
Potentials of the Dry Granulation by Roller Compaction
Yujie Zhang
University of Edinburgh, Scotland, U.K.
Keywords: Roller Compaction, Dry Granulation, Agglomeration, Pharmaceutic Engineering, Drug Production.
Abstract: The latest trends in the field of pharmaceutical engineering are regarding roller compaction. Roller
compaction technology is a well-established strategy particularly involved in the medical industry. This
developing process has a great impact on the manufacturing of oral solid dosage forms while containing high-
quality active pharmaceutical ingredients (API). When the medical industry tends to develop a tablet, there
are three basic processing routes that have been considered: direct compression, dry granulation, and wet
granulation. Among these three high-efficiency productive ways, dry granulation is a process that compresses
powdery ingredients into tablets without adding any liquid solution. Dry granulation is carried out using either
slugging or roller compaction techniques. This pharmaceutic process has potentials for drug development as
it has been proved to continuously manufacture tablets by working with compaction machines. Roller
compaction employed to dry granulation can provide more advantages compared with other processing
methods. However, there are still have some challenges that this technology and researchers need to face.
Improvements on the dry granulation by roller compaction technology would give a great contribution to drug
development and goods production.
1 INTRODUCTION
The manufacture of processing solids into
pharmaceutical tablets is a multi-stages assembly line
whereby scientist has used this process for hundreds
of years. Most compressed tablets are required to go
through several procedures started from ingredient
dispensing. The ingredients will be divided by
accuracy, mixed with excipients through blenders,
and powders aggregated into granules. The dry
granulation is then compressed and coated into tablets
by using compaction techniques. Nowadays, there are
typically three similar routes for solid dosage
processing, including direct compression, dry
granulation, and wet granulation. Three alternative
procedures share similarities and differences. Among
these routes, direct compression is the simplest
process by bleeding the powder of API with excipient
particles and then compressing them directly into a
tablet machine. Although direct compression is the
most economical way in the manufacturing industry,
it cannot apply in many cases due to the segregation
of particles during routine processing. Most
manufacturing industries prefer granulation instead of
direct compression.
Granulation is a process which helps to avoid
segregation within particles. It is carried out in order
to let powder particles adhere to each other, resulting
in a high density of products which is known as
granules. Dry granulation is processed with no aid of
liquid blenders whereas wet granulation is required.
In the dry granulation process, powder particles
compacted by a force, causing adhesion. The powder
would be dried and physically milled to form granules
(Stutzman, 2020). Dry granulation is suitable in the
case of APIs that are hydrophilic and sensitive to heat,
which wet granulation cannot approach. Granulation
is a more challenging process than direct compression
because it requires higher techniques and more
complex steps. Researchers need to consider the
impact of high pressure from compaction machines
whether it would induce physical or chemical change
on APIs. However, the advantages of dry granulation
cannot be ignored, especially involved roller
compaction technology.
Zhang, Y.
Potentials of the Dry Granulation by Roller Compaction.
DOI: 10.5220/0011202700003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 303-307
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
303
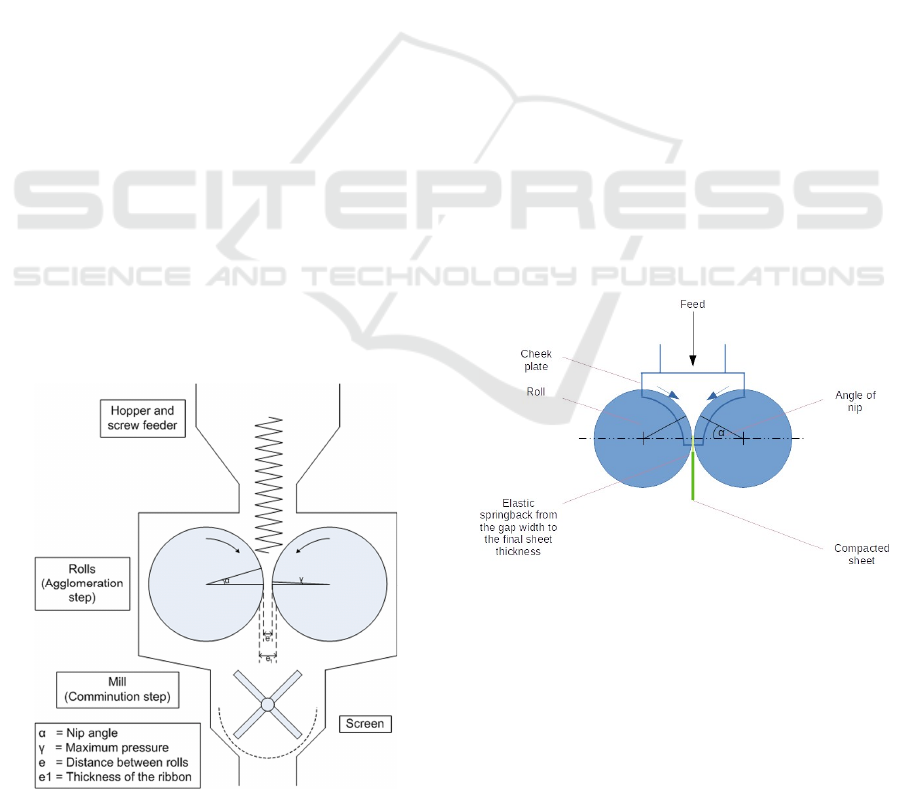
2 DRY GRANULATION BY
ROLLER COMPACTION
Roller compaction technology is considered a well-
known and economic granulation method for drug
development. Dry granulation is a continuous method
with a low amount of energy required and suitable for
compounds that are sensitive to heat (Peter et al.
2010). During the process of dry granulation,
intragranular excipients would be compacted through
a roller compaction machine and result in granules.
Dry granulation has the same prior procedures with
direct compression, but the resulting powder would
continue to go through additional compaction and
sequentially form dried granules. The most important
parameters involved in this process are powder
feeding, pressure control, roll surface, and gap region,
which take place in the machine of roller compaction.
The common way for manufacturing granulation is
roller compaction, where the powder is compressed
into a ribbon before segregation happens. The powder
is fed into a feed hopper first and then passes through
a tube named screw feeder. After that, particles with
a bleed of the API and the excipients would load
through two counter-rotating rollers within the
compactor machine (Stutzman, 2020). Two rollers
keep rotating in order to squeeze the powders within
a small gap between rollers, generating a dried, solid
ribbon. The solid ribbon is then milled into small
fragments by crusher, blended with extragranular
excipients, and given an end product of tablets by
compressing the mixture, the process can be seen in
Figure 1, raw material is fed into the machine with a
final product of tablet (Hudon et al. 2019).
Figure 1: Process of roller compaction within the compactor.
Dry granulation by roller compaction is separated
into two alternative steps. The first step is
agglomeration started from putting the raw material
into the machine. The flowing powder is passed
through the feed hopper by using a feed screw to
transport it into the rollers, result in a solid form of a
ribbon. The second step is called the size reduction
step which compresses the ribbon into granules (Teng
et al. 2009). Each step plays an important role in the
granulation process. Variation happens during the dry
granulation that would affect the final quality of
tablets. Granulator speed is a possible variable that
would decrease the production of fine materials (Rana
et al. 2011). By understanding the principles of
compaction roller press, different zones are shown
within the compactor, as can be seen from Figure 2.
Two rotating rollers are the key point by applying a
force to compact powder into the solid ribbon. Unique
angle and specific shape of rollers that would allow
producing the amount of solid product. During the
agglomeration step, powder pass through the
compaction zone starts at the first zone, the nip
region. Particles within the power would break down
under the force of two plates. Then, powders would
be squeezed into small pieces of fragments under the
pressure of two rolls. Fragmentation takes place in the
roll gap and releases ribbon that bonds by dried
particles into the next stage (Rana et al. 2011). In this
whole process, the main compaction force is carried
out by the two rollers as it solves the big problem
about adhesion between particles.
Figure 2: Compaction zone of two rollers within the
compaction machine.
Instead of using liquid binders to stick each
particle, dry granulation depends on the compound
itself. During the compaction process, the power
suffered from great pressure would become easier to
be broken down. It has been indicated that materials
pass through the two rolls which give a very high
pressure, allowing to completely compact powder and
result in dried flakes. Also, the smooth surface of
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
304

rollers is beneficial for particles sticking and reduced
the use of lubricant. The different surfaces of rolls
would have more or less gripping force for the
compaction (Pietsch, 2002). The technology of
manufacturing granules from raw material has been
adapted to many industries since it was considered as
an economic way. Researchers need to control
parameters in roller compaction so that the machine
would effectively produce tablets. The density of the
ribbons is a relative parameter that has the ability to
control the flowability of granules and the
compactibility of final products (Peter et al. 2010).
Many results show that a large density of the ribbon
would manufacture a small number of fine granules
after granulation, thus there would have a better
flowability but a low rate of compactibility.
Comparing the quality of granules with the products
obtained from direct compression, there would be a
reduction in tablet strength due to the loss of
compactibility.
3 ADVANTAGES OF DRY
GRANULATION
The most obvious difference between dry granulation
and wet granulation is that dry granulation is not
required for liquid content. The major advantage of
dry granulation is that the process is suitable for the
API or excipients which are sensitive to heat and
moisture. This dry formulation of the drug satisfies
the compound which has a low melting point or
contains hydrophilic contents. Dry granulation is the
benefit to the manufacturing industry which
progresses a more economic and less equipment way
compared with wet granulation. Another advantage of
roller compaction is that the technique improves the
flow properties of powders. Throughout many
pharmaceutical applications, the main goal is to
increase the flowability of medical powder to achieve
a fast compressing of tablets. Researchers usually use
the compressibility index to calculate the flowability
of granules, with a method named The United State
Pharmacopeia (USP) (Anshul et al. 2017). Results
and data would then be collected from the
compressibility index calculation by using the
equation in Figure 3.
Figure 3: An equation used to calculate compressibility
index (CI).
The equation shows that bulk and trapped
densities can be determined per the procedure within
the USP. By calculating the compressibility index
(CI) in a different situation, results indicate that the
roller speed has an impact on CI. Variables include
roller forces that would change the flowability,
suggesting stronger ribbons produce by the strong
roller forces (Anshul et al. 2017). Furthermore, roller
compaction prevents particle segregation during the
granulation. The process of granulation forces the
powder to pass through two rotating rollers. By
squeezing and milling the mixtures, the density of
granules would increase so that each particle would
stick to the other tightly (Rana et al. 2011).
4 DISADVANTAGES AND
CHALLENGES
Roller compaction becomes a new trend in the drug
manufacturing industry. Although dry granulation by
roller compaction presents many benefits to the
economy and productivity, there are still have some
challenges that researchers need to overcome. One of
the major challenges during dry granulation is the loss
of compactibility in the double compaction (Heiman
et al. 2017). Researchers found the details of the loss
of tabletability by using microcrystalline cellulose
(Sun et al. 2007). Granules size enlargement on
tabletability would change the bonding within the
tablets. In the paper of Sun and Changquan Calvin
(2008), phenomenon of larger particles perform a
lower tabletability after the granulation process has
been addressed. To verify the phenomenon of loss of
compactibility, Sieve analysis has been introduced
which is a common method that used to detect the
powder particle size. Levels of different variables in
the designed experiments are showed in Table 1
(Herting et al. 2007). Experiments used fraction of
Theo and porosity of the ribbons to obtain the levels
of Microcrystalline cellulose (MCC) and theophylline
(Theo) since MCCs affect the flowability of granules
and Theo is another API ingredient used in particle
size (Herting et al. 2007). Sieve method can divide
granules in order to make a comparison between
variables in the granules of roller compaction. The
results of sieve analysis are usually presented in
median diameter of particles (μm). Based on the
results in Table 1, there was a relationship between
Fraction of Theo and MCC, with an increasing
percentage of fraction of Theo, the change on
granules size enlargement influenced by MCC is less
but significant (Herting et al. 2007). Subsequently,
Potentials of the Dry Granulation by Roller Compaction
305

the results shown that enlargement of granules also
reduced the tabletability after the granulation process
(Sun et al.2006). Therefore, granules size
enlargement plays an important role in causing the
reduced tabletability and this become one of the major
disadvantages of roller compaction through dry
granulation.
Table 1: Levels of variables for measuring the influence of particle size in the designed experiments.
Level -1 -0.33 0 +0.33 +1
MCC (μm)
21 - 56 - 106
Theo (μm)
7 - - - 110
Fraction (%)
25 41.67 50 58.33 75
Porosity (%) 20 26.67 30 33.33 40
Other disadvantages including the process is too
slow compare with direct compression. Direct
compression has the same characteristic as an absence
of liquid binder within the process. However, dry
granulation has additional steps of transforming the
raw materials into granules by go through the
compactor. The machine would cost more money and
time which is a burden for some drug industries.
5 CONCLUSIONS
In conclusion, the dry granulation technique has
drawn the attention of many pharmaceutical
industries because of the advantages of dry
granulation by roller compaction compared with other
compaction technology. The agglomeration step of
drug formulation briefly illustrates how powder of
APIs and excipients pass through the compactor and
result in dried granules. During the dry granulation,
the absence of liquid content gives a major advantage
to this process which becomes suitable for heat and
moisture products. Dry granulation also prevents
particle segregation and contributes to flowability.
Although the dry granulation process may lose
tabletability, researchers still believe the process has
the potentials to overcome the disadvantages.
ACKNOWLEDGEMENTS
I am very grateful to the Prof. Axel for his enormous
contribution towards the project. Professor Axel
provided me with encouragement and patience
throughout the duration of this research project.
Thanks to the teacher assistant Ben Wei who always
solve my question in patience.
REFERENCES
Anshul Gupte, et al. Comparative binder efficiency
modeling of dry granulation binders using roller
compaction. Drug Development and Industrial
Pharmacy, vol 43, no. 4, pp. 574-583, 2017.
Heiman, Johanna, et al. Roller Compaction of Hydrophilic
Extended Release Tablets—Combined Effects of
Processing Variables and Drug/Matrix Former Particle
Size. AAPS PharmSciTech, vol. 16, no. 2, pp. 267–77,
2015.
Hudon, Sophie, et al. Evaluation of a Dry Coating
Technology as a Substitute for Roller Compaction for
Dry Agglomeration Applications in the Pharmaceutical
Industry. Journal of Pharmaceutical Innovation, vol. 14,
no. 3, pp. 286–303, 2019.
Michael G. Herting, Peter Kleinebudde. Roll
compaction/dry granulation: Effect of raw material
particle size on granule and tablet properties.
International Journal of Pharmaceutics, vol. 338, pp.
110-108, 2007.
Peter, Stefanie, et al. Roller compaction/Dry Granulation:
Use of the Thin Layer Model for Predicting Densities
and Forces During Roller Compaction. Powder
Technology, vol. 199, no. 2, pp. 165–75, April 2010.
Pietsch, Wolfgang. Agglomeration Processes: Phenomena,
Technologies, Equipment / Wolfgang Pietsch.
Weinheim: Wiley-VCH, 2002.
Rana, Khokra, et al. Overview on roll compaction/dry
granulation process. Pharmacologyonline, vol. 3, pp.
286-298, November 2011.
Teng, Yue, et al. Systematical Approach of Formulation
and Process Development Using Roller Compaction.
European Journal of Pharmaceutics and
Biopharmaceutics, vol. 73, no. 2, pp. 219–29, 2009.
Sun C., Calvin. On the Mechanism of Reduced
Tabletability of Granules Prepared by Roller
Compaction. International Journal of Pharmaceutics,
vol. 347, no. 1, pp. 171–72, 2008.
Stutzman, Todd., Roller Compaction: New Trends,
Challenges and Solutions: When Developing a Tablet
or Capsule, Three Widely Used Technologies Are
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
306

Often Considered to Produce Solid Dosage Forms:
Direct Compression, Wet Granulation and Dry
Granulation. Manufacturing Chemist (London: 1981),
vol. 91, no. 11, pp.34, November 2020.
Sun C. Calvin, Micah W. Himmelspach. Reduced
tabletability of roller compacted granules as a result of
granule size enlargement. Journal of Pharmaceutical
Sciences, vol. 95, pp. 200-206, 2006.
Potentials of the Dry Granulation by Roller Compaction
307
