
Cloning, Expression and Sequence Analysis of PbeHSFA1d in Pyrus
betulifolia
Cong Jin
a
and QiaoHui Guo
b
School of Life Science and Food Engineering , Meicheng Street, Huaian, China
Keywords: Pyrus Betulifolia, Pbehsfa1d Gene, Gene Cloning, Abiotic Stress, Expression Analysis.
Abstract: Heat shock protein is a kind of transcription factors widely existing in plants, which plays a major role in
regulating plant growth and responses to environmental stress. In order to study the sequence characteristics
of HSF gene from pear and its response to abiotic stress, PbeHSFA1d gene was isolated from Pyrus
betulifolia by RT-PCR. Sequence analysis of PbeHSFA1d was carried out by bioinformatics software, and
real time quantitative PCR was used to detect the expression level of PbeHSFA1din different tissues and its
response patterns to various abiotic stresses. The results reflected that the ORF of PbeHSFA1d was 1548 bp
in length, 516 amino acids were encoded, and there were 52 phosphorylation sites in the predicted protein,
but the glycosylation site was not found. Domain analysis showed that PbeHSFA1d contained a conserved
N-terminal DNA binding domain (DBD), a bipartite oligomerization domain (HR-A/B), an activation
domain (AHA), a nuclear localization signal (NLS) and a nuclear export signal (NES), which are typical
structural features of plant HSF protein family. Moreover, PbeHSFA1d was close to MdHSFA1d, PaHSF8,
PpHSF8 and PdHSFA1d in genetic relationship. PbeHSFA1d was localized to the nucleus by subcellular
localization. The results of RT-PCR demonstrated that the expression of PbeHSFA1d in stem was higher
than that in flower, leaf and root, and the transcript levels of PbeHSFA1d were induced by high temperature,
dehydration, low temperature and salt. The results of this study will provide a theoretical basis for further
exploration of the function in abiotic stress of PbeHSFA1d.
1 INTRODUCTION
1
During the last decades, heat has gradually become
an important environmental factor seriously
affecting plant growth and yield with global
warming. Therefore, plants have formed a series of
molecular and physiological mechanisms to resist
environmental stress in the long-term evolution
process (Gall 2015). As an important regulator of
gene expression, transcription factors participate in a
set of plant protection mechanisms under
environmental stresses, indicating that transcription
factors play an essential role in improving plant
stress resistance, such as bZIP, NAC, bHLH and
HSF (Tang 2020).
The response of plants to heat is a extremely
complex process, which involves a wide range of
genes that related to abiotic stress, signal
transduction, material transport, photosynthesis,
a
https://orcid.org/0000-0001-6732-0648
b
https://orcid.org/0000-0002-3098-8166
protein metabolism and carbohydrate metabolism
gene (Tian 2021). Since the first plant HSF (heat
shock transcription factor) gene was cloned from
tomato, there are 21, 52, 25 and 30 members in
Arabidopsis, soybean, rice and maize, respectively
(Yoshida 2011). As a large transcription factor
family in plants, HSF is the core regulator of heat
stress response genes in higher plants (Qiao 2015).
HSF could specifically recognize and bind heat
shock elements, and then activate the expression and
transcription of downstream heat shock protein
genes to produce heat shock proteins, so as to
improve the heat resistance of plants. HSF has five
typical functional domains: DBD, HR-A/B domain,
NLS, NES and AHA (Li 2020). HSF can be further
divided into A, B and C types in plants, and A-type
HSF contains AHA structure, which mostly plays a
positive regulatory role in the response to heat stress,
while most of B-type HSF play a negative regulatory
role in the response to heat stress. Several studies
have shown that HSF could respond not only to heat,
but also to cold, drought, oxidative stress and salt
Jin, C. and Guo, Q.
Cloning, Expression and Sequence Analysis of PbeHSFA1d in Pyrus betulifolia.
DOI: 10.5220/0011200700003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 277-282
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
277

(Tian 2021, Liu 2021). HSFA1b enhances heat
tolerance in wheat and Arabidopsis through
jasmonate signalling pathway and OPR3 (Tian
2020). AtHSFA2 is involved in drought and
oxidative stress in Arabidopsis (Nishizawa 2006). In
addition, AtABI3 positively regulates the expression
of AtHSFA9 by directly binding to its promoter, but
AtHSFA9 is negatively regulated by AtIAA27
(Kotak 2007).
So far, although many HSF transcription factors
that respond to plant development and abiotic stress
have been identified, but most of these studies focus
on Arabidopsis and crops, and there are still few
studies on HSF transcription factors in woody
plants. Pyrus betulifolia is a heat-tolerant species,
making it an ideal source to isolate gene. In this
study, the full length of PbeHSFA1d of Pyrus
betulifolia was cloned by reverse transcription PCR.
The sequence analysis was carried out by
bioinformatics software, and the tissue expression
level and the pattern of expression to abiotic stress
were studied by quantitative PCR, in order to prove
the function and stress resistance mechanism of
PbeHSFA1d.
2 MATERIALS AND METHODS
2.1 Plant Materials
Healthy and uniform seedlings were collected from
60-day-old Pyrus betulifolia, which were exposed to
abiotic stresses of low temperature (4℃), high
temperature (40℃), dehydration and salt (200
mmol·L
-1
NaCl).
2.2 Stress Treatments
Temperature stresses were carried out by placing the
seedlings in a growth incubator at the set 4℃or 40℃
for 0, 6, 12, 24, 48 h, respectively. Before
dehydration and salt treatment, the roots of seedlings
were placed in a beaker containing distilled water,
which were grown in a growth chamber at 25°C for
1 day, had a 16-h light/8-h dark photoperiod. For the
dehydration treatment, the seedlings were put on the
dry filter papers dried at 25°C in an artificial climate
chamber, with relative humidity of 40.0% for 0, 0.5,
1, 3 and 6 h. Salt treatment was applied by
transferring the seedlings to flasks with 200 mmol·L
-
1
NaCl solution for 0, 6, 12, 24 and 48 h. At least 50
seedlings were used in each treatment, and the fully
expanded leaves were collected from randomly 3-4
seedlings at set time points. All the samples of each
treatment were mixed and put into the liquid
nitrogen frozen immediately, then stored at -80°C
for further experiments.
2.3 Gene Isolation
Based on the pear genome database, a sequence with
a complete opening reading frame, high degree of
similarity to MdHSFA1d was obtained, named as
PbeHSFA1d. The 2000 bp upstream of the
transcription start site of PbeHSFA1d gene was
selected as the promoter sequence. Based on the two
sequences, specific primers GSP1 and GSP2 were
designed for amplifying the gene and promoter by
using cDNA and DNA in the leaves of Pyrus
betulifolia as templates, respectively. The PCR
mixture in a total 20 μL reaction volume, contained
100 ng cDNA, 10 μL I-5
TM
2X High-Fidelity Master
Mix, 0.4 μM of a pair of specific primers and
nuclease-free water. PCR was performed by a
program as follows: initial denaturation at 98°C for
1 min, 35 cycles of 98°C for 10 s, 56°C for 15 s,
72°C for 90 s, and 72°C for 10 min. The PCR
product of gene cloning was purified and cloned into
pCAMBIA1300 vector to generate a fusion
construct (pCAMBIA1300-PbeHSFA1d) and
sequenced in Sangon. The promoter of PbeHSFA1d
product was subcloned into pMD-18T vector and
sequenced in Sangon.
2.4 Sequence Analysis of PbeHSFA1d
The molecular weight and isoelectric point (pI) were
calculated by Expasy
(http://web.expasy.org/compute_pi/); The cis acting
elements in the promoter that related to
environmental stress was detected by the online tool
of Plant CARE; Subcellular localization was
analyzed by software Softberry; The secondary and
three-dimensional structures of PbeHSFA1d protein
were predicted by software Sopma and SWISS-
MODEL (Waterhouse 2018), respectively; The
protein modification patterns were analyzed by
software Netphos 3.1 server and Dictyglyc 1.1
server; The multiple alignments of the deduced
amino acid sequence were carried out by the Clustal
W program (Jin 2017); The protein functional
domain was analyzed by software Pfam;
Phylogenetic tree was constructed by the Maximum
Likelihood method using MEGA 6.0.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
278

2.5 Analysis of PbeHSFA1d Gene
Expression Characteristics
Based on the confirmed PbeHSFA1d sequence, a
pair of specific expression primers (GSP3) were
designed. Quantitative Real-Time PCR (qRT-PCR)
was performed to analyze the expression levels in
different tissues and expression patterns under
various abiotic stresses of PbeHSFA1d, the primer
of internal reference gene was GSP4. The PCR
solution in a total 20 μL reaction volume, contained
10 ml of SYBR-Green PCR Master Mix, 0.25 mM
of each primer, 100 ng of cDNA, and nuclease-free
water. Quadruple qPCR was carried out in a
LightCycler 480 Real-Time PCR System, the PCR
reaction conditions were as follows: 95 °C for 5 min,
then 40 cycles of 94 °C for 10 s, 60 °C for 30 s,
72 °C for 30s, and 72 °C for 3 min. Relative gene
expression levels of the amplified products were
normalized to Pyrus TUB-b2 to minimize variation
in the cDNA template and the relative gene
expression data were calculated by the 2
− ΔΔCT
method.
2.6 Statistical Analysis
The experimental results are at least three
independent replicates in this study, shown as mean
± SE. Experimental data were analyzed using SPSS
software and statistical difference was compared
based on Duncan’s multiple range test.
Table 1: Primer sequences.
Primer Primer Sequence (5ʹ-3ʹ) Function
GSP1-F GAGAACACGGGGGACTCTAGAAT
GGAGGCTGCTAATAACAAC
Gene
cloning
GSP1-R GCCCTTGCTCACCATGGATCCCAC
CCCTTTGGTCTCTGATG
GSP2-F AACAAATGAACGGTATTGGTAA
Promoter
cloning
GSP2-R CAATTCTTTTATTGTTCTCTGTG
GSP3-F GAGCATCAATCGGCGTAA
QRT-
PCR
GSP3-R CTGCACCATTGTTTGTAGCT
GSP4-F TGGGCTTTGCTCCTCTTAC
Internal
control
GSP4-R CCTTCGTGCTCATCTTACC
3 RESULTS
3.1 Sequence Analysis of PbeHSFA1d
The target fragment was amplified by using specific
cloning primers with the cDNA from the leaves of
Pyrus betulifolia as the template, sequencing results
showed that the fragment was consistent with the
PbeHSFA1d. The full length of is 1548 bp, encoding
516 amino acids.
In order to further speculate the potential
function of PbeHSFA1d, the cis acting elements of
promoter were analyzed. The results showed that a
various of cis acting elements related to
environmental stress (MBS, LTR, STRE, TC-rich
repeats, ARE and so on) and hormone (ABRE,
TCA-element, ERE, TGACC-motif, GARE-motif,
and so on) in the promoter, which suggested that
PbeHSFA1d might play a key role in plant defense
against abiotic stresses and response hormones.
3.2 Sequence and Bioactivity Analysis
of PbeHSFA1d
The predicted molecular weight of PbeHSFA1d
was about 57.141 kDa, the theoretical isoelectric
point was 4.8, the instability index was 56.67, and
the hydrophilicity coefficient was - 0.697, indicating
that PbeHSFA1d belonged to unstable hydrophilic
protein; The results of signal peptide analysis
showed that there was no signal peptide sequence in
PbeHSFA1d, which belonged to non-secretory
protein; The prediction of subcellular localization
showed that PbeHSFA1d was mainly located in the
nucleus. The results of protein secondary structure
prediction showed that PbeHSFA1d contained
32.75% α- Helix, 9.11% extension chain, 5.62% β-
Corner and 52.52% random coil; The protein tertiary
structure of PbeHSFA1d was analyzed by using HSF
protein template (PDB ID:1fbu.1) in SWISS-
MODEL software, ratio of the coverage of the
constructed model and template was 50% and the
amino acid coverage range was 41-116 aa.
Phosphorylation and glycosylation, as the
common protein modifications, may greatly affect
the protein structure and function in the process of
plant growth and development. The phosphorylation
analysis of PbeHSFA1d showed that 52
phosphorylation sites exceeded the probability
threshold, including 40 serine sites and 12 threonine
sites, and there were dense phosphorylation sites in
the amino acid sequence range 290-350 aa (Figure
1). Glycosylation prediction analysis showed that
there were no sites that exceed the probability
threshold, which indicated glycosylation of
PbeHSFA1d protein was highly likely.
Cloning, Expression and Sequence Analysis of PbeHSFA1d in Pyrus betulifolia
279
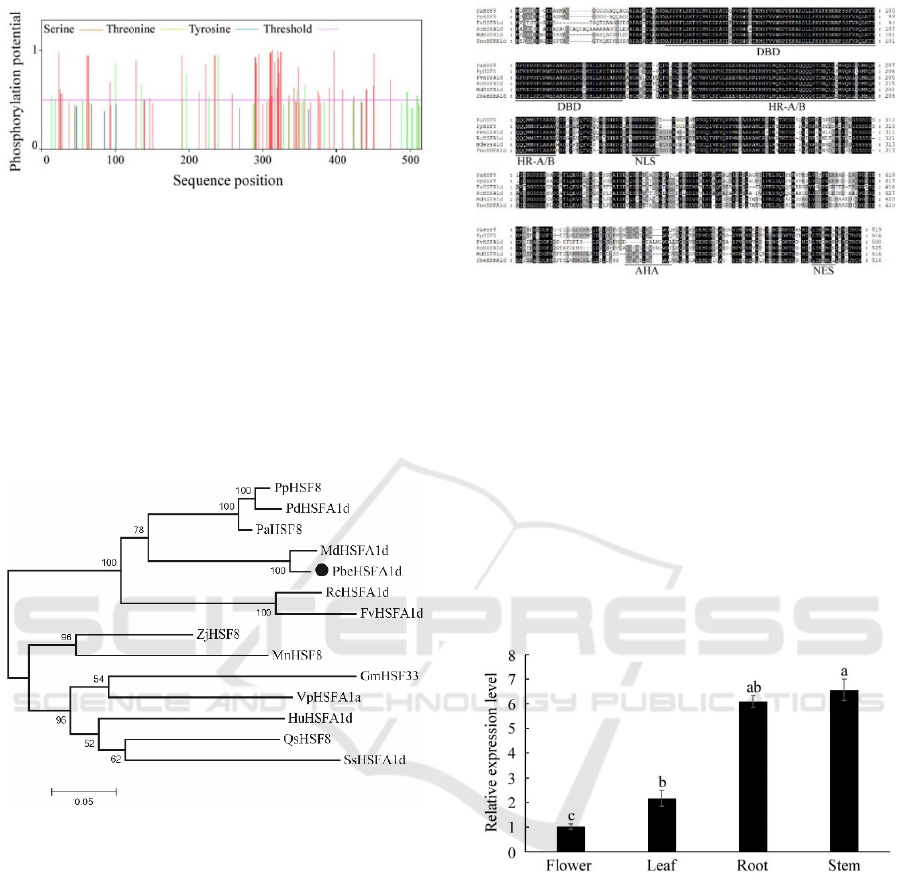
Figure 1: Prediction of phosphorylation site in amino acid
sequence of PbeHSFA1d protein.
3.3 Phylogenetic and Sequence
homology Analysis of PbeHSFA1d
Phylogenetic analysis showed that PbeHSFA1d had
a close relationship with MdHSFA1d, PaHSF8,
PpHSF8 and PdHSFA1d of Rosaceae, and clustered
together; Besides that, PbeHSFA1d had a distant
relationship with ZjHSF8, GmHSF33 and
SsHSFA1d (Figure 2).
Figure 2: Phylogenetic tree of PbeHSFA1d and HSFs of
other species.
Multiple sequence alignment analysis was
performed between PaHSF8, PpHSF8, FvHSFA1d,
RcHSFA1d, MdHSFA1d and PbeHSFA1d, the
results showed that the sequence similarity
respectively was 77.50%, 79.03%, 73.29%, 73.84%
and 95.16%, which indicated PbeHSFA1d had high
homology with these five species and including a
conserved N-terminal DBD domain, a HR-A/B
domain, a NLS, AHA and NES. In addition,
PbeHSFA1d possessed an AHA domain which is
specific to A-type HSF transcription factors in
plants, all of those proved that PbeHSFA1d was a A-
type HSF transcription factor (Figure 3).
Figure 3: Multiple alignment of HSF proteins.
3.4 Tissues Specific Expression of
PbeHSFA
HSFs were expressed in different tissues of plant,
and with a certain degree of tissue specificity.
Therefore, the expression levels of PbeHSFA1d in
Pyrus betulifolia different tissue of flower, leaf, stem
and root were analyzed (Figure 4). The result
indicated that PbeHSFA1d was expressed in all
tested tissues, with the highest transcription level in
stem and the least in flower, which demonstrated the
significant tissue expression specificity of
PbeHSFA1d.
Figure 4: Expression of PbeHSFA1d in various tissues.
3.5 Expression Characteristics of
PbeHSFA1d under Different
Stresses
The qRT-PCR was performed to detect the
tanscription levels of PbeHSFA1d under various
treatments, including low temperature (4℃), high
temperature (40℃), salt and dehydration. For all
treatments, the expression of PbeHSFA1d was
higher than the initial level after treatment. Under
low temperature stress, PbeHSFA1d was
continuously induced and reached the highest level
at 24 h and declined at the last day (Figure 5A).
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
280
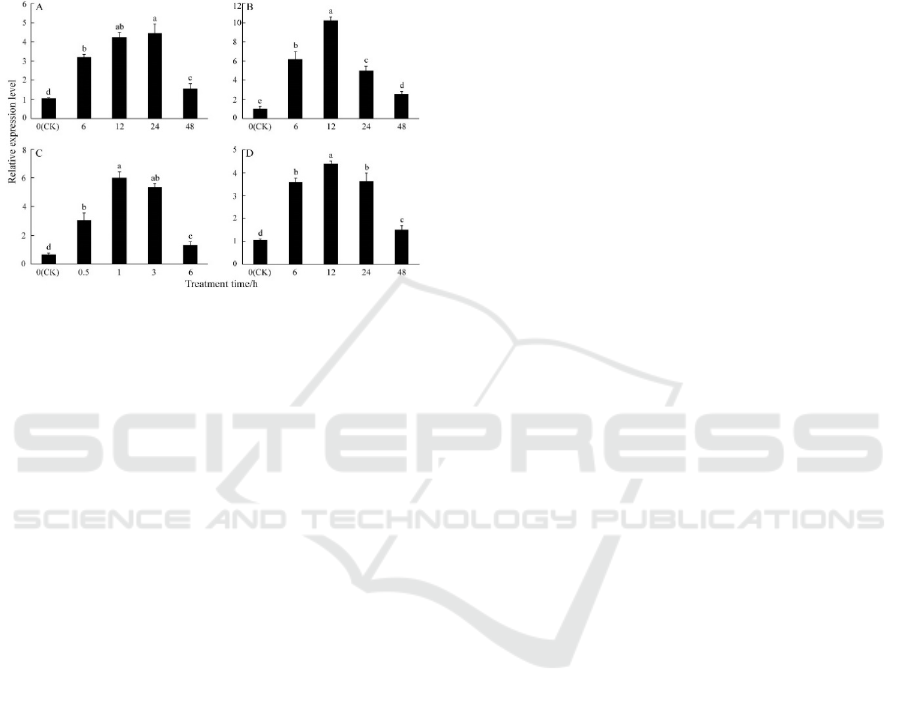
Under heat stress, expression profile was progressive
elevated until the highest level was reached at 12 h
(Figure 5B). As can be seen in Figure 5C,
PbeHSFA1d was sharply induced within 1 h, which
was 6 times that of initial level. The expression of
PbeHSFA1d reached the maximum level at 12 h
with salt treatment and then decreased continuously
(Figure 5D).
Figure 5: Relative expression pattern of PbeHSFA1d
under various abiotic stresses.
4 DISCUSSION
With the intensification of greenhouse effect, heat
stress has become one of the major limiting factors
that adversely affect plant development and crop
yield. Under heat stress, HSF can regulate the
expression of heat shock genes and produce HSP,
which is considered to be the central transcription
factor to resist heat stress. Although several HSFA1d
genes have been isolated, there are differences in
tissue expression patterns and response levels under
abiotic stress (Tang 2020, Ohama 2017). Identifying
HSFA1d in different species will lay a foundation
for further study of the function and application.
In this study, the PbeHSFA1d of Pyrus betulifolia was
cloned, the ORF has a total length of 1548 bp, encodes
516 amino acids and contains 52 phosphorylation sites,
which suggest PbeHSFA1d will play extensive role in the
regulation of phosphorylated proteins (Shen 2019). Cis
acting elements are mainly involved in gene expression
regulation, several stress-related cis acting elements such
as MBS, LTR, STRE and ARE are predicted in the
PbeHSFA1d promoter, which are also found in the
promoter of AtHSFA1d that in response to a variety of
abiotic stresses, indicates the PbeHSFA1d may be
involved in the pathway of resistance to environmental
stress (Liu 2021). Multiple sequence alignment results
show that both PbeHSFA1d and homologous genes
contain the A-type HSFs key domains of DBD, HR-A/B,
NLS, AHA and NES, which are also the main motifs for
HSF to perform its functions (Li 2020). Evolutionary
analysis show that PbeHSFA1d is closely related to
MdHSFA1d, PdHSFA1d, PpHSF8 and PaHSF8, implying
that PbeHSFA1d is highly conservative in the process of
evolution.
HSFs are distributed in many tissues of plant and show
the tissue-specific expression. In this study, the expression
of PbeHSFA1d is higher in the stem and root, but lower in
the leaf and flower, which is similar to the tissue-specific
expression of PpHSF5 (Tan 2021). On the contrary, the
expression of CsHSFB2b is higher in fruit and leaf (Zhang
2020). The difference of tissue expression among
homologous genes may be related to their main
physiological functions. Several studies have shown that
the transcription level of HSFs is induced by a variety of
abiotic stresses, which affect the stress resistance of plants.
AtHSFA1d responds to chilling stress and promotes
hypocotyl elongation via enhancing expression of
ribosomal protein genes (Liu 2021). MdHSFA8A is
induced by drought and modulates flavonoid synthesis
(Wang 2020). Overexpression of TaHSFA2d and
AtHSFA2 improve salt tolerance by regulating stress
response (Chauhan 2013). As a major heat stress
transcription factor, AtHSFA1d improves heat tolerance
by regulating related gene expression (Higashi 2013). In
this study, PbeHSFA1d of Pyrus betulifolia is involved in
the response to cold, dehydration, heat and salt,
demonstrating that PbeHSFA1d may play an essential role
in the response to abiotic stress. However, the action
mechanism of PbeHSFA1d resistance to abiotic stress
needs to be further explored.
5 CONCLUSIONS
In this study, PbeHSFA1d was isolated from Pyrus
betulifolia and proved to be a A-type HSF protein by
sequence analysis. PbeHSFA1d was clustered with
MdHSFA1d, PaHSF8, PpHSF8 and PdHSFA1d in
evolutionary relationship. The expression of
PbeHSFA1d was the lowest in the flower and the
highest in the stem, showing significant tissue
expression specificity. PbeHSFA1d was induced by
low temperature, heat, salt and dehydration stress,
indicating that PbeHSFA1d may be involved in the
process of resistance to abiotic stress. In brief, the
present work lays a foundation for identifying the
function of PbeHSFA1d and other more work in the
future.
ACKNOWLEDGEMENTS
This work was supported by the National Natural
Science Foundation of China (31801829), the
Natural Science Foundation of Jiangsu Province
(BK20170463, BK20170462, BK20181062).
Cloning, Expression and Sequence Analysis of PbeHSFA1d in Pyrus betulifolia
281

REFERENCES
Chauhan, H., Khurana, N., Agarwal, P., Khurana, J.P.,
Khurana, P. (2013). A seed preferential heat shock
transcription factor from wheat provides abiotic stress
tolerance and yield enhancement in transgenic
Arabidopsis under heat stress environment. PLoS One.
8(11), e79577.
Gall, H. L., Philippe, F., Domon, J.M., Gillet, F., Pelloux,
J., Rayon, C. (2015). Cell wall metabolism in response
to abiotic stress. Plants. 4(1), 112-166.
Higashi, Y., Ohama, N., Ishikawa, T., Katori, T., Shimura,
A., Kusakabe, K., Yamaguchi-Shinozaki, K., Ishida,
J., Tanaka, M., Seki, M. (2013). HsfA1d, a protein
identified via FOX hunting using Thellungiella
salsuginea cDNAs improves heat tolerance by
regulating heat-stress-responsive gene expression.
Molecular plant. 6(2), 411-422.
Jin, C., Li, K.Q., Xu, X.Y., Zhang, H.P., Chen, H.X.,
Chen, Y.H., Hao, J., Wang, Y., Huang, X.S., Zhang,
S.L. (2017). A novel NAC transcription factor,
PbeNAC1, of Pyrus betulifolia confers cold and
drought tolerance via interacting with PbeDREBs and
activating the expression of stress-responsive genes.
Frontiers in plant science. 8, 1049.
Kotak, S., Vierling, E., Baumlein, H., Koskull-Doring, P.
(2007). A novel transcriptional cascade regulating
expression of heat stress proteins during seed
development of Arabidopsis. The Plant Cell. 19(1),
182-195.
Li, M., Xie, F., Li, Y., Gong, L., Luo, Y., Zhang, Y.,
Chen, Q., Wang, Y., Lin, Y., Zhang, Y. (2020).
Genome-Wide analysis of the heat shock transcription
factor gene family in Brassica juncea: structure,
evolution, and expression profiles. DNA and cell
biology. 39(11), 1990-2004.
Liu, H., Zhang, Y., Lu, S., Chen, H., Wu, J., Zhu, X., Zou,
B., Hua, J. (2021). HsfA1d promotes hypocotyl
elongation under chilling via enhancing expression of
ribosomal protein genes in Arabidopsis. The New
phytologist. 231(2), 646-660.
Nishizawa, A., Yabuta, Y., Yoshida, E., Maruta, T.,
Yoshimura, K., Shigeoka, S. (2006). Arabidopsis heat
shock transcription factor A2 as a key regulator in
response to several types of environmental stress. The
Plant journal. 48(4), 535-547.
Ohama, N., Sato, H., Shinozaki, K., Yamaguchi-
Shinozaki, K. (2017). Transcriptional Regulatory
Network of Plant Heat Stress Response. Trends in
plant science. 22(1), 53-65.
Qiao, X., Li, M., Li, L., Yin, H., Wu, J., Zhang, S.L.
(2015). Genome-wide identification and comparative
analysis of the heat shock transcription factor family
in Chinese white pear (Pyrus bretschneideri) and five
other Rosaceae species. BMC plant biology. 15, 12.
Shen, Q., Zhan, X., Yang, P., Li, J., Chen, J., Tang, B.,
Wang, X., Hong, Y. (2019). Dual activities of plant
cGMP-Dependent protein kinase and its roles in
Gibberellin signaling and salt stress. The plant cell.
31(12), 3073-3091.
Tan, B., Yan, L., Li, H., Lian, X., Cheng, J., Wang, W.,
Zheng, X., Wang, X., Li, J., Ye, X. (2021). Genome-
wide identification of HSF family in peach and
functional analysis of PpHSF5 involvement in root
and aerial organ development. PeerJ. 9, e10961.
Tang, R., Gupta, S.K., Niu, S., Li, X.Q., Yang, Q., Chen,
G., Zhu, W., Haroon, M. (2020). Transcriptome
analysis of heat stress response genes in potato leaves.
Molecular biology reports. 47(6), 4311-4321.
Tian, F., Hu, X.L., Yao, T., Yang, X., Chen, J.G., Lu,
M.Z., Zhang, J. (2021). Recent advances in the roles
of HSFs and HSPs in heat stress response in woody
plants. Frontiers in plant science. 12, 704905.
Tian, X., Wang, F., Zhao, Y., Lan, T., Yu, K., Zhang, L.,
Qin, Z., Hu, Z., Yao, Y., Ni, Z. (2020). Heat shock
transcription factor A1b regulates heat tolerance in
wheat and Arabidopsis through OPR3 and jasmonate
signalling pathway. Plant biotechnology journal.
18(5), 1109-1111.
Wang, N., Liu, W., Yu, L., Guo, Z., Chen, Z., Jiang, S.,
Chen, X. (2020). HEAT SHOCK FACTOR A8a
modulates flavonoid synthesis and drought tolerance.
Plant Physiology. 184(3), 1273–1290.
Waterhouse, A., Bertoni, M., Bienert, S., Studer, G.,
Tauriello, G., Gumienny, R., Heer, F.T., Rempfer, C.,
Bordoli, L. (2018). SWISS-MODEL: homology
modelling of protein structures and complexes.
Nucleic acids research. 46(1), 296-303.
Yoshida, T., Ohama, N., Nakajima, J., Kidokoro, S.,
Mizoi, J., Nakashima, K., Maruyama, K., Kim, J.M.,
Seki, M., Todaka, D. (2011). Arabidopsis HsfA1
transcription factors function as the main positive
regulators in heat shock-responsive gene expression.
Molecular genetics and genomics. 286(5-6), 321-332.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
282
