
Study on HPLC Fingerprint and Chemical Pattern Recognition of
Guizhi Shaoyao Zhimu Granules
Shujing Zheng
1a
, Zhenglong Li
1b
, Jia Wang
1c
, Yang Li
1d
, Xiangyang Leng
2,* e
and Shumin Wang
1,* f
1
College of Pharmacy, Changchun University of Chinese Medicine, Changchun, Jilin, China
2
Changchun University of Chinese Medicine, Changchun, Jilin, China
*
lengxiangy@163.com,
*
wangsm@ccucm.edu.cn
Keywords: Guizhi Shaoyao Zhimu Granules, HPLC Fingerprint, Chemical Pattern Recognition, Similarity Evaluation.
Abstract: Objective: To establish the fingerprint of Guizhi Shaoyao Zhimu Granules, and evaluate its quality in
combination with the chemical pattern recognition method, so as to provide a reference for the quality
control of the preparation. Method: Use Waters XSelect HSS T3 (4.6 mm ×250 mm, 5 μm)
chromatographic column; mobile phase is acetonitrile-0.1% phosphoric acid aqueous solution; gradient
elution; flow rate is 1.0 min·m L
–1
; column temperature is 35℃; the detection wavelength was 210 nm; the
injection volume was 10 μL. Ten batches of Guizhi Shaoyao Zhimu Granules fingerprints were established,
and similarity evaluation, cluster analysis, principal component analysis and orthogonal partial least squares
discriminant analysis were performed. Results: The established HPLC fingerprint of Guizhi Shaoyao Zhimu
Granules identified 16 common peaks, and identified ephedrine hydrochloride, D-pseudu-ephedrine
hydrochloride, mangiferin, paeoniflorin, liquiritin, and 5-O-methylvisammioside , cinnamic acid,
glycyrrhizic acid, 6-ginger phenol; 9 batches of Guizhi Shaoyao Zhimu Granules fingerprint pattern and
control pattern similarity are all> 0.990, cluster analysis and principal component analysis results are
basically the same, the orthogonality is the smallest. The two-fold discriminant analysis method screened
out 9 quality difference markers. Conclusion: The method is simple and reliable, and can provide a
reference for the quality control of Guizhi Shaoyao Zhimu Granules.
1 INTRODUCTION
1
Guizhi Shaoyao Zhimu Granules is a traditional
Chinese medicine compound preparation made from
9 medicinal materials of Cinnamomum cassia,
Paeonialactiflora, Glycyrrhiza uralensis, Ephedra
sinica, Zingiber officinale Roscoe, Atractylodes
macrocephala, Anemarrhena asphodeloides,
Saposhnikovia divaricata, and Aconitum
carmichaelii. It has the effects of expelling wind and
dampness, warming menstruation and dispelling
cold
,
the main clinical treatment of rheumatoid
arthritis, gouty arthritis and knee osteoarthritis and
a
https://orcid.org/0000-0003-3509-8362
b
https://orcid.org/0000-0003-4352-3023
c
https://orcid.org/0000-0002-6166-7883
d
https://orcid.org/0000-0003-4579-939X
e
https://orcid.org/0000-0001-6385-5370
f
https://orcid.org/0000-0002-0730-4475
other diseases. The preparation is composed of 9
medicinal materials with complex ingredients, and
only controlling the content of one or several of
them cannot reflect the quality level of the
preparation as a whole. The fingerprint of traditional
Chinese medicine is a comprehensive and
quantifiable identification method, which can
comprehensively evaluate the quality and stability of
traditional Chinese medicine and its preparations as
a whole, and provide effective means for its quality
control. However, fingerprints have shortcomings
such as large amount of information, fuzzy data, and
difficult analysis, so it is necessary to use
chemometric method to reduce the dimension of the
data. Chemical pattern recognition technology is a
comprehensive technology that analyzes information
with the help of computers, which can quantify the
entire fingerprint information, so as to more
objectively reflect the differences in the quality of
Chinese medicine, and achieve the purpose of
Zheng, S., Li, Z., Wang, J., Li, Y., Leng, X. and Wang, S.
Study on HPLC Fingerpr int and Chemical Pattern Recognition of Guizhi Shaoyao Zhimu Granules.
DOI: 10.5220/0011191700003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 139-145
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
139

comprehensive control of the quality of Chinese
medicine. Therefore, in this study, the HPLC
fingerprint of Guizhi Shaoyao Zhimu Granules was
established to characterize the types of chemical
components at the overall level. At the same time,
cluster analysis, principal component analysis and
orthogonal partial least squares discriminant analysis
were combined to analyze each sample. The main
markers for the quality difference of Guizhi Shaoyao
Zhimu Granules of different batches are screened to
provide a scientific basis for its quality control.
2 INSTRUMENTS AND
MATERIALS
2.1 Instrument
Shimadzu LC-20AT system (SPD-M20A PDA
detector, SIL-20A autosampler): Japan Shimadzu
Technology Co., Ltd.; MS105DU electronic
balance: METTLER TOLEDO Instrument Co., Ltd.;
TGL16M high-speed centrifuge: Hunan Kaida
Scientific Instrument Co., Ltd.; Ultrasonic Cleaner:
Tianjin Autosines Instrument Co., Ltd.
2.2 Material
Liquiritin, glycyrrhizic acid, paeoniflorin, 6-ginger
phenol, mangiferin, cinnamic acid, 5-O-
methylvisammioside reference substance (Chengdu
Desite Biotechnology Co., Ltd., batch numbers are
DST200412-009, DSTDG000601, DSTDS007001,
DST190716-027, DST190305-031, DST190413-
045, DST190213-006, all with a purity of 98.0%);
Ephedrine hydrochloride and D-pseudu-ephedrine
hydrochloride reference substances (China Institute
for Food and Drug Control, batch numbers are
171241-201809 and 171237-201809, respectively,
with a purity of 100.0%). Anemarrhena
asphodeloides, Glycyrrhiza uralensis and Aconitum
carmichaelii were purchased from Hebei Renxin
Pharmaceutical Co., Ltd.; Cinnamomum cassia,
Paeonialactiflora, Atractylodes macrocephala and
Saposhnikovia divaricata were purchased from
Anguo Anxing Chinese Medicine Decoction Pieces
Co., Ltd.; Ephedra sinica was purchased from Inner
Mongolia Pukang Pharmaceutical Co., Ltd.;
Zingiber officinale Roscoe was purchased From Jilin
Hongjian Pharmacy. Guizhi Shaoyao Zhimu
Granules, batch numbers are 200913, 200924,
201107, 201113, 201118, 201125, 210319, 201012,
210402, 201206, serial number S1~S10, laboratory
self-made. Acetonitrile is chromatographically pure,
water is pure water, and other reagents are
analytically pure.
3 METHODS AND RESULTS
3.1 Chromatographic Conditions
Waters XSelect HSS T3 column (4.6 mm × 250 mm,
5 μm); mobile phase: acetonitrile (A)-0.1%
phosphoric acid water (B), gradient washout (0-5
min, 95.5% B; 5-8 min, 95.5% B → 91% B; 8-13
min, 91% B → 82% B; 13-28 min, 82% B → 80%
B; 28-35 min, 80% B → 77% B; 35-43 min, 77% B
→ 72% B; 43-51 min, 72% B → 57% B; 51-59 min,
57% B → 30% B; 59-64 min, 30% B→ 5% B; 64-
70 min, 5% B; 70-73 min, 5% B→95.5% B; 73-80
min, 95.5% B). Flow rate: 1.0 min·m L
–1
; column
temperature: 35℃; injection volume: 10 μL;
detection wavelength: 210 nm.
3.2 Solution Preparation
3.2.1 Mixed Reference Solution
Accurately weigh the appropriate amount of
ephedrine hydrochloride, D-pseudu-ephedrine
hydrochloride, mangiferin, paeoniflorin, liquiritin, 5-
O-methylvisammioside, cinnamic acid, glycyrrhizic
acid, and 6-ginger phenol reference substance.
Dissolve with methanol to prepare a single reference
solution containing the above-mentioned control
quality concentrations of 1.008, 1.012, 1.024, 1.007,
1.013, 1.008, 1.000, 1.001, 1.015 mg·m L
–1
; take an
appropriate amount of each of the above single
reference solution and dilute with methanol to
prepare a mixed reference solution with mass
concentrations of 10.08, 10.12, 10.24, 10.07, 10.13,
10.08, 10.00, 10.01, and 10.15 µg·m L
-1
.
3.2.2 Test Solution
Take an appropriate amount of Guizhi Shaoyao
Zhimu Granules, grind it into small pieces, take 0.8
g, accurately weigh it, place it in a stoppered
erlenmeyer flask, accurately add 5 ml of methanol,
ultrasonically treat (220 V, 50 Hz) for 20 min,
10,000 rpm, centrifuge for 10 min, take the
supernatant, and pass through a 0.22 μm
microporous membrane to get it.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
140
3.2.3 Single Medicinal Solution
Weigh about 50 g of Guizhi Shaoyao Zhimu
Granules prescription medicinal materials
Anemarrhena asphodeloides, Glycyrrhiza uralensis,
Aconitum carmichaelii, Cinnamomum cassia,
Paeonialactiflora, Atractylodes macrocephala,
Saposhnikovia divaricata, Ephedra sinica, and
Zingiber officinale Roscoe respectively, and prepare
each single medicinal material sample according to
the prescription process of the preparation. Prepare a
single medicinal solution according to the method
under "3.2.2", and get it.
3.3 Methodological Review
3.3.1 Precision Test
Precisely draw the same test solution, inject 6 times
continuously, and record the chromatogram. With
liquiritin as the reference peak, the relative retention
time and relative peak area of each shared peak were
calculated. The RSD of the relative retention time of
each chromatographic peak was less than 0.74%
(n=6), and the RSD of the relative peak area was less
than 2.90% (n=6), indicating that the precision of the
instrument was good.
3.3.2 Stability Test
Precisely draw the same test solution, inject samples
at 0, 4, 8, 12, 18 and 24 h, and record the
chromatogram. With liquiritin as the reference peak,
the relative retention time and relative peak area of
each shared peak were calculated. The relative
retention time RSD of each common peak was less
than 0.69% (n=6), and the RSD of the relative peak
area was less than 2.80% (n=6), indicating that the
test product was stable within 24 hours.
3.3.3 Repeatability Test
Take the same batch of Guizhi Shaoyao Zhimu
Granules, prepare 6 test solution solutions according
to the method under "3.2.2", inject 6 samples for
determination. With liquiritin as the reference peak,
the relative retention time and relative peak area of
each shared peak were calculated. The relative
retention time RSD of each common peak was less
than 0.27% (n=6), and the RSD of the relative peak
area was less than 2.68% (n=6), indicating that the
method has good repeatability.
3.4 Establishment of Fingerprint Atlas
and Evaluation of Similarity
3.4.1 Establishment of Fingerprint Atlas
Take 10 batches of Guizhi Shaoyao Zhimu Granules
and inject them for determination under the
chromatographic conditions under "3.1". Analyzed
by "Chinese Medicine Chromatographic Fingerprint
Similarity Evaluation System (2012 Edition)", with
S1 sample chromatogram as the reference map, the
time width is set to 0.1 min, and the multi-point
calibration is set to automatically match, and the
median method is used to generate the overlay map
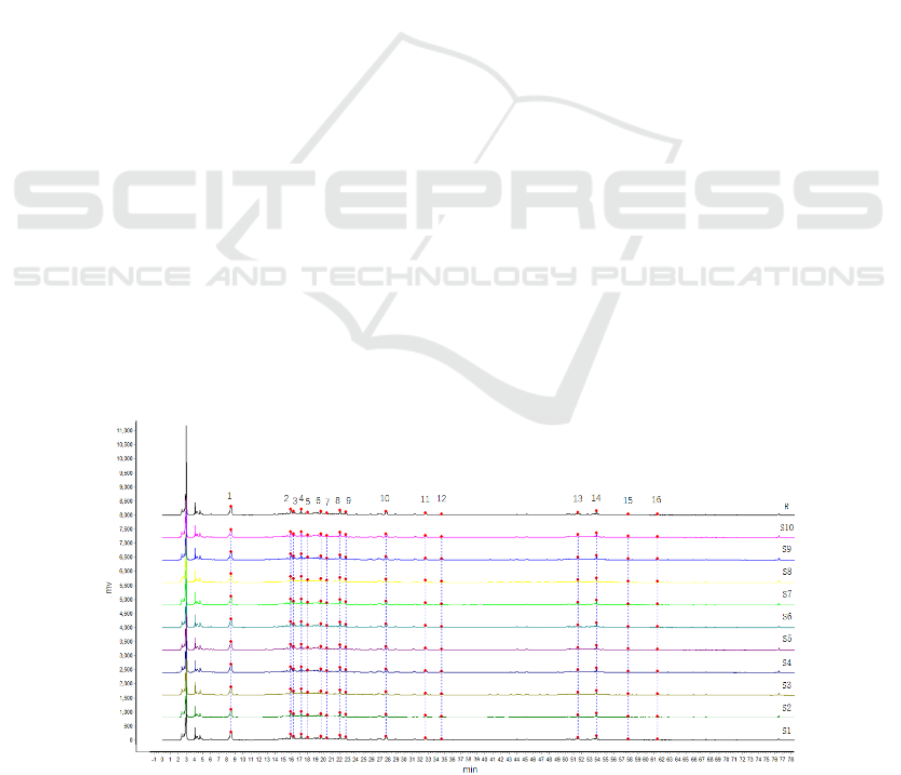
and the control map, as shown in Figure 1. A total of
16 common peaks were calibrated, of which peak
No. 10 (liquiritin) had a good separation effect and
was in the middle position, so it was taken as the
reference peak.
Figure 1: HPLC Superimposed Chromatogram (S1-S10) and Control Chromatogram (R) of 10 batches of Guizhi Shaoyao
Zhimu Granules.
Study on HPLC Fingerprint and Chemical Pattern Recognition of Guizhi Shaoyao Zhimu Granules
141
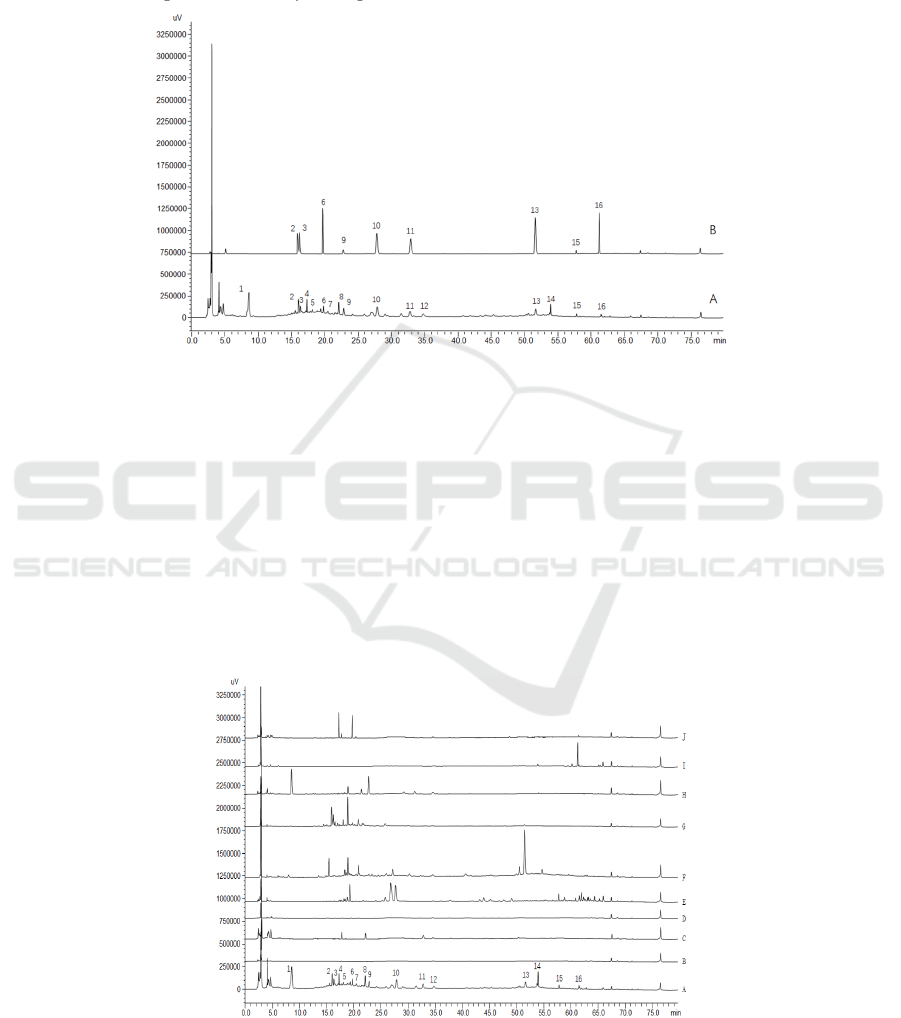
3.4.2 Identification of Common Peaks
Measure the test solution and the mixed reference
solution under the chromatographic conditions under
"3.1", and compare the retention time of each peak
to identify 9 components, namely 2 (ephedrine
hydrochloride), 3 (D-pseudu-ephedrine
hydrochloride), 6 (mangiferin), 9 (paeoniflorin), 10
(liquiritin), 11 (5-O-methylvisammioside), 13
(cinnamic acid), 15 (glycyrrhizic acid), 16 (6-ginger
phenol), see Figure 2.
2.ephedrine hydrochloride 3. D-pseudu-ephedrine hydrochloride 6.mangiferin 9.paeoniflorin 10.liquiritin 11.5-O-
methylvisammioside 13.cinnamic acid 15.glycyrrhizic acid 16.6-ginger phenol
Figure 2: HPLC chromatograms of test solution (A) and mixed reference solution (B).
3.4.3 Attribution of Shared Peaks
By comparing the HPLC chromatograms of the
single medicinal solution and the test solution, the
common peaks of the fingerprints are assigned to the
medicinal materials, as shown in Figure 3.Peaks 1, 9
are from Paeonialactiflora; peaks 2, 3, and 5 are
from Ephedra sinica; peaks 4, 6, and 7 are from
Anemarrhena asphodeloides; peaks 8, 11 are from
Saposhnikovia divaricata; peaks 10 and 15 are from
Glycyrrhiza uralensis; peak 12 is shared by
Saposhnikovia divaricata, Glycyrrhiza uralensis,
Paeonialactiflora, and Ephedra sinica; peak 13 is
derived from Cinnamomum cassia; peaks 14 and 16
are derived from Zingiber officinale Roscoe. The
results show that the preparation has a good
correlation with the single medicinal material.
A. test solution B. Atractylodes macrocephala C. Saposhnikovia divaricata D. Aconitum carmichaelii E. Glycyrrhiza
uralensis F. Cinnamomum cassia G. Ephedra sinica H. Paeonialactiflora I. Zingiber officinale J. Anemarrhena
asphodeloides
Figure 3: HPLC chromatograms of test solution and single medicinal materials.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
142
3.4.4 Fingerprint Similarity Evaluation
The similarity evaluation system of fingerprints of
traditional Chinese medicine (2012 edition) was
used to calculate the similarity of fingerprints of 10
batches of Guizhi Shaoyao Zhimu Granules, as
shown in Table 1. The similarities of 10 batches of
preparations are all greater than 0.990, indicating
that the similarity between batches of the
preparations is good and the quality is relatively
stable.
Table 1: Table of similarity evaluation results of 10 batches of Guizhi Shaoyao Zhimu Granules.
Peak
numbe
r
S1 S2 S3 S4 S5 S6 S7 S8 S9 S10 R
S1 1.000 1.000 0.996 0.996 0.996 0.997 0.999 0.993 0.999 0.996 0.999
S2 1.000 1.000 0.996 0.996 0.996 0.998 0.999 0.994 0.999 0.996 0.999
S3 0.996 0.996 1.000 1.000 0.999 0.998 0.995 0.990 0.995 0.992 0.998
S4 0.996 0.996 1.000 1.000 0.999 0.998 0.995 0.991 0.995 0.991
0.998
S5 0.996 0.996 0.999 0.999 1.000 0.998 0.995 0.992 0.996 0.990 0.998
S6 0.997 0.998 0.998 0.998 0.998 1.000 0.997 0.994 0.997 0.993 0.999
S7 0.999 0.999 0.995 0.995 0.995 0.997 1.000 0.996 1.000 0.996 0.999
S8 0.993 0.994 0.990 0.991 0.992 0.994 0.996 1.000 0.996 0.992 0.996
S9 0.999 0.999 0.995 0.995 0.996 0.997 1.000 0.996 1.000 0.995 0.999
S10 0.996 0.996 0.992 0.991 0.990 0.993 0.996 0.992 0.995 1.000 0.996
R 0.999 0.999 0.998 0.998 0.998 0.999 0.999 0.996 0.999 0.996 1.000
3.5
Chemical Pattern Recognition
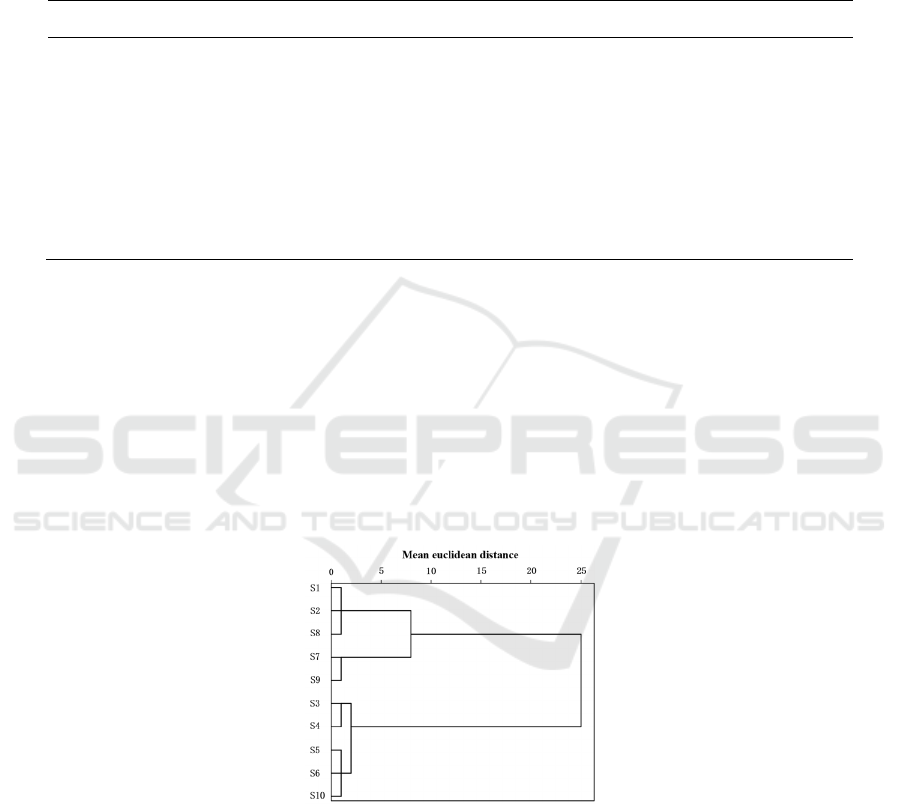
3.5.1 Cluster Analysis (HCA)
Using SPSS 20.0 software, taking the peak areas of
16 common peaks in 10 batches of Guizhi Shaoyao
Zhimu Granules as variables, the original data
matrix of 10×16 order was obtained, and the Ward
method combined with mean euclidean distance was
used as the metric to perform cluster analysis, see
Figure 4. When the mean euclidean distance is 10,
10 batches of samples can be grouped into two
types: samples S1, S2, S7, S8, and S9 are grouped
into one type; samples S3, S4, S5, S6, and S10 are
grouped into one type. When the squared Euclidean
distance is 5, 10 batches of samples are aggregated
into three categories: samples S1, S2, and S8 are
aggregated into the first category; samples S7 and
S9 aggregated into the second category; samples S3,
S4, S5, S6, and S10 aggregate it is the third
category.
Figure 4: Cluster analysis tree of 10 batches of Guizhi Shaoyao Zhimu Granules.
3.5.2 Principal Component Analysis (PCA)
Using the common peak area as a variable, SPSS
20.0 software was used to perform principal
component analysis on 10 batches of Guizhi
Shaoyao Zhimu Granules, and 4 principal
components were extracted (eigenvalues> 1), and
the cumulative variance contribution rate was
88.609%, which is good Represents most of the
information in the fingerprint, see Table 2. Use
SIMCA 13.0 software to draw a principal
component score chart, as shown in Figure 5. The
results show that 10 batches of samples can be
roughly divided into 3 categories, which are
basically consistent with the cluster analysis results,
and further verify the classification results of the
cluster analysis.
Study on HPLC Fingerprint and Chemical Pattern Recognition of Guizhi Shaoyao Zhimu Granules
143
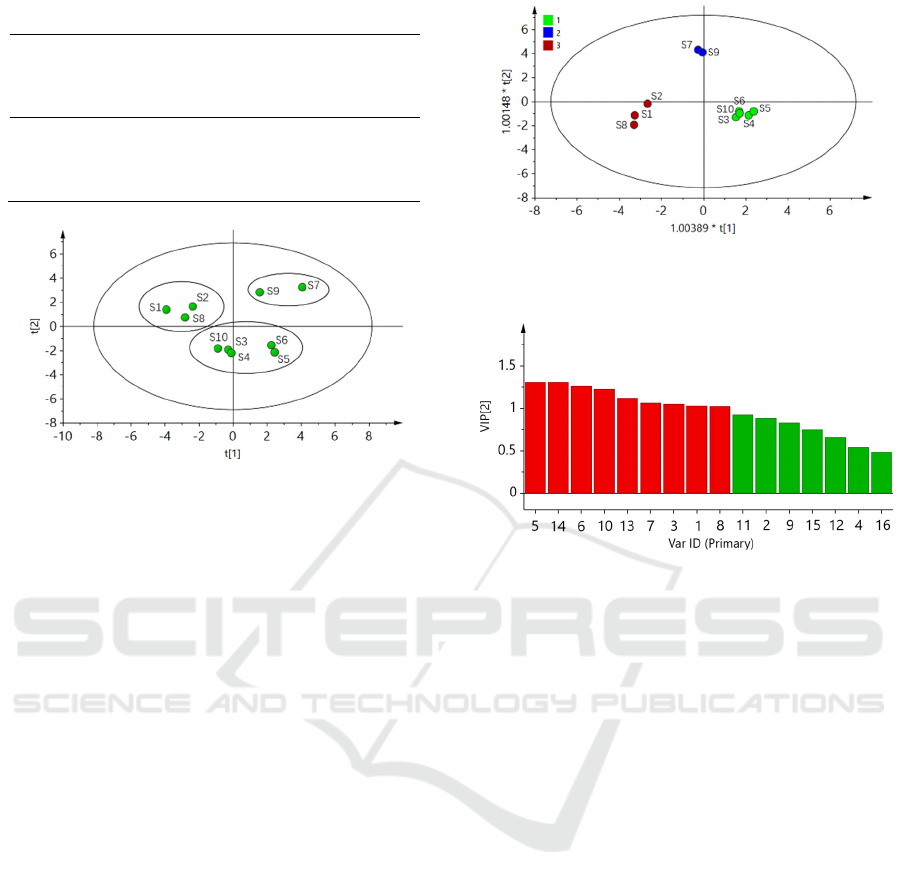
Table 2: Principal component eigenvalues and variance.
Element Eigenvalues Variance
contribution
rate /%
Cumulative
variance
contribution
rate /%
1 6.688 41.801 41.801
2 4.748 29.674 71.475
3 1.466 9.163 80.638
4 1.275 7.971 88.609
Figure 5: Principal component analysis score chart of 10
batches of Guizhi Shaoyao Zhimu Granules.
3.5.3 Discriminant Analysis of Orthogonal
Partial Least Squares (OPLS-DA)
OPLS-DA analysis of common peak area of 10
batches of Guizhi Shaoyao Zhimu Granules using
SIMCA 13.0 software. Under this model,
RX2=0.794, RY2=0.974, and model prediction
parameters Q2=0.905, all greater than 0.5. It shows
that the OPLS-DA model established in this research
is stable and has strong predictive ability. The 10
batches of samples are divided into 3 categories
(Figure 6), which is consistent with the results of
cluster analysis and principal component analysis. In
order to further screen out the components that cause
differences in 10 batches of samples, the variable
importance projection (VIP) method was used for
analysis, and the VIP values of 16 common peaks in
the OPLS-DA model were extracted. The
compounds with VIP values greater than 1
summarize the sample classification. The rate is
greater than 50%, which is a marker of difference.
Therefore, the differential markers of 10 batches of
Guizhi Shaoyao Zhimu Granules have
chromatographic peaks 5, 14, 6 (mangiferin), 10
(liquiritin), 13 (cinnamic acid), 7, 3 (D-pseudu-
ephedrine hydrochloride), 1, 8, see Figure 7.
Figure 6: OPLS-DA score diagram of 10 batches of
Guizhi Shaoyao Zhimu Granules.
Figure 7: VIP value of each chromatographic peak in
OPLS-DA model.
4 CONCLUSIONS
The similarity of 10 batches of Guizhi Shaoyao
Zhimu Granules is above 0.990, indicating that the
correlation between the batches is good, but the
results of cluster analysis and principal component
analysis show that there are certain differences
between the sample groups, which may be different
from the original formulation used. The origin of
medicinal materials is related to the quality
difference between batches. The chromatographic
peaks 5, 14, 6 (mangiferin), 10 (liquiritin), 13
(cinnamic acid), 7, 3 (D-pseudu-ephedrine
hydrochloride), and 1, 8 were selected by orthogonal
partial least square discriminant analysis. In the
quality control of Guizhi Shaoyao Zhimu Granules,
the main marker ingredients that cause differences
between the preparations should focus on the quality
changes of these ingredients. In summary, the HPLC
fingerprint of Guizhi Shaoyao Zhimu Granules
established in this study combined with the chemical
pattern recognition method can provide a reference
for its overall quality evaluation.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
144

ACKNOWLEDGEMENTS
This study was financial supported by the Science
and Technology Development Plan Project of Jilin
Province (20190304059YY).
REFERENCES
DIAO, J. Y. (2018). Recent progress on correlation
between fingerprints and pharmacodynamics of
traditional Chinese medicines. J. Journal of
Pharmaceutical Research. 37(03), 165–168.
HE, R. J. (2017). A Survey of Guizhi Shaoyao Zhimu
Decoction in Treating Knee Joint Osteoarthritis. J.
Chinese folk medicine. 26(11), 63–65.
HAN, Q. W. (2020). Quality evaluation of Shenlian
capsules by HPLC fingerprint and pattern recognition.
J. Chinese Journal of Pharmaceutical Analysis. 40(07),
1300–1308.
HUANG, C. (2019). Discussion on modern
pharmacological effects of Guizhi Shaoyao Zhimu
Decoction based on "Limb Arthralgia". 30(04), 949–
950.
LI, C. C. (2021). Q-Marker prediction of Xiaoyan Tuire
Granules based on HPLC fingerprint and network
pharmacology. J. Chinese Traditional and Herbal
Drugs. 52(13), 3885–3895.
LIN, Y. (2018). UPLC fingerprint spectrum, clustering
and principal component analysis of Xiaoer Chaigui
Tuire Granules. J. Chinese Pharmacy. 29(04), 474–
477.
LIN, X. L. (2017). UPLC fingerprint spectrum, clustering
and principal component analysis of Xiaoer Chaigui
Tuire Granules. J. Chinese Traditional Patent
Medicine. 39(03), 551–555.
LIU, X. R. (2011). Advances and Application on
Chemical Pattern Recognition in Pharmaceutical
Analysis. J. Journal of Mathematical Medicine.
24(01), 101–104.
LIU, H. (2021). HPLC fingerprint and chemical pattern
recognition of Jingulian Capsules. J. Chinese
Traditional and Herbal Drugs. 52(14), 4185–4192.
QIAO, L. F. (2021). Progress in clinical application of
Guizhi Shaoyao Zhimu Decoction in orthopedics and
traumatology. J. Modern Journal of Integrated
Traditional Chinese and Western Medicine. 30(16),
1814–1818.
SHI, S. H. (2019). Formula Syndrome Analysis of Guizhi
Shaoyao Zhimu Decoction. J. Acta Chinese Medicine
and Pharmacology. 47(03), 27–28.
SUN, L. L. (2017). Application progress on chemical
pattern recognition in quality control of Chinese
materia medica. J. Chinese Traditional and Herbal
Drugs. 48(20), 4339–4345.
WANG, Q. (2021). HPLC fingerprint and chemical
pattern recognition of Xingpi Yang’er granules. J.
Chinese Journal of Pharmaceutical Analysis. 41(06),
1083–1090.
YUAN, M. R. (2019). Study on UPLC Fingerprint of
Xiaoer Resuqing Granules and Determination of 5
Components. J. Chinese Journal of New Drugs.
28(12), 1517–1522.
Study on HPLC Fingerprint and Chemical Pattern Recognition of Guizhi Shaoyao Zhimu Granules
145
