
Analysis on Intestinal Microbiota in Rheumatoid Arthritis
Ruining Peng
School of Engineering and Applied Sciences, University of Pennsylvania, Philadelphia, PA 19104, U.S.A.
Keywords: Rheumatoid Arthritis, Intestinal Microbes, RA Pathogenesis, Immune System, Probiotics.
Abstract: Rheumatoid Arthritis (RA) is a prevalent symmetrical facet joint involved systemic autoimmune disease,
which is mainly characterized by swelling and deformity of the joints, causing mobility problems and
affecting living quality. Factors such as heredity, environment, and microbial infection often lead to the onset
of RA, which is also related to immune dysfunctions, but the specific pathogenesis is still unclear. Tens of
billions of microbes in the gastrointestinal tract, the biggest immune organ, participate in immunological
regulation of the human body, changes in the composition and function of which are closely related to
rheumatoid diseases such as RA. Hence, this review focuses on the relationship between intestinal microbes
and RA. Based on the analysis and summary of existing materials, it is found that the composition of microbes
in the gastrointestinal tract in RA patients is different from that in healthy group. Reductions in Bacteroides
and increases in Prevotella are strongly correlated with disease in new-onset untreated rheumatoid arthritis
(NORA) subjects. In addition, intestinal microbes and their metabolites affect the production and
differentiation of regulatory T cells (Treg cells). Besides, these substances also disrupt the balance between
Treg and helper T cells, induce the release of pro-inflammatory factors, interfere with the host immune system,
and get involved in the occurrence of autoimmune diseases. The study of the intestinal-articular axis provides
a new perspective of RA pathogenesis.
1 INTRODUCTION
RA is a chronic systemic immune disease with
synovitis and pannus as the main pathological
manifestations. The global incidence of RA is about
1%. Previous studies believe that RA susceptibility
genes are the most common cause of the disease.
However, recent epidemiological studies have
reported that the proband-wise concordance rate of
RA in monozygotic twins is lower than that in
dizygotic twins (Svendsen, Anders et al 2002),
indicating that the environment should be the
environment instead of genetic factor play a role in
such autoimmune diseases. Environmental changes,
such as humid climate, imbalance the immune
homeostasis through epigenetic modification, leading
to RA and other rheumatoid disorders (Calabresi,
Emanuele et al. 2018).
With in-depth research on microorganisms and
new understandings of their functions in the human
body, the relationship between microbes and RA has
gradually been revealed. The early hypothesis was
that Europeans were exposed to a certain version of
the pathogen in America that led to the RA on-set;
recent studies have found that RA's pathogenesis may
involve the colonization of specific microorganisms
in the human body. It has been reported that RA
might be caused by intestinal flora participating in
cell, especially immunological cells, interactions,
changing immune homeostasis, triggering
inflammatory reactions.
Changes in the composition of intestinal microbes
in RA patients, pathogenesis involved
microorganisms and their role in immune regulation,
and the application prospects of probiotics as new
treatments are current research hotspots. This review
collects, analyzes the existing research results,
discusses the genetic and environmental
susceptibility factors, changes in the microbial
composition in RA patients, the participation of
intestinal flora in human immunity, and the possible
role of RA pathogenesis. As the specific pathogenic
cause of RA is still unclear, the relationship between
gut microbes and RA will provide new research
perspectives and new ideas for adjuvant therapeutic
methods.
238
Peng, R.
Analysis on Intestinal Microbiota in Rheumatoid Arthritis.
DOI: 10.5220/0011197100003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 238-243
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

2 RA PATHOGENESIS
RA is a systemic immunological disease, which
pathogenesis includes innate immune dysfunction
and acquired immune responses, involving antigen-
presenting cells, self-reactive T cells, and antibodies.
Thus immunological dysfunction should be the
leading cause of joint damages and RA.
Cytokines and inflammatory mediators, for
example, tumor necrosis factor α (TNF-α),
interleukin-17 (IL-17), and granulocyte-macrophage
colony-stimulating factor (GM-CSF) could induce a
strong inflammatory response, play a key role in RA
pathogenesis, which is one of the critical floating
activity indicators for RA diagnosis and prognosis.
Besides, cytokines and their signaling pathways work
as effective therapeutic targets applied in RA clinical
treatment.
The high level of serum TNF-α, one of the
inflammatory factors involved in the pathogenesis of
RA, indicates the active stage. TNF is mainly
produced by activated mononuclear macrophages in
the synovial membrane, and its various effector
functions, such as inflammatory effects, are related to
RA pathogenesis. TNF triggers the activation of
several immune cells (leukocytes, endothelial cells,
etc.) as well as the production of a series of cytokines
and chemokines (IL-1, IL-6, IL-8, GM-CSF, etc.)
(McInnes, Iain B, and Georg Schett. 2007) TNF also
drives the differentiation of osteoclasts. Meanwhile,
it inhibits the formation and function of osteoblasts,
which disrupts the balance between bone formation
and destruction, causing damages to tissues in the
patient's joints. Study shows that THF antagonist can
clinically reduce the production of several cytokines,
slow down inflammatory responses, and relieve joint
damages.
The production of self-reactive antibodies is
another decisive factor in the progression of RA
disease. B cells, induced by the antigen-antibody
recognition process, stimulate T cell activation,
initiate immune responses, and trigger the production
of autoantibodies (for example, rheumatoid factors),
which will then precipitate with IgG leading to the
onset and progression of RA. Regulatory B cells
(Breg cells) direct Th cells to develop into the
memory T cell and reduce inflammatory T cells'
proliferation, inhibiting RA progression. Since it is
well-known that the imbalance between pro- and anti-
inflammatory T cells is closely correlated with the
RA disease. Th 17 cell, a new subtype of CD4+ T cell,
could induce autoantibody through the secretion of
IL-17, which is one of the pro-inflammatory factors
that amplify inflammatory responses. It has been
reported that the large amount of IL-17 positively
correlated with RA severity. Sun J, et al. found that
metallothionein-1 (MT-1) can correct the relationship
between pro- and anti-inflammatory T cells, alleviate
the pathological symptoms (for example, synovitis)
of RA by inhibiting Th17 cell while inducing the
proliferation of Treg cells (Sun, Li, Li, Ding, Liu,
Chen, Zhang, Qi, Du, Huang 2018).
3 RA SUSCEPTIBILITY
FACTORS
RA is closely related to the genetic background while
also involves several other susceptibility factors.
Among them, the body's immunity and microbial
infection are at the central position; endocrine and
environmental factors also increase RA's
susceptibility (Figure 1) (Deane, Kevin D et al. 2017).
Certain microorganisms, such as Epstein-Barr virus
and parvovirus B19, infect the human body and then
mediate autoimmune responses via polypeptide
fragments; or work as an initiating factor, first cause
local inflammatory responses (for example,
pharyngitis and sinusitis), causing immunity to start
the regulation process to fight against inflammation
but might trigger systemic immune disease including
RA as well (Mathew, Ashish Jacob, and Vinod
Ravindran. 2015). In addition, microbial infection
may change the micro-ecological environment in the
oral cavity and gastrointestinal tracts, which disrupts
the normal composition of microorganisms,
interferes with immune regulation, leading to the
enhanced susceptibility of RA. Except for microbial
infections, the incidence of RA in women is
significantly higher than that in men at the same age,
indicating endocrine and gender might participate in
RA progression. Finally, cold and humid climates,
smoking, obesity, etc. can also aggravate disease
conditions.
Analysis on Intestinal Microbiota in Rheumatoid Arthritis
239
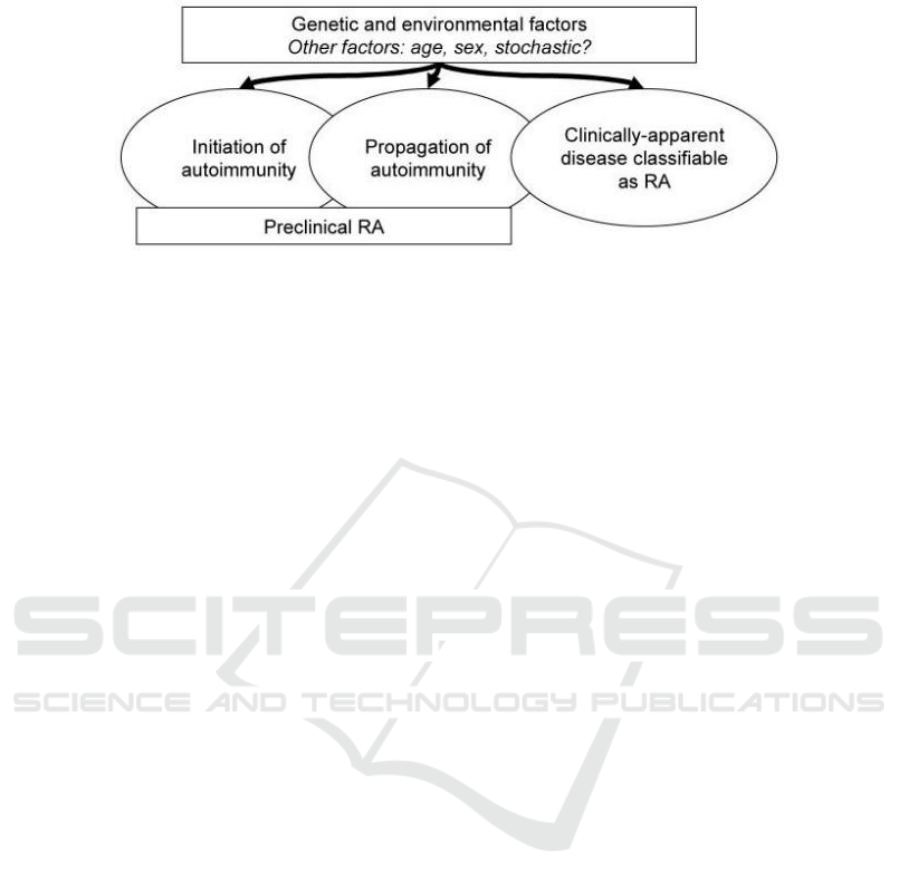
Figure 1: A general model of RA susceptibility factors.
4 RA AND GASTROINTESTINAL
MICROBES
Joint deformities and dysfunction are common
clinical manifestations of RA. While patients in the
earlier stage only have elevated serum autoantibodies
level with no obvious clinical synovitis. And research
suggests that synovial self-reactive immunity in oral
mucosa, lungs, intestines, etc., may cause systemic
autoimmune diseases, including RA (Mankia,
Kulveer, and Paul Emery. 2015). Wu et al. found that
mice under sterile conditions had alleviated RA
symptoms with the reduced number of Th17 cells and
serum self-reactive antibody titers. When using
intestinal segmented filamentous bacteria (SFB)
infected these mice, Th17 cells in lamina propria and
self-reactive antibody recovered. It indicated the
critical functions of intestinal microbes in regulating
pro- and anti-inflammatory T cells and the
progression of autoimmune diseases. Still, it should
not be ignored that the difference between human and
mouse intestinal microbiota. Analysis with RA
patient’s saliva and stool samples using sub-genome
shotgun sequencing and whole-genome association
studies (MGWAS) demonstrates the imbalance of
microbiota in both oral cavity and gastrointestinal
tract. Specifically, Haemophilus spp. decrease and
Lactobacillus salivarius increase significantly
compared to the control, indicating the probable role
of such microorganisms in autoantibody production
and disease activity.
When the environment changes, the mutual
relationship in microbial flora will be disrupted;
conditioned pathogens gain an ability to cause
disease. Prevotella is a commensal bacterium locates
ubiquitously in mucous membranes in healthy
subjects and rarely causes inflammation.
Epidemiological studies have found Prevotella can
lead to periodontitis which associates with an
increased risk of systemic immune diseases. Research
by Maeda Y, et al. showed that patients in RA early-
stage carry Prevotella dominated intestinal flora
(Maeda, Kurakawa, Umemoto, Motooka, et al. 2016),
which correlated with the decreased population of
Bacteroides and other beneficial bacteria. Thus,
intestinal microbe plays an essential role in RA
pathogenesis. In addition, infection of Prevotella goes
along with the increased number of Th17 cells in
mice, causing severe arthritis symptoms (Maeda,
Kurakawa, Umemoto, Motooka, et al. 2016). And
Prevotella can induce secretion of IL-6 and IL-23,
stimulate the proliferation of bone marrow-derived
dendritic cells, and promote five times higher IL-17.
It can be seen that changes in intestinal flora
correspond to RA disease activity.
5 THE ROLE OF
GASTROINTESTINAL
MICROBES IN RA
PATHOGENESIS
The onset and progression of RA are related to
dysfunctional immunity, while the specific
mechanism is still unclear. It is believed that the
imbalance of Treg cells and Th cells, which can cause
immune system disorders, might get involved in RA
development and disease activity. The typical healthy
human digestive tract is planted with a larger
population of Treg cells as well as 1014
microorganisms, which is ten times the number of
human cells. These large, various microbes have
significantly enriched the diversity of the host
genome, encoding 3.5 million genes, which is about
150 times more complex than the host self-genes
(Figure 2) (Cresci, Gail A, and Emmy Bawden.
2015). It has been reported that intestinal microbes
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
240
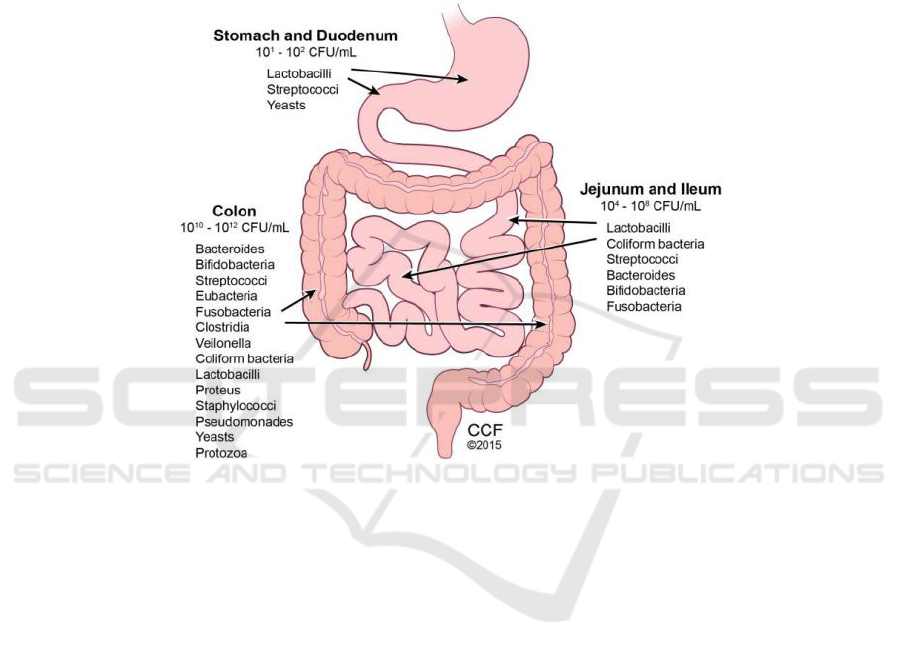
and these genes can encode proteases that host cannot
possess, which play an important role in regulating
host metabolic functions (Kho, Zhi, and Sunil K Lal.
2018). Intestinal microbes and their metabolites
affect the production and proliferation of Treg cells,
that is, by interfering with immune cells
differentiation to disrupt the normal capacity of
immune system and trigger the development of
systemic immune disease. It is also agreed that
intestinal microbes participate in cell
communications, induce inflammatory responses via
mucosal barrier devastation caused by mutual
recognition of immunological molecules. In
summary, intestine-colonized microbes can affect the
production of Treg cells, induce releases of pro-
inflammatory mediators using molecular simulation
mechanisms, interfere with the body’s immune
regulation functions, then initiate systemic
autoimmune diseases, including RA.
Figure 2: Typical microorganisms colonized human gastrointestinal tracts.
The host immune system is functioned by various
immune mediators, cells, and complicated biological
processes to defend against pathogen invasions and
diseases. When biological, pharmaceutical,
environmental factors simulate the host,
inflammatory responses will immediately be initiated
to fight against infected tissues, and repair processes
will then start. Breg cells differentiate in response to
early inflammation and restrain excessive
inflammatory responses through the secretion of IL-
10. Studies showed that in the inflammatory stage of
RA, intestinal microbes can regulate the
differentiation of Breg cells colonized in immune
tissues and organs, such as spleen and mesenteric
lymph nodes, by releasing IL-1β and IL-6.
Antibiotic-treated mice (ABX mice) and specific
pathogen-free mice (SPF mice) developed a lower
number of IL-1β and IL-6, compared to the
conventionally housed mice (CNV mice), in response
to the induction of arthritis. Besides, compared to the
control, ABX mice showed severer arthritis
symptoms with a lower-functional small number of
Breg cells due to changes in the composition of
intestine-colonized microbiota. It indicated the
participation of microorganisms in regulating
immune responses, inducing Breg cells
differentiation and retraining excessive inflammation
could alleviate RA progression (Rosser, Elizabeth C
et al. 2014). Another study showed that germ-free
mice (GF mice) developed milder arthritis (WuHJ,
IvanovII, DarceJ, etal. 2010). In addition, GF mice
had lower number of splenic autoantibody-secreting
cells, Th17 cells and Treg cells, as well as lower
serum self-reactive antibody titers. After the infection
of intestinal-specifically colonized single commensal
SFB, Th17 cell populations in lamina propria and
serum autoantibody titers have recovered.
Colonization of SFB can induce the proliferation of
CD4+ T helper cells (Th17 cells), which produce IL-
17 and IL-22 in lamina propria. Additionally,
together with the up-regulation of acute subtype
serum amyloid, activation of dendritic cells and
production of other immune mediators lead to mouse
joints destruction (Ivanov II, Atarashi K, Manel N, et
Analysis on Intestinal Microbiota in Rheumatoid Arthritis
241

al. 2009). Animals fed with Lactobacillus casei
developed milder level of pro-inflammatory factors
(IL-1β, IL-2, IL-6, IL-12, etc.), TNF-α, and IFN-γ;
together with increased population of IL-10 and TGF-
β ameliorate arthritis (Vaghef-Mehrabany, Elnaz et
al. 2013). It suggested the beneficial effects of
intestinal-specifically colonization of certain
microbes on the amelioration of RA symptoms via
regulating immune functions. In conclusion,
intestine-planted microbes have significant impacts
on the maintenance of normal immune function;
certain or conditioned pathogens can disrupt host
immune structure and affect immune tolerance,
leading to the occurrence and development of
diseases.
Intestinal microorganisms can affect host
immunity via their metabolites. Short-chain fatty
acids (SCFAs), mainly produced by the
decomposition of carbohydrates, are among the most
important microbial metabolites linked with immune
regulation. As a key energic source for intestinal
epithelial cells and intestinal microbes, SCFAs are
also involved in cell differentiation, anti-
inflammatory responses, and many other critical
metabolic processes and are therefore of great
significance in regulating host immunity. Studies
showed that SCFAs recruit granulocytes, thus
aggravate local inflammation. The host can detect
and respond to the appearance of SCFAs using the
surface located G-protein-coupled receptor 41 and
43, which then promotes Treg cells differentiation
and clustering, enhances the IL-10 production, and
thereby inhibits inflammatory responses (Lopez,
Christopher A et al. 2014). SCFAs can also stimulate
mucin 2 expression in intestinal epithelial cells. It
may play an important role in mucoprotective
function (Willemsen, L E M et al. 2003).
Above, microbes may affect the host immune
metabolism process mainly in two ways:
microorganisms and microbial metabolites. Both can
enhance the susceptibility of RA through, for
example, inducing immune cell differentiation, pro-
inflammatory mediator releases. Microbes and their
metabolic products may become new therapeutic
targets in RA clinical treatment.
6 MICROBIOME IN RA
TREATMENT
Probiotics, living microorganisms that are believed to
be beneficial, change the microbial composition of
the human body, influence disease progression.
According to clinical researches, eight weeks of oral
administration of Lactobacillus casei leads to
significantly reduced disease activity index of RA
patients. Also, patients developed lower serum
inflammatory factors (TNF-α, IL-6, IL-12) with
increased serum regulatory cytokine IL-10 (Vaghef-
Mehrabany, Elnaz et al. 2013). Many other studies,
however, demonstrated that probiotics could not
significantly change inflammatory parameters (such
as erythrocyte sedimentation rate, TNF-α, IL-6, IL-
10) and oxidative stress indicators (total antioxidant
capacity and malondialdehyde); while it did improve
the RA disease activity index (Aqaeinezhad Rudbane,
Seyed Mohammad et al. 2018). The current research
evidence is not sufficient to prove the effectiveness of
probiotic interventions in RA treatment, which still
needs extended animal and clinical trials for further
evaluation. Besides, screening for the best bacteria
species and optimizing the dose intake is also
necessary.
Probiotics can change intestinal flora. While, due
to the larger quantities of microorganisms that
colonize gastrointestinal tracts, oral probiotics can
sometimes be difficult to achieve the desired effects.
Therefore, fecal flora transplantation has become an
alternative way to adjust patient’s intestinal microbial
composition. It helps reorganize the micro-
environment of intestinal tract via transplanting
healthy people’s intestinal flora into the patients in
need, thereby improving disease conditions caused by
disorders of intestinal microbes.
In recent years, traditional DMARDs,
methotrexate represented, treatments have played a
certain role in controlling RA development.
However, such therapeutic methods cannot cure the
disease, the long-term use of which will also increase
the risk of infections and cancers. By contrast, oral
probiotics and fecal transplantation are much safer,
with more advantages in inhibiting inflammation and
improving arthritis symptoms.
7 CONCLUSIONS
Human intestinal microbiota is closely related to the
host immune system. It participates in the occurrence
and development of autoimmune diseases such as RA
by interfering with immune cell differentiation,
inflammatory mediator, and self-reactive antibody
production, while the specific mechanism of which
remains to be explored. And due to the relationship
between intestinal flora disorders and incidence of
RA, it’s plausible to predict the disease occurrence
and evaluate disease stages via detecting the human
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
242

intestinal microenvironment, which is helpful to
disease control. In addition, advanced techniques,
such as high-throughput sequencing, make precision
medicine achieved. Patients can obtain targeted
treatments, targeted use of certain probiotics, specific
changes in daily diet, etc. Future studies could
combine multiple analyses (microbiota, genomics,
and proteomics) to explore mechanisms involved
intestinal microorganisms with host immune system.
It would deepen the current understanding of the
intestinal microenvironment, leading to discovering
new biomarkers for RA diagnosis and prognosis,
providing an innovative reference for staged
treatment of RA.
REFERENCES
Aqaeinezhad Rudbane, Seyed Mohammad et al. “The
efficacy of probiotic supplementation in rheumatoid
arthritis: a meta-analysis of randomized, controlled
trials.” Inflammopharmacology vol. 26,1 (2018): 67-
76. doi:10.1007/s10787-017-0436-
Calabresi, Emanuele et al. “One year in review 2018:
pathogenesis of rheumatoid arthritis.” Clinical and
experimental rheumatology vol. 36,2 (2018): 175-184.
Cresci, Gail A, and Emmy Bawden. “Gut Microbiome:
What We Do and Don't Know.” Nutrition in clinical
practice: official publication of the American Society
for Parenteral and Enteral Nutrition vol. 30,6 (2015):
734-46. doi:10.1177/0884533615609899
Deane, Kevin D et al. “Genetic and environmental risk
factors for rheumatoid arthritis.” Best practice &
research. Clinical rheumatology vol. 31,1 (2017): 3-18.
doi:10.1016/j.berh.2017.08.003
Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal
Th17 cells by segmented filamentous bacteria. [J]. Cell,
2009, 139: 485–498.
Kho, Zhi Y, and Sunil K Lal. “The Human Gut Microbiome
- A Potential Controller of Wellness and Disease.”
Frontiers in microbiology vol. 9 1835. 14 Aug. 2018,
doi:10.3389/fmicb.2018.01835
Lopez, Christopher A et al. “Collateral damage:
microbiota-derived metabolites and immune function
in the antibiotic era.” Cell host & microbe vol. 16,2
(2014): 156-163. doi:10.1016/j.chom.2014.07.009
Maeda Y, Kurakawa T, Umemoto E, Motooka D, et al.
Dysbiosis Contributes to Arthritis Development via
Activation of Autoreactive T Cells in the Intestine. [J].
Arthritis & rheumatology (Hoboken, N.J.), 2016,
68(11).
Mankia, Kulveer, and Paul Emery. “Is localized
autoimmunity the trigger for rheumatoid arthritis?
Unravelling new targets for prevention.” Discovery
medicine vol. 20,109 (2015): 129-35.
Mathew, Ashish Jacob, and Vinod Ravindran. “Infections
and arthritis.” Best practice & research. Clinical
rheumatology vol. 28,6 (2014): 935-59.
doi:10.1016/j.berh.2015.04.009
McInnes, Iain B, and Georg Schett. “Cytokines in the
pathogenesis of rheumatoid arthritis.” Nature reviews.
Immunology vol. 7,6 (2007): 429-42.
doi:10.1038/nri2094
Rosser, Elizabeth C et al. “Regulatory B cells are induced
by gut microbiota-driven interleukin-1β and
interleukin-6 production.” Nature medicine vol. 20,11
(2014): 1334-9. doi:10.1038/nm.3680
Sun J, Li L, Li L, Ding Li, Liu X, Chen X, Zhang J, Qi X,
Du J, Huang Z. Metallothionein-1 suppresses
rheumatoid arthritis pathogenesis by shifting the
Th17/Treg balance. [J]. European journal of
immunology, 2018.
Svendsen, Anders J et al. “Relative importance of genetic
effects in rheumatoid arthritis: historical cohort study
of Danish nationwide twin population.” BMJ (Clinical
research ed.) vol. 324,7332 (2002): 264-6.
Vaghef-Mehrabany, Elnaz et al. “Probiotic
supplementation improves inflammatory status in
patients with rheumatoid arthritis.” Nutrition (Burbank,
Los Angeles County, Calif.) vol. 30,4 (2014): 430-5.
doi:10.1016/j.nut.2013.09.007
Willemsen, L E M et al. “Short chain fatty acids stimulate
epithelial mucin 2 expression through differential
effects on prostaglandin E(1) and E(2) production by
intestinal myofibroblasts.” Gut vol. 52,10 (2003):
1442-7. doi:10.1136/gut.52.10.1442
WuHJ, IvanovII, DarceJ, etal. Gut-
residingsegmentedfilamentous bacteria drive
autoimmune arthritis via T helper 17 cells[J].
Immunity, 2010, 32(6): 815-827.
Analysis on Intestinal Microbiota in Rheumatoid Arthritis
243
