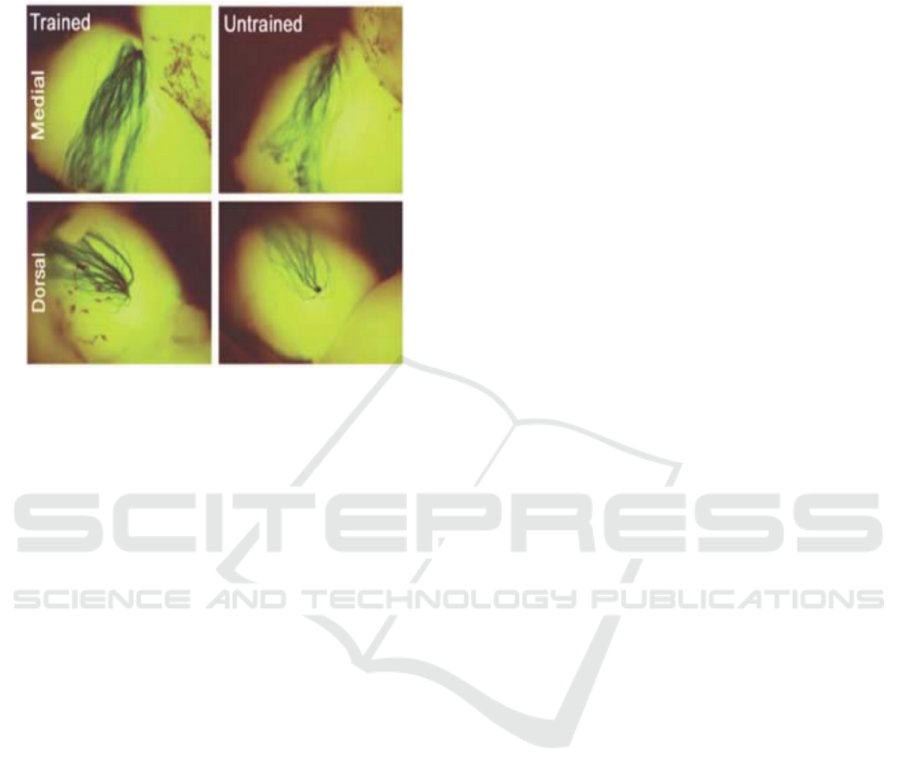
after the odor fear conditioning test in the dorsal and
medial areas. Therefore, these acetophenone-
sensitive neurons can be identified and isolated to
find the piRNA that has increased in abundance.
Figure 3: Increased M71 axon density and glomerular size
with fear conditioning in the medial and dorsal pairs of M71
glomeruli (Jones, 2008).
2.3
Determination of the piRNA-X
To ensure that the found piRNA-X is indeed
associated with the neuronal circuit and memory
formation, the piRNA-X is conditionally removed in
the olfactory bulb. The piwi protein that works
together with the piRNA is knocked out by using
CRE recombinase to remove the gene encoding for
the piwi protein. Therefore, piRNA-X will no longer
have an effect. Lastly, a fear conditioning test is
conducted to see if the inhibition of the piRNA-X has
any effect on the memory formation of acetophenone.
2.4
Mutant piRNA
To see if piRNAs can migrate from the brain to the
germline a mutant piRNA-X is created using a virus
vector to add a piRNA DNA to a group of mice
embryos. The mutant piRNA-X differs from the
initially found piRNA-X by a nucleotide achieved by
using the CRE loxP system. Then the mutant piRNA
strand can be activated using the CRE recombinase
enzyme in the olfactory bulb region of the adult mice.
Following this, immunoprecipitation is used to
see if the mutant piRNA-X can still perform the same
task as the original piRNA-X. First, a crosslinker is
used to link the piwi protein to the mutant piRNA-X
and test if they can interact. Then specific antibodies
are used to separate the piwi-piRNA complexes from
the cells. We elute to separate the mutant piRNA-X
from the piwi protein and check if the mutant piRNA-
X is present with the RT-PCR test.
2.5
Testing for the Migration of
piRNA-X
The RT-PCR test is used to check if the mutant
piRNA-X is present in the sperm cells. First, a
constant sequence of nucleotides is joined to the 3'
end of the mutant piRNA-X. A primer
complementary to the constant region binds to it and
the reverse transcriptase can come along and form the
DNA strand. A second primer specific to the mutant
piRNA-X binds to the DNA strand and reverse
transcriptase forms the cDNA of the mutant piRNA-
X. The sample is then placed into the PCR machine.
2.6
Further Experiments + Controls
Lastly, the offspring of the experimental groups are
tested to see if they were able to inherit the memory
of the acetophenone fear-conditioning test. The first
group of mice consists of the offspring of parents who
have undergone the fear-conditioning test and have
the mutated piRNA-X. The second experimental
group consists of offspring of parents who have
undergone the fear-conditioning test but do not have
the mutant piRNA-X. The third experimental group
consists of offspring of parents who have not
undergone the FC test but have the mutant piRNA-X.
These experiments are to prove that the mutant
piRNA-X does not interfere with the regular function
of the piRNA-X and the original piRNA-X is
functional with the addition of the mutant piRNA-X
too.
3 POSSIBLE RESULTS
3.1
Odor Fear-conditioning
From the first FC tests, some predictions of the results
can be drawn. We expect that the first group of mice
will show strong freezing behavior when exposed to
acetophenone after the fear conditioning test. If the
mice do not display a freezing behavior the same
procedure will be repeated. The second group of mice
should not show sensitivity to the odor and the third
group of mice should not have fluorescent neurons
(Jones et al., 2008).
Figure 4 shows how the results of the freezing
behavior look like in the mice in response to odor
PiRNAs Involved in the Memory Formation of Fear-conditioning Tests Migrating from the Brain to the Germline
363
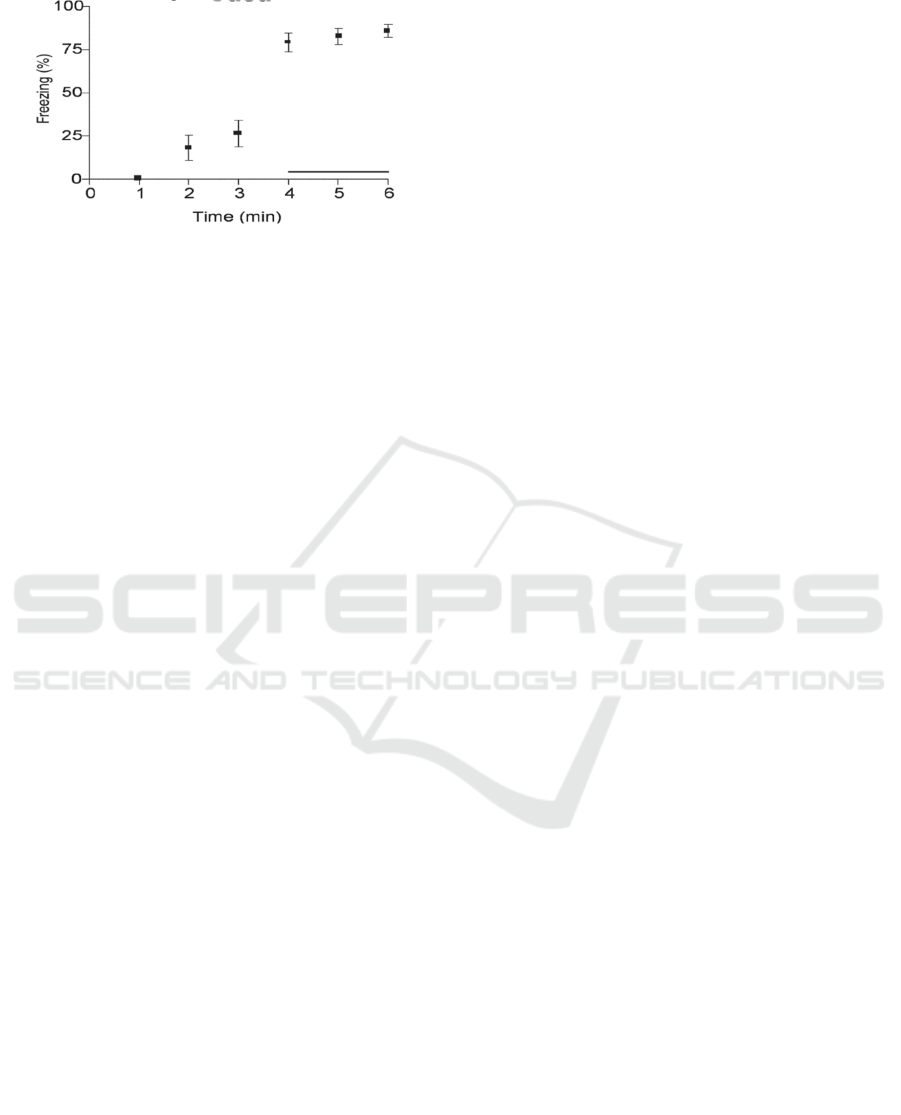
stimulus after training.
Figure 4: Freezing in response to CS presentation on Day 2
after training. (Sweatt, 2010).
3.2
Sequencing and Identification of
piRNA
After DNA sequencing, the machine should give us
the nucleotide base sequence of the piRNA that has
increased in abundance the most, the piRNA-X.
3.3
Determination of piRNA
We expect that after the inhibition of piRNA the mice
will not be able to form a memory of the odor FC test,
they will not display freezing behavior. Therefore, we
have found the correct piRNA that is involved in the
FC neuronal circuit.
3.4
Mutant piRNA
The PCR machine can give 2 different results:
positive or negative. If the PCR machine shows a
positive result then we know that the cDNA is present
in the sample and the mutant piRNA-X has migrated
from the brain to the germline. If the PCR machine
gives a negative result, then the cDNA is not present
in the sample and the piRNA has not migrated from
the olfactory bulb to the germline.
3.5
Offspring
We would expect that the offspring of the first and
second experimental groups would display similar
increased freezing behavior to acetophenone, whilst
the offspring of the third experimental group would
not display freezing behavior on exposure to
acetophenone.
4 DISCUSSION
One possible limitation of using DNA sequencing to
obtain the piRNA sequences is that several piRNAs
can be identified that have increased in amount. In
this case, we would have to choose which piRNA to
mutate and eliminate. If after the inhibition of the 1st
piRNA the mice can still exhibit enhanced sensitivity
to acetophenone then a 2nd piRNA must be inhibited
until the mice stops exhibiting freezing behavior and
the piRNA then can be mutated.
Furthermore, one other limitation of creating the
mutant piRNA is that changing the base sequence of
the piRNA can result in the mutant piRNA having a
different function to the original strand or can lead to
damage to the animal since piRNAs are only 26-28
nucleotides long and a slight change to the sequence
can lead to consequences.
5 CONCLUSION
In conclusion, if the mutant piRNA is present in the
sperm cells, then the piRNA associated with fear odor
conditioning has migrated from the brain to the sperm
cells and memory of the odor used in th fear
conditioning test can be inherited by offspring.
If the mutant piRNA is not present in the sperm
cells, then the piRNA associated with odor fear
conditioning has not migrated from the brain, and
offspring will not inherit the memory of
acetophenone.
This work could therefore confirm that piRNAs
are related to transgenerational memory and migrate
from the brain to the germline. In the future, the
pathway and mechanism of the migration of piRNAs
are nevertheless yet to be confirmed. We are currently
thinking that perhaps piRNAs are transported in
exosomes around the body or piwi-piRNA complexes
can travel around the body to the germline. Moreover,
future studies can also focus on structures such as the
hippocampus and amygdala which are known to be
involved in FC memory formations, and possibly the
transgenerational memory.
REFERENCES
Dias, B. G., & Ressler, K.J. (2013). Parental olfactory
experience influences behaviour and neural structure in
subsequent generations. Nature Neuroscience, 17(1),
89-96. https://doi.org/10.1038/nn.3594.
Jones, S. V., Choi, D. C., Davis, M., & Ressler, K. J. (2008).
Learning-Dependent Structural Plasticity in the Adult
Olfactory Pathway. Journal of Neuroscience, 28(49),
13106–13111. https://doi.org/10.1523/jneurosci.4465-
08.2008.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
364

Moore, R. S., Kaletsky, R., & Murphy, C. T. (2019).
Piwi/PRG-1 Argonaute and TGF-β Mediate
Transgenerational Learned Pathogenic Avoidance.
Cell, 177(7), 1827–1841.e12.
https://doi.org/10.1016/j.cell.2019.05.024.
Rajasethupathy, P., Antonov, I., Sheridan, R., Frey, S.,
Sander, C., Tuschl, T., & Kandel, E. (2012). A Role for
Neuronal piRNAs in the Epigenetic Control of
Memory-Related Synaptic Plasticity. Cell, 149(3),
693–707. https://doi.org/10.1016/j.cell.2012.02.057.
Sweatt, J. D. (2010). Rodent Behavioral Learning and
Memory Models. In Mechanisms of Memory (2nd ed.,
pp. 76–103). Academic.
https://doi.org/10.1016/C2009-0-03605-8.
PiRNAs Involved in the Memory Formation of Fear-conditioning Tests Migrating from the Brain to the Germline
365
