
Application of Enzyme in Pharmaceutical Engineering
Ouyang Tu
YK PAO School, Shanghai, 200333, China
Keywords: Biocatalyst, Enzyme, Pharmaceutical Engineering, Drugs, Lipase.
Abstract: Enzymes have found massive applications as every industry is becoming more environmentally friendly. In
recent decades, the pharmaceutical industry has successfully applied enzymes in drug manufacturing as
catalysts and as a part of API. Biocatalytic progress is mild and green compared to the chemical catalytic
process. This paper discovers the new pharmaceutical engineering progress from three aspects: the
development of biocatalysts, potential risks of biocatalytic process, and a significant biocatalyst-lipase. This
paper also explores the process through recent experiments and achievements, discusses the advantages and
disadvantages of enzyme-catalyzed processes through comparisons with the original chemical catalysts. The
research on biocatalysts helps more scientists and students to learn about the latest techniques and
achievements in pharmaceutical engineering. It’s possible to solve problems associated with complex
molecules and pollutions created by chemical catalyzed processes. The application of enzyme is a crucial
improvement in developing drugs.
1 INTRODUCTION
The enzyme has become an important part of the
food, feed, chemical, biological and pharmaceutical
industries. The application and development of
recombinant DNA and bioengineering using various
enzymes in the past decades (Goutam, 2016), such as
using genetically engineered bacteria to make human
insulin, made huge progress in manufacturing drugs.
As enzymes are environmental-friendly,
biodegradable, and sustainable, biocatalysts are
established as a better alternative to original chemical
catalysts (Liang et al. 2016). Therefore, enzymes are
not only applied in API, multiple kinds of enzymes
are useful in the chemical synthesis of complex
molecules and the improvement of drug qualities. The
conditions of producing enzyme are mild
temperature, normal pressure, and neutral pH level,
which saves cost, energy, and improves the stability
and safety of the production comparing to the
chemical catalytic process. The catalytic efficiency is
100 times higher at least (Andrew et al. 2016). With
these advantages, many biocatalytic and enzymes
have been used for commercial benefits. It’s one of
the reasons for the fast development of
biopharmaceutics. However, it’s still a niche tool in
the whole pharmaceutical engineering. This paper
focused on three aspects of recent achievements and
risks in utilize biocatalysts and enzymes, including
one of the most special hydrolytic enzymes, lipase.
The importance of lipases exceeded the status of
proteases and amylases as the complexity of API
continuously increases, according to the enzyme’s
advantage in chemical synthesis (Saxena et al. 1999).
Different types of medicine required specific
biocatalytic as they have unique chemical functions
of API. Sophisticated compounds can be achieved by
late-stage modification, which requires biocatalysts.
Discovering the suitable biocatalyst through the
process of biocatalytic retrosynthesis followed by
engineering is the basic route. The theory of how to
form multi-functional compounds while ensuring
their reactivity is important (Elvira et al. 2021). Even
though it seems like a time-consuming and expensive
process, it’s still much quicker and cheaper than
finding a new chemical synthesis for the catalytic
process (Andrew et al. 2016).
In the recent development, biocatalysts are
usually used during producing small molecule
intermediates and APIs. Biocatalysts are efficient in
the entire process of developing new medicines,
especially with complex drug targets. Despite the
benefits, there are several risks to be considered.
Investigating potential hazards in the process of API
synthesis using biocatalysts is crucial, as the quality
of drugs should be ensured. There will be some
Tu, O.
Application of Enzyme in Pharmaceutical Engineering.
DOI: 10.5220/0011196900003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 231-237
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
231

residual enzymes in the drug, and these might be
harmful to certain patients. It’s essential to develop
appropriate residual enzyme control strategies when
the drug enters clinical trial to assure the quality by
raising the purity of API (Andrew et al. 2016).
Lastly, lipase is one of the most outstanding
micro-origin enzymes that is known for its massive
contributions and wide application as a biocatalyst
(Rohit et al. 2013). The reason behind this is its
unique attributes. Microbial lipases can be
biocatalytic in both aqueous and non-aqueous
reactions, which differentiates lipases from proteases
and amylases. Its huge potential in organic synthesis
determines its remarkable importance. A magnificent
usage of lipases in the production of chiral drugs,
anticholesterol, and anti-Alzheimer’s are two
examples (Ramesh et al. 2001). As lipase is a micro
origin enzyme, it can be easily extracted from bacteria
with unlimited supplies at any time. Also, it’s
relatively cheap. Lipases are suitable for commercial
applications (Rohit et al. 2013).
The job content of developing medicines with
biocatalysts is using biocatalytic retrosynthesis to
identify suitable synthons, then design the
corresponding biocatalysts. This process needs to be
repeated for every enzyme because each one of them
has unique and distinct composition (Elvira et al.
2021). The details of the process will be discussed in
the next section. The application of biocatalysts is
significant in two perspectives: sustainable
development and medical development. Firstly,
the enzyme-catalyzed process saves an enormous
amount of energy and costs comparing to chemical
catalytic progress. Most enzymes are fairly cheap to
purchase, and the reaction only requires mils
conditions (Andrew et al. 2016). Enzymes are also
biodegradable, so zero pollution is released from the
production. Recently, scientists discovered that when
enzymes are conjugated to stimuli-responsive
polymers, they can protect enzymes by changing their
structures and manage enzymes’ activities when
facing external stimuli. Therefore, enzymes can be
extracted from the reaction mixture effortlessly and
be reused in later reactions (Truppo, 2017). Secondly,
as enzymes are basically proteins from the human
body, they usually have better therapeutical effects.
Protein, hormone, and polypeptide’s molecular
weight is over 10000u, which exceeds the most
advanced techniques of organic chemistry.
Biocatalysts are exquisitely selectivity in protein
engineering, which lead to the success in
manufacturing new drugs with complicated formulas
and solving the bottlenecks in organic chemistry
(Truppo, 2017). By decreasing the price of drugs,
more people can afford the treatment. Overall, the
health condition of the whole population will
increase.
2 LITERATURE REVIEW
2.1 Development and Advantages of
Biocatalyst and LSMs
In organic chemistry, the C-H bond is the
fundamental of all compounds. Before enzymes
emerge, the selective modification of the C-H bond is
the top challenge. In early-stage functionalization, the
modification starts from a nonfunctionalized
compound. After multiple steps, the compound is
attached to the same functional group (Figure 1).
There’s no diversification. In late-stage
functionalization with biocatalyst, targeted
modification of C−H and C−heteroatom bonds
becomes realistic. The site-specific transformations
with functional groups on the compound offer
diversification at the final synthetic step. With
various possibilities of multi-functional compounds,
many new drugs are discovered. However, the main
drawbacks of this method are cross-reactivity and
incompatibility due to the different chemical
properties of different atoms (Elvira et al. 2021).
Figure 1: Progress of Early-stage functionalization VS
Late-stage functionalization
Another advantage of applying enzymes is that
enzymes are functional for the synthesis of complex
metabolites in aqueous media with no requirements
of extra costs on protection. An example of metal
catalysis is catalytic metallodrugs, which contain
artificial nucleases and artificial proteases. With a
catalytic metal center and a targeting domain,
catalytic metallodrugs can overcome the limitation of
normal drugs binding reversibly to their targets
(Joyner et al. 2013). This development can
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
232
significantly improve the effectiveness and reduce the
toxicity of drugs (Robinson et al. 2004).
Before the usage of biocatalysts, every substance
requires specified biocatalysts. Using biocatalytic
retrosynthesis on target molecules, which is
disconnecting chemical bonds to identify reasonable
synthons for biocatalysis. The results can be verified
by computer-aided synthesis planning. There are four
ways in the pyramid to screen, design, discover or
engineer the suitable enzyme in order to satisfy the
tailored biocatalytic manufacture (Figure 2). Genetic
engineering, rational mutagenesis, and detect unseen
biocatalysts from nature are three common, potent
methods to increase biocatalyst diversity. Ancestral
sequence reconstruction is a new method. The
artificial ancestors of known enzymes are likely
endued with higher robustness and can accept
extended types of substrates (Elvira et al. 2021).
Figure 2: Process of developing tailored biocatalysts.
Even though the bond energy of C-H does not
always have a positive correlation with the reactivity
of the compound, the bond strength still decides the
selectivity of activation in most of the reactions. The
C-H bond is directed functionalization is affected by
a metal ion. Comparing to catalyst-controlled
functionalization, biocatalyst protects the C-H bonds
by using a site-specific modification (Figure 3).
Therefore, in this example, the reactivity of the
hydrocarbon compounds manufactured by directed
functionalization is probably lower than hydrocarbon
compounds manufactured by catalyst-controlled
functionalization (Elvira et al. 2021).
Figure 3: Directed functionalization and catalyst-controlled functionalization.
The development of enzyme makes the
manufacture of complex compounds with high yields
possible. In 2011, a survey shows that amide
couplings are applied to 16% of all reactions in
medicinal chemistry. As one of the most commonly
used motif, the improvement in biocatakysts are
crucial. The biosynthesis of tabtoxin discovers a
miscellaneous ATP-grasp enzyme, TabS. It is able to
constitute variety of dipeptides from unprotected
amino acids. The suitable dipeptides including 136
Application of Enzyme in Pharmaceutical Engineering
233
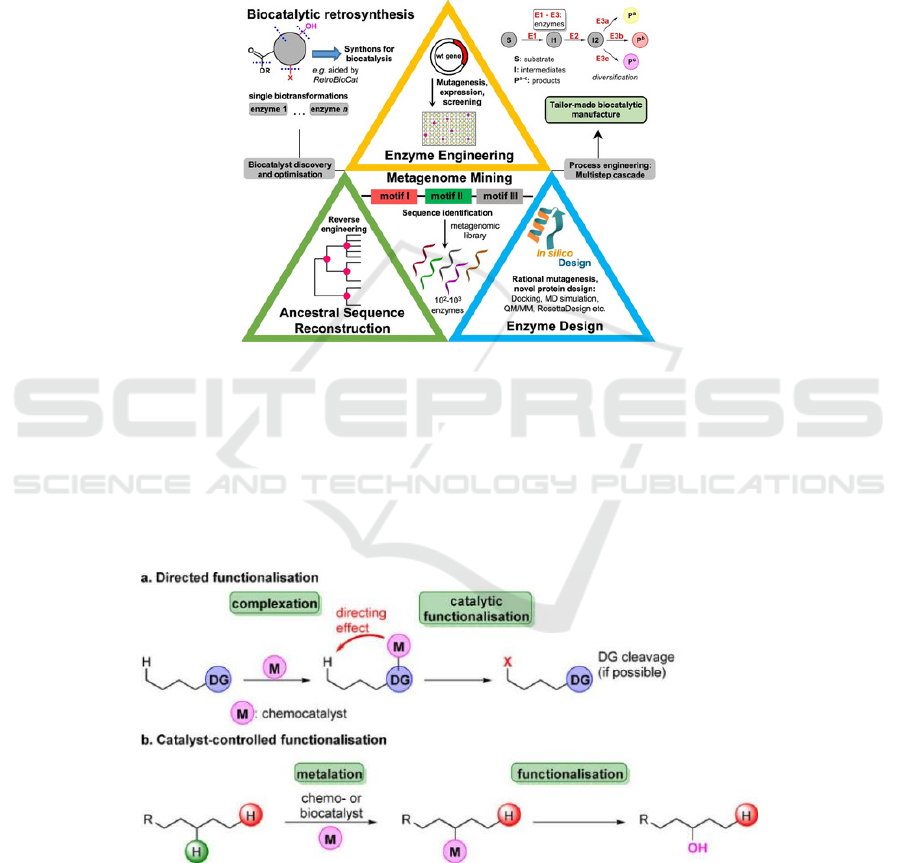
types of amino acid combinations is a supreme
challenge for chemical engineering. The original
method to produce 244 is by the usage of different
biocatalytic approaches on prochiral ketone precursor
(242) or the racemic amine (rac-244). The application
of AspRedAm, which produce 244 from 242 and
propargylamine (243), has the conversion of 97% in
Figure 4 (Elvira et al. 2021).
Figure 4: Biocatalytic approaches for (R)-rasagiline (244) synthesis.
2.2 Quality of Drug Manufactured by
Biocatalyst
For the procedure of selecting appropriate enzymes,
biocatalytic retrosynthesis and a reliable database can
be helpful. Publishing detailed rules and guidelines of
biocatalytic retrosynthesis can require chemists to
have a deeper understanding of the structures and
properties of the molecule as they need to investigate
the potential transformations and applicable
intermediates for the biocatalytic process. The
support of computer-aided synthesis planning can
ensure the enzymes are viable. A database containing
information on identified and practiced biocatalytic
reactions are also extremely conducive to chemists. If
they can check the safety, scalability, substrate scope,
conversions, and productivity of a range of suitable
biocatalysts before the real experiment, so time and
money can be saved. As engineering, a workable
biocatalyst is already more cost-effective and less
time-consuming than chemical catalysts without the
database, the positive effect on the whole
manufacturing process’ efficiency, sustainability, and
safety will assure the drug quality (Andrew et al.
2013).
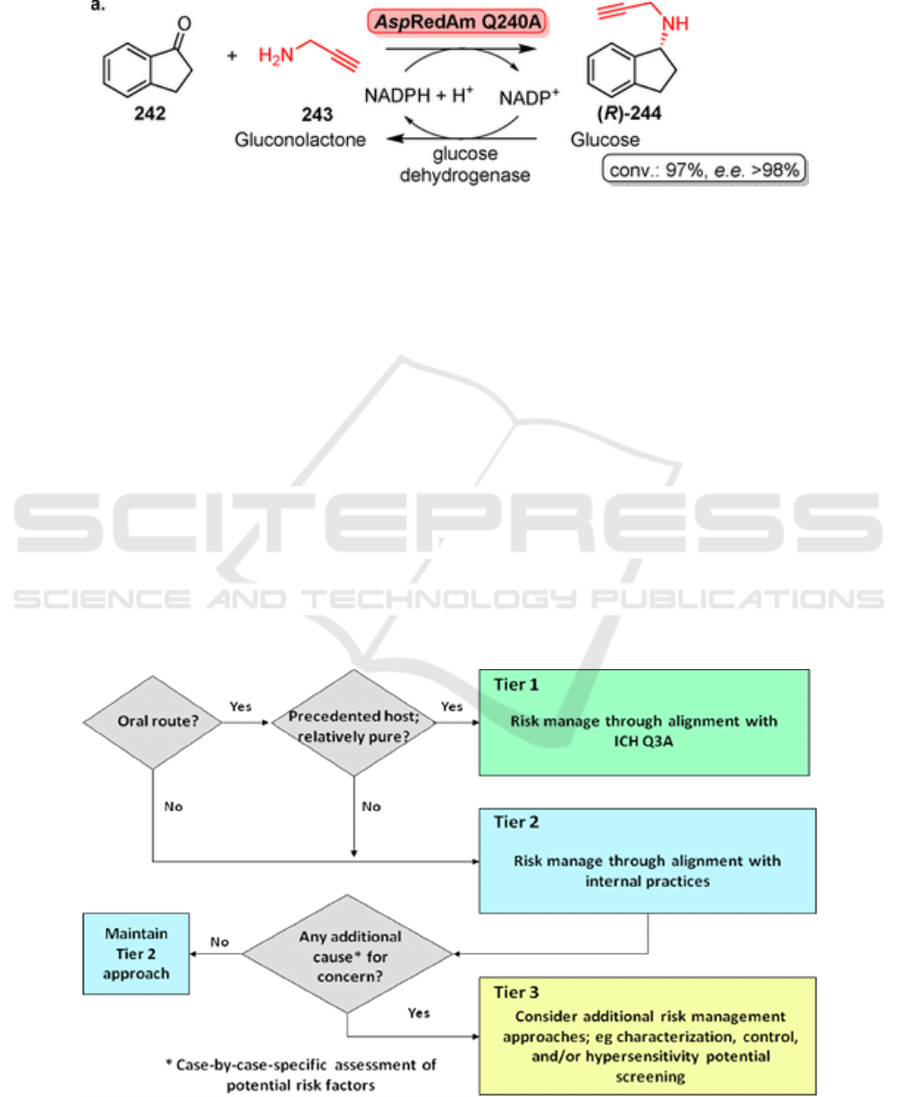
After the usage of biocatalysts, some residue
enzymes are possibly left in API. The property and
safety data of the protein residues should be
considered in science-based risk assessment. For
example, amino acids and peptides are not toxic.
Furthermore, ease patients should fill an individual
risk assessment according to their condition to clarify
their endurance to protein residues (Andrew et al.
2013) (Figure 5).
Figure 5: Basic tiered risk assessment.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
234
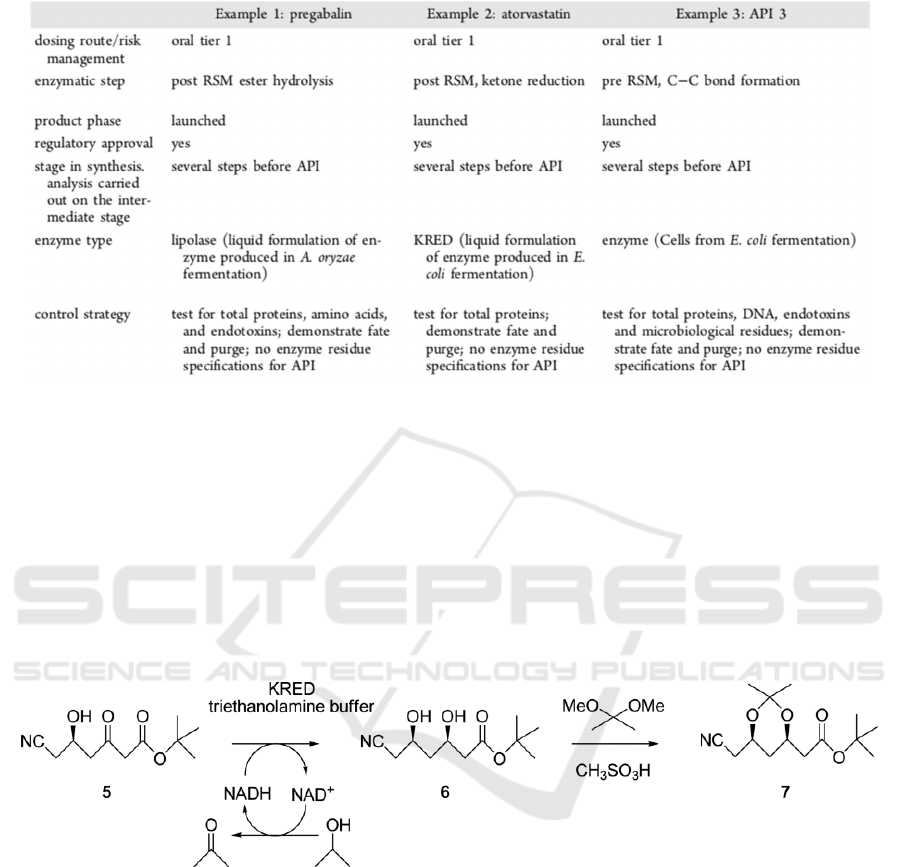
Table 1: Enzyme Fate Across Three API Projects.
During the manufacture of API, continuous
operations are enabled to remove residue enzymes.
E.g., filtration, distillation, and pH adjustment. None
of the residue proteins from enzyme preparations are
expected to pass through the operations with the API.
Therefore, the anticipation is the absence of residue
enzymes in intermediates and APIs in Table 1. The
production of atorvastatin is a piece of supportive
evidence. A biocatalytic process is carried out for the
synthesis of Acetonide 7, which is a crucial
intermediate in the formation of atorvastatin as can be
seen from Figure 6. The procedure was implemented
by adding acetone to recombinant E, an organic layer
containing diol 6, and an aqueous phase containing
the enzyme are formed. The analysis of crude 6
samples and isolated intermediate 7 both from 4 lots
have the result of no perceivable protein (Bradford
protein assay, LOD < 0.01%). This data demonstrates
that separating aqueous and organic phases can
practically remove residue enzymes, which proves
the absence of residue enzymes in atorvastatin. All
agencies responsible for drug registration approved
the strategies of not assaying residue enzymes in APIs
(Andrew et al. 2016).
Figure 6: Chemoenzymatic synthesis of pregabalin.
Pregabalin, which is the API in Lyrica, is also
manucfactured through a chemoenzymatic process.
as can be seen from Table 1. 3 samples form 10 lots
are tested with Bradford protein assay, and 20 lots are
tested with the Micro BCA assay for total proteins.
The result is no residue protein are perceived with
LOD 0.04% w/w and LOD < 0.1). After
derivatization with EZfaast, 24 commercial lots of
pregabalina are also analyzed with LC/MS/MS. None
of the samples have amino acidsabove the LOD
(0.05%) (Andrew et al. 2016).
2.3 Contributions of Lipase
Lipases have become the top choice in enzymes for
organic chemists, pharmacists, and other professors
because of their unique properties. The activity of
lipases can be easily controlled as it only works at the
oil-water interface in Figure 7. Fats are only
hydrolyzed in this certain condition. By adding an
emulsifier, followed by stirring, the interface area
will increase constantly until its limitation (Saxena et
al. 1999). Therefore, the efficiency of API
manufacturing and the efficacy of drugs containing
lipases can reach the optimum.
Application of Enzyme in Pharmaceutical Engineering
235

Figure 7: lipolytic reaction at the oil-water interface.
According to the properties mentioned above,
lipases are very popular in organic synthesis. Lipases
can be applied in the discovery or development of
drugs using the method of organic synthesis.
Chemists can manage the speed of the reactions easily.
Additionally, catalysts are always expensive, so
lipases enabled more chemists to implement more
researches without worrying about costs by the
technique of crude enzyme preparation. The
regiospecificity and strong tolerance to a variety of
organic substances make lipases even more suitable
and capable for the catalyst of organic synthesis as
most of the enzymes do not have these two properties
(Saxena et al. 1999).
Lipases also have magnificent contributions to
chiral drugs due to their enantioselective and
regioselective nature. The effectiveness and efficacy
of many drugs rely on chirality. With the ability of
selective reaction at functional group and the
preference of forming enantiomers, preparing chiral
drugs intermediates using biocatalysts is well
recognized (Rohit et al. 2013). For example, one of
the key intermediates in the synthesis of an
antihypertensive drug, Diltiazem, is successfully
produced after lipases solve the hydrolysis of epoxy
ester alcohols (Saxena et al. 1999).
Most of the biocatalysts have disadvantages, such
as being easily affected by heat, poor stability, require
a neutral pH level and room temperature. It is simple
for enzymes to become denatured. Although enzymes
are relatively cheap, they should be employed for at
least 3 months or 30 batches. Any accidental denature
that happened is considered a loss. Nowadays,
scientists are trying to discover thermostable
biocatalysts as thermo stabilization is a necessary step
to improve the robustness of enzymes (Shakya et al.
2018). Robustness of enzymes is especially vital
because designing biocatalysts requires enzymes to
constantly expose in the probably unstable
environment of organic reactions, which increases the
chance of denaturing and inactivation. Normally,
thermo stabilization is achieved by accumulating
numbers of mutations in directed evolution. However,
the potential risk is to sacrifice the catalytic function
(Shakya et al. 2018). Thermophilic lipases have been
discovered in recent decades. It can endure harsh
conditions while keeping the former advantages. Also,
it is extracted from various microorganisms, for
example, escherichia coli (Rohit et al. 2013).
Microbial origin lipases then become one of the best
choices in enzymes.
3 CONCLUSION
The emerging, developssment and application of
biocatalysts in pharmaceutical industries have
effectively made the industry more advanced and
more reliable. LSF is the fundamental support of
successful drug developments. The achievement of
complex scaffolds, which stimulates the
diversification of compounds, determines the huge
progress made in modern synthetic organic
chemistry. Comparing to chemical catalytic progress,
biocatalysis is more sustainable, cost-effective, and
environmentally friendly. Testing in the API
specification is not necessary, because the basic
chemical operations can purge residue proteins.
Therefore, the possible risks of residue enzymes don't
really exist. Lipases are one of the most important
biocatalysts. The special properties of lipases solved
many crucial problems and produced many complex
intermediates that have no resolution in the chemical
industry now. It is not just applicable in various
industries, but also the best catalyst to many
pharmaceutics. Despite the significant achievements
of lipases, more researches are needed to completely
understand it.
Biocatalysts will probably be more important and
widespread in pharmaceutical engineering by
assessing the potential commercial benefits that
enzymes can create. The whole process of
engineering biocatalysts will have huge
improvements and become significant on drug
discovery. The most crucial part is to apply computer-
aided synthesis planning in recognizing possible
synthons and choose the most efficient process to
design the suitable biocatalysts (Elvira et al. 2021).
With the tremendous efforts of some drug companies
inventing drugs with enzyme-catalyzed processes, the
environment will be better when we dispose of less
toxic gases and chemical products. More complex
diseases can be treated and more people will ve cured.
The application of biocatalysts offers something more
beneficial than engineering tanglesome molecules or
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
236

solving problems in organic chemistry. The
healthcare and life expectancy in the world will
slowly increase. Scientists and pharmaceutics have
only learned the tip of the iceberg, there are so much
to explore, expand and reinforce.
ACKNOWLEDGMENTS
Writing a paper about new techniques can be very
challenging for a high school student. I want to
appreciate Professor Axel for teaching me basic
knowledge of pharmaceutical engineering, so I have
the chance to explore this topic. My TA, Ben, also
explains and expands professor’s contents, which
enables me to understand and write about the niche
topic. Ms. Wang helps me to state and paraphrase my
ideas into a paper, so I should be thankful to her, too.
Lastly, my parents are always being supportive and
helpful. Without them, I might not have the courage
and capacity to write this paper. Once again, I would
like to express my heartfelt thanks to everyone who
helped me.
REFERENCES
Andrew S. Wells, John W. Wong, Peter C. Michels, David
A. Entwistle, Keith Fandrick, Gregory L. Finch,
Animesh Goswami, Heewon Lee, Stefan Mix, Thomas
S. Moody, Long Pang, Robert K. Sato, Nicholas J.
Turner, and Timothy J. Watson. (2016). Case Studies
Illustrating a Science and Risk-Based Approach to
Ensuring Drug Quality When Using Enzymes in the
Manufacture of Active Pharmaceuticals Ingredients for
Oral Dosage Form, pp.594-600.
Elvira Romero, Bethan S. Jones, Bethany N. Hogg, Arnau
Rué Casamajo, Prof. Martin A. Hayes, Prof. Sabine L.
Flitsch, Prof. Nicholas J. Turner, Dr. Christian
Schnepel. (2021). Enzymatic Late-Stage
Modifications: Better Late Than Never.
Eric J. Moore, Dmitri Zorine, William A. Hansen, Sagar D.
Khare and Rudi Fasan. (2017). Enzyme Stabilization
via Computationally Guided Protein Stapling, National
Academy of Sciences, Vol. 114, No. 47, pp. 12472–
12477.
Goutam Brahmachari. (2016). Biotechnology of Microbial
Enzymes, Academic Press, pp.6.
Joyner J. C., Cowan J. A. (2013). Target-directed catalytic
metallodrugs, Brizillian Journal of Medical and
Biological Research, Vol.46, No. 6.
Ramesh N. Patel. (2001). Enzymatic Synthesis of Chiral
Intermediates for Drug Development.
Robinson, Mark A., Stuart T. Charlton, Philippe Garnier,
Xiang-tao Wang, Stanley S. Davis, Alan C. Perkins,
Malcolm Frier, Ruth Duncan, Tony J. Savage, David A.
Wyatt, Susan A. Watson, Benjamin G. Davis, Robert
Langer. (2004). “LEAPT: Lectin-Directed Enzyme-
Activated Prodrug Therapy, National Academy of
Sciences, Vol. 101, No. 40, pp. 14527–14532.
Rohit Sharma, Vishal Thakur, Monika Sharma, Nils-Kåre
Birkeland. (2013). Biocatalysis Through Thermostable
Lipases: Adding Flavor to Chemistry, Thermophilic
Microbes in Environmental and Industrial
Biotechnology, Springer, Dordrecht, pp.905-927.
Saxena, R., Ghosh, P., Gupta, R., Davidson, W., Bradoo,
S., Gulati, R. (1999). Microbial lipases: Potential
biocatalysts for the future industry, Current Science,
Vol. 77, No. 1, pp. 101–115.
Shakya, Akhilesh Kumar, Kutty Selva Nandakumar. (2018).
An update on smart biocatalysts for industrial and
biomedical applications, Journal of the Royal Society,
Vol. 15, No. 139.
Truppo, Matthew D. (2017). Biocatalysis in the
Pharmaceutical Industry: The Need for Speed, ACS
medicinal chemistry letters, Vol. 8, No. 5, pp. 476-480.
Youyun Liang, Mingzi M. Zhang, Ee Lui Ang, Huimin
Zhao. (2016). Biocatalysis for Drug Discovery and
Development.
Application of Enzyme in Pharmaceutical Engineering
237
