
Conditions for a Microbial Consortium for the Biological
Degradation of Plastic Polymers
Xinyue Liu
1a
and Finn Stirling
2b
1
Wuhan Britain China School, Wuhan, China
2
The University of Cambridge, U.K.
Keywords: Biodegradation, Polymer, Plastic, Consortium, Microbiology.
Abstract: Plastic pollution is one of the most serious environmental problems the world faces. Conventional
approaches to plastic waste, such as landfilling, incineration and recycling are not sufficient to maintain an
equilibrium between plastic production and decomposition. Since plastics can provide an energy source for
microbes, biotic degradation could contribute a solution towards excessive plastic waste. Over the last 30
years, hundreds of species have been described to degrade one or more of the six most common polymer
types. By combining multiple types of species to form consortias, the efficiency of degradation can be
synergistically increased. In this study, we consider the factors that would contribute to a microbial
consortium, and what combinations of species would most effectively degrade plastic polymers. Abiotic
factors such as temperature, oxygen level, pH, UV exposure, moisture level, carbon availability and
abundance of trace elements are considered. The origin of individual microbes is considered with regard to
their compatibility. The usefulness and shortcomings of reported degradation efficiencies is discussed. A
theoretical consortium is recommended, taking into account individual strain decomposition efficiency and
the comprehensiveness of plastic degradation.
1 INTRODUCTION
1
Plastic pollution is one of the greatest environmental
concerns of our time, adversely affecting most
biomes and wildlife across the globe. The growth in
volume of plastic production alongside the longevity
of most plastic polymers have both contributed to
this issue. Annual plastic production has increased
rapidly since 1940, with global plastic production
reaching 365 million tons in 2019. The most
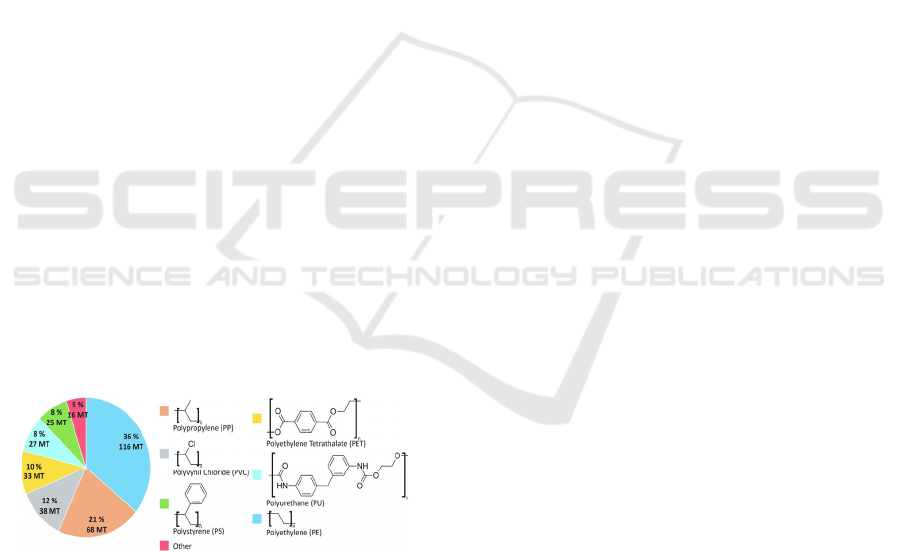
common plastics polymers, which account for about
80% of worldwide plastic production, are
polyethylene (PE), polypropylene (PP), polyvinyl
chloride (PVC), polystyrene (PS), polyethylene
terephthalate (PET) and polyurethane (PU). It has
been predicted that in the next decade, if there are no
improvements to manage the pollution, 99 million
tons of plastic waste will end up in the environment
by 2030.
Due to its longevity in nature, and its propensity
to be used as a disposable item, the output of plastic
a
https://orcid.org/0000-0001-5123-0402
b
https://orcid.org/0000-0002-5960-4429
polymers vastly outpaces its natural degradation.
Plastic can take from 20 to 500 years to decompose,
depending on its structure and chemical
composition. The longevity of plastic is estimated to
be hundreds to thousands of years, and is likely to be
even longer in deep sea and non-surface polar
environments.
2 METHODS
Conventional approaches to plastic waste, including
landfilling, incineration and recycling, all come with
their own drawbacks. Land filling can lead to
contamination of earth's surface and result in
anaerobic production of methane gas which
contributes to climate change. Furthermore, because
of the large footprint required by landfills, the
habitat of animals may be affected and displaced. In
addition, landfilling produces leachate that pollutes
the surrounding water and soil. The incineration of
waste plastic material produces toxic gases, which
can result in human health complications such as
lung disease and carcinomas. During the process of
198
Liu, X. and Stirling, F.
Conditions for a Microbial Consortium for the Biological Degradation of Plastic Polymers.
DOI: 10.5220/0011195700003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 198-203
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All r ights reserved

burning plastic, dangerous chemicals like
hydrochloric acid, sulfur dioxide, dioxins, furans and
heavy metals can be discharged. These kinds of
emissions are known to be carcinogenic and can
cause respiratory diseases. The burning of
polystyrene polymers from food packaging releases
styrene which can be absorbed through the skin and
the lungs. When it comes to plastic recycling, there
are several problems that need to be addressed. First,
it generates economically low yields, with
complications such as the removal of dyes, fillers
and other additives increasing the cost of recycling.
Second, individual polymer types require their own
specific pathways to be recycled, therefore plastic
waste needs to be sorted before efficient processing
can take place. These factors make recycling
suboptimal for many countries (Suchismita
Satapathy 2017).
The abiotic degradation of plastics takes place
through four main pathways, oxidation, photolysis,
hydrolysis and mechanical shearing (Figure 1). The
mechanical shearing of polymers is influenced by a
wide variety of environmental factors such as
weathering and freeze thaw cycles. Plastic waste
gradually experiences cracking, surface erosion,
abrasion, and breakdown to mesoplastics (~5–20
mm), large microplastics (~1–5 mm), small
microplastics (~20–999 μm), and nanoplastic (<1
μm) sized pieces. In the presence of water,
hydrolysis of those plastics with heteroatoms in their
backbone causes cleavage of ester bonds, allowing
the rapid breakdown of PET or PU to their monomer
constituents. Exposing polymers to UV radiation
causes photodegradation, and is considered the
primary method of degradation for discarded plastic
pollution. UV radiation provides the energy to break
C-H or C-C bonds, forming a highly reactive radical
group. Alternatively, UV radiation can lead to the
dechlorination of a C-Cl bond in PVC, releasing
chlorine. The first step of polymer oxidation is free
radical formation, often initiated by UV mediated
breaking of C-C and C-H bonds. The carbon
centered free radicals that are generated can then
react with free oxygen in the environment, leading to
the formation of peroxy radicals; or react with the
polymer, causing the formation of more carbon
centered free radicals. In an effort to quench the free
radicals, this often results in chain linking and chain
scission reactions in the polymer backbone.
Figure 1: Four major pathways of abiotic polymer
degradation. “R” represents a polymer chain.
Plastics present a poor food source for most
organisms, but there are those that have evolved the
cellular machinery required. Plastic polymers
synthesised from fossil fuels are a relatively new
addition to world ecosystems, and generally
represent an inefficient reservoir of energy. As such,
there has not been the opportunity for biology to
evolve an effective means of degrading them. The
millions of tons of plastic that ends up in the sea,
landfills and other environments has created a
reservoir of potential energy for microbial life. The
microbial degradation of plastic plays a not
insignificant role in polymer degradation, with over
400 species having been identified to date. The
general biodegradation mechanism for polymer
breakdown can be summarised in five steps;
secretion, adsorption, release, uptake and
degradation (Figure 2). Enzymes such as PETase,
Cutinase, or perhaps alkane hydroxylases are
excreted by the cell into the extracellular. These
enzymes will then adsorb to the hydrophobic
polymer surface, a process facilitated by the
formation of biofilms. The enzymes secreted can
then bind to their potential substrates, the polymers,
and interact with them. Enzymes can successfully
break down the large polymers into smaller
segments or monomers which are then released.
These smaller fragments can then be uptaken into
the cell through specific importers. Once in the
cytoplasm, additional specific enzymes may be
required to enact modification before the polymer
fragments can be incorporated into cellular
metabolism.
Conditions for a Microbial Consortium for the Biological Degradation of Plastic Polymers
199
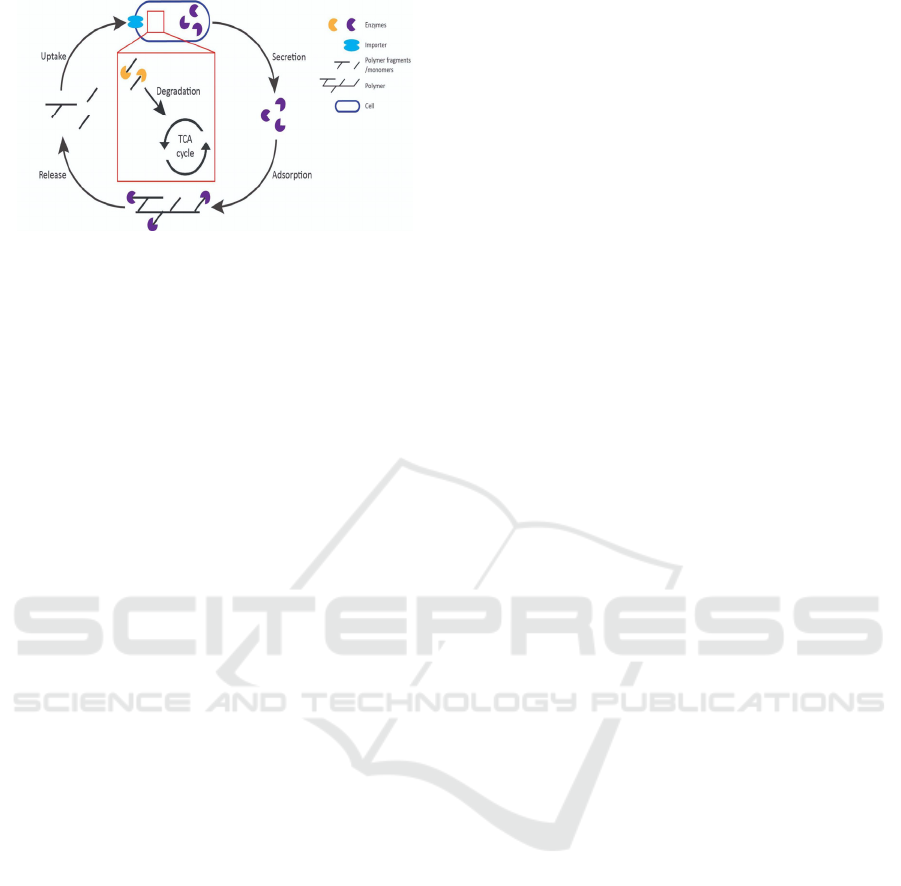
Figure 2: Proposed mechanism for microbial led
biodegradation of polymers.
Microorganisms will often exist in nature in
established consortiums, allowing for powerful
benefits to all its members, including the capacity to
degrade substances. Cultures with multiple species
have better communal properties like robustness and
division of labor. Various components are required
for the biological breakdown of plastics (Figure 2),
and dividing their production over multiple species
could lead to synergistic effects. Robustness is the
trait of microbes which enables them to survive in
varieties of unstable environments. Such a property
is presented in lichens, a composite organism which
can include bacteria, fungi, and algae. When algae
and fungi are alone, they both suffer from oxidative
damage during desiccation. But when in a
consortium, both enable up-regulation of protective
systems. Without the contact with fungus, the algae
can tolerate only low levels of light, and their
photoprotective systems are not upregulated; in the
absence of algae, the glutathione-based antioxidant
system of fungus is slow and invalid. There are
already some successful applications of consortium
in the field of plastic degradation. Individual
bacterial strains were isolated from various faecal
samples, and tested both individually and in
consortium for their capacity to degrade
polyethylene or polyethylene and polypropylene.
Individually, degradation rates ranged from 15.5%
to 29% over the course of the assay, but in
consortiums of four they achieved 75% efficiency of
degradation for polyethylene and 56% for
polypropylene.
3 DISCUSSIONS
In January 2021, Gambarini et. al. published an
extensive and theoretically comprehensive database
describing every microbial strain that has been
reported to degrade any type of plastic polymer to
date. This database is continually updated as new
publications come out. For this work, we have
analysed all species from this database that degrade
one or more of the 6 predominant types of “non-
biodegradable” plastics; PE, PP, PVC, PS, PET or
PU.
Incorporating organisms across the different
kingdoms into a consortium will likely allow for a
wider degradation coverage of polymer types and
additives. Since different types of organisms with
varying enzymes and chemical substances have the
ability of degrading different parts of the plastic,
they are able to decompose larger proportions of
plastic if they can work as a consortium. As a
comparison, if a human wants to share a banana with
a chimpanzee, they are only able to consume the
banana pulp; if the human can share the banana with
ants which can consume the skin, the whole banana
may be decomposed in a rapid fashion. The
degradation of plastic can be divided into several
steps, such as degrading long chains and small
chains, or the backbone of a polymer vs the
monomers released, or a hydrophobic bacteria that
facilitates interactions between the polymer and
other organisms. Different kinds of organisms can
be responsible for each part. For example, some
insects have been identified that are able to chew
plastic debris into smaller parts, assisting with
mechanical shearing, before the bacteria in their gut
break down the plastic enzymatically.
Microbial strains isolated from a similar
environmental location have a higher chance of
successfully cultivating in a single, compatible,
minimal media. When considering a consortium,
organisms isolated from the same kind of
environment are likely to have a higher
compatibility than organisms collected from
disparate environments. For example, Samples
collected from water (particularly ocean water) are
unlikely, though not impossible, to be compatible
with samples from soil. In addition, an organism's
capacity to grow in a minimal medium with a
limited carbon source will likely contribute to its
efficiency at degrading plastic polymers. With
plastic polymers generally being a poor source of
energy, if an alternative energy source is present
then it will be consumed preferentially first.
Therefore, if an organism has been shown to grow
successfully in a minimal medium with plastic
polymers as the sole carbon source, this would be a
positive sign. The growth environment should also
contain the essential trace elements, such as alkali
metals, transition metals or phosphates. Since there
is little experimental evidence observing
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
200
consortiums of polymer degrading microbes
growing together, it is hard to say which strains will
be compatible so it is worth trying out a variety of
combinations.
For a consortium to be effective, all organisms
must be able to successfully grow at equivalent
temperatures, with higher temperatures being
preferable for thermodynamic optimisation. The
higher the temperature the organism can survive at,
more likely that faster degradation will occur. The
thermal compatibility of different organisms is
heavily dependent on the environmental niche that
organism was isolated from, and its thermophilic
potential.
Additional environmental factors to be
considered are pH and oxygen level. A significant
part of polymer degradation is hydrolysis (Figure 3),
which can be influenced by the pH value. In an
acidic environment, the carbonyl group of an ester
bond will be protonated, allowing for an easier
nucleophilic attack. This process is particularly
relevant for polymers with heteroatoms in their
backbones, such as PET and PU (Fig 1). Oxygen is
also an important consideration for the degradation
of plastic, playing a role in both abiotic and biotic
degradation. For abiotic degradation, oxidation and
incorporation of oxygen into the polymer structure
both require the presence of oxygen. Biotically, for
most microbes, oxygen is preferential or even
necessary for survival. It is also worth noting that
the vast majority of polymer degradation studies
have been carried out in an aerobic environment, as
experimental convenience favours this condition. It
is possible that anaerobic metabolism could offer
alternative pathways.
Figure 3: The proportion of waste, non-fibrous plastic
mass attributed to the most common forms of plastic, from
2015. MT= million tons.
Reported degradation efficiency gives an
indication of the potential of an organism, but comes
with the considerable caveat that degradation studies
are far from standardized, or even reliable.
Theoretically, we would choose bacteria that have a
higher degradation rate over a shorter period of time,
for a more efficient breakdown of material.
However, many reported strains have no data
supporting their degradation rate, and those that do
differ vastly in the substrates used or the
methodology carried out. In addition, different
studies vary in their pretreatment of the plastic
substrate used with thermal, acidic UV or no
treatment employed. It can be difficult to determine
if an observed weight loss or CO₂ capture is due to
the degradation of the polymer additives in the
plastic. Moreover, degradation rates are often
reported as a single value after a time course, and
therefore it cannot be determined if degradation will
continue at the same rate or if an asymptote is
reached.
To create the optimal conditions for the
biodegradation of polymers, a variety of potentially
incompatible environmental factors will need to be
considered and balanced. These factors include
temperature, oxygen level, pH, UV exposure,
moisture level, carbon availability and abundance of
trace elements. In order to control the temperature,
heating elements or ventilation can be used to
maintain the temperature within an optimum range.
To prevent an oxygen gradient forming, mechanical
rotation may be necessary, although this may
become prohibitively expensive on larger scales. pH
will need to be monitored and kept within
biologically acceptable limits using additives. UV
levels are expensive to control artificially therefore
natural light will be a more viable method. Moisture
levels can be controlled using the addition of water.
The consortium should be provided with the
necessary trace elements generally required for
microbial growth, and should not be provided with
an alternative carbon source such as sugars or fats
that would be consumed preferentially over the
polymers. It is important to keep in mind that
exposure to sunlight as a UV source will provide
complications when trying to maintain an optimum
temperature and moisture level, therefore a balance
must be struck.
Genera that occur more frequently could be more
reliable and compatible, with multiple related
species all reported with degradation capacity. When
strains capable of degrading polymers are analysed,
some genera occur much more frequently than
others. The three most common genera are
Aspergillus, Bacillus, and Pseudomonas. There are
16, 18 and 19 species for each, reported across 18,
18 and 20 publications, respectively. Species that
originate from the same genus will have closer
genotypes, which means that their habitat and
environmental needs for trace elements will be more
compatible, so being able to select multiple species
from the same genus could prove advantageous. It
Conditions for a Microbial Consortium for the Biological Degradation of Plastic Polymers
201

could be argued that because these genera are found
more frequently to degrade plastics, they are the
genera which are best adapted for it. However, it is
more likely that laboratory based screening has a
strong bias for strains that thrive in standard
laboratory conditions.
Several species have been identified that have
been observed to degrade multiple types of polymer,
which could prove advantageous when considering a
consortium. Only three types of bacteria or fungi
have the ability to break down three or more types of
plastic Bacillus cereus, Bacillus gottheilii, and
Phanerochaete chrysosporium. Bacillus cereus and
Phanerochaete chrysosporium have each been
observed to degrade three types of polymer (PE,
PET and PS and PE, PP and PVC respectively),
while Bacillus gottheilii degrades 4 types of polymer
(PE, PP, PS and PET) If all these species can be
utilized, then with the help of just these three
species, five of the six main plastics can be broken
down. Since two of them are from the same genus,
they are likely to be compatible and both of them are
isolated from soil/sediment environments. With a
smaller number of species, biotic degradation and
the use of consortia could be more practical for
large-scale use. However, since related research is
still limited, some organisms that can break down
multiple plastics may not have been observed to
yet.Although reported degradation rates are not
always reliable or comparable, there are some
species with such a high reported rate that they are
worth additional consideration. For example, four
species that have a reported weight loss for PE
above 50% are Penicillium chrysogenum,
Penicillium oxalicum, Microbacterium paraoxydans,
and Pseudomonas aeruginosa. The two fungal
species, P. chrysogenum and P. oxalicum, were
found to degrade 55 % and 59 % of a PE sheet over
a 90 day period. P. aeruginosa and M.
paraoxydansare bacteria found to degrade PE with a
50.5% and 61% weight loss recorded after 60 days
at room temperature. These four species have all
been isolated from soil samples, and have an optimal
growth temperature of around 28 °C and have
similar growth conditions, so it would be reasonable
to have them in the same consortium. However, it is
important to remember that methodologies for
degradation rates vary greatly between publications,
making exact comparisons and conclusions hard to
draw. Nevertheless, it is probably worth considering
these four species for a consortium that is required to
degrade PE.
In order to select the right organisms for a
consortium, compatibility, efficiency and
degradation comprehensiveness need to be
considered. As previously mentioned, B. cereus and
B. gottheilii are both soil microbes capable of
degrading multiple types of polymer. Ideonella
sakaiensis is soil microbe whose PET degrading
activity is well characterized, as is Acinetobacter
baumannii. The soil fungus Aspergillus flavus could
be considered for the decomposition of PU. High
levels of PE degradation could be covered by one or
more of the soil microbes P. chrysogenum, P.
oxalicum, M. paraoxydans, or P. aeruginosa. By
using these organisms the six predominant forms of
plastic (PE, PP, PU, PS, PVC and PET) can be
degraded by a range of organisms all capable of
growing in a similar environment.
4 CONCLUSIONS
If implemented correctly, and at a significant scale,
biodegradation of plastic waste through microbial
consortiums could present an efficient, economical
and environmentally sound response to the world’s
ever increasing plastic waste crisis. However, we do
not recommend that this approach be taken on as an
alternative to reducing the current polymer
production levels, rather as a method for reducing
the waste that already exists.
ACKNOWLEDGEMENTS
If any, should be placed before the references
section without numbering.
REFERENCES
Aamer Ali Shah, Fariha Hasan, Abdul Hameed, and Safia
Ahmed. 2008. Biological degradation of plastics: a
comprehensive review. Biotechnol. Adv. 26, 3 (May
2008), 246–265.
C. Ioakeimidis, K. N. Fotopoulou, H. K. Karapanagioti,
M. Geraga, C. Zeri, E. Papathanassiou, F. Galgani,
and G. Papatheodorou. 2016. The degradation
potential of PET bottles in the marine environment:
An ATR-FTIR based approach. Sci. Rep. 6, (March
2016), 23501.
David K. A. Barnes, Francois Galgani, Richard C.
Thompson, and Morton Barlaz, 2009 Accumulation
and fragmentation of plastic debris in global
environments, Philos. Trans. R. Soc. Lond. B Biol.
Sci. 364, 1526 (July 2009), 1985–1998.
David R. Lighthall Steven Kopecky, David R. Lighthall,
and Steven Kopecky. 2000. Confronting the Problem
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
202

of Backyard Burning: The Case for a National Ban.
Society & Natural Resources 13, 157–167.
DOI:https://doi.org/10.1080/089419200279171
Heera Rajandas, Sivachandran Parimannan, Kathiresan
Sathasivam, Manickam Ravichandran, and Lee Su
Yin. 2012. A novel FTIR-ATR spectroscopy based
technique for the estimation of low-density
polyethylene biodegradation. Polymer Testing 31,
1094–1099.
DOI:https://doi.org/10.1016/j.polymertesting.2012.07.
015
I. Kranner, W. J. Cram, M. Zorn, S. Wornik, I.
Yoshimura, E. Stabentheiner, and H. W. Pfeifhofer.
2005. Antioxidants and photoprotection in a lichen as
compared with its isolated symbiotic partners.
Proceedings of the National Academy of Sciences
102, 3141–3146. DOI:
https://doi.org/10.1073/pnas.0407716102
Kyungjun Min, Joseph D. Cuiffi, and Robert T. Mathers.
2020. Ranking environmental degradation trends of
plastic marine debris based on physical properties and
molecular structure. Nat. Commun. 11, 1 (February
2020), 727.
Nupur Ojha, Neha Pradhan, Surjit Singh, Anil Barla,
Anamika Shrivastava, Pradip Khatua, Vivek Rai, and
Sutapa Bose. 2017. Evaluation of HDPE and LDPE
degradation by fungus, implemented by statistical
optimization. Sci. Rep. 7, (January 2017), 39515.
Paolo Bombelli, Christopher J. Howe, and Federica
Bertocchini. 2017. Polyethylene bio-degradation by
caterpillars of the wax moth Galleria mellonella. Curr.
Biol. 27, 8 (April 2017), R292–R293.
Roland Geyer, Jenna R. Jambeck, and Kara Lavender
Law. 2017. Production, use, and fate of all plastics
ever made. Science Advances 3, e1700782.
DOI:https://doi.org/10.1126/sciadv.1700782
Stephanie B. Borrelle, Jeremy Ringma, Kara Lavender
Law, Cole C. Monnahan, Laurent Lebreton, Alexis
McGivern, Erin Murphy, Jenna Jambeck, George H.
Leonard, Michelle A. Hilleary, Marcus Eriksen, Hugh
P. Possingham, Hannah De Frond, Leah R. Gerber,
Beth Polidoro, Akbar Tahir, Miranda Bernard,
Nicholas Mallos, Megan Barnes, and Chelsea M.
Rochman. 2020. Predicted growth in plastic waste
exceeds efforts to mitigate plastic pollution. Science
369, 6510 (September 2020), 1515–1518.
Stephanie G. Hays, William G. Patrick, Marika Ziesack,
Neri Oxman, and Pamela A. Silver. 2015. Better
together: engineering and application of microbial
symbioses. Curr. Opin. Biotechnol. 36, (December
2015), 40–49.
Victor Gambarini, Olga Pantos, Joanne M. Kingsbury,
Louise Weaver, Kim M. Handley, and Gavin Lear.
2021. Phylogenetic Distribution of Plastic-Degrading
Microorganisms. mSystems 6, 1 (January 2021).
DOI:https://doi.org/10.1128/mSystems.01112-20
Conditions for a Microbial Consortium for the Biological Degradation of Plastic Polymers
203
