
Anti-fatigue Effects of Polysaccharides from Morchella esculenta
Fuwei Yin
1 a
and Lantao Liu
2,* b
1
School of Physical Education, Hunan Normal University, Changsha 410012, China
2
Department of Physical Education, Central South University, Changsha 410083, China
Keywords:
Polysaccharides, M. esculenta, Anti-Fatigue, Mechanism, Forced Swimming Test.
Abstract:
Fatigue has caused indirect damage to the body or a direct cause of disease, which has led to the research
and development of anti-fatigue natural medicines and supplements that have become a hot spot at home
and abroad. The aim of present work was to evaluate the anti-fatigue effects of polysaccharides from M.
esculenta (PMe) by using a swimming exercise animal model. The mice were assigned into a normal control
group and three PMe treatment groups. The treatment groups received different doses of PMe (100, 200,
and 400 mg/kg) through gastric gavage once per day for 4 weeks, and normal control group received
distilled water. On the last day of the experiment, the forced swimming test was performed, and fatigue-
related biochemical parameters were analyzed. The results showed that PMe significantly prolonged
(p<0.05) the swimming time to exhaustion, reduced (p<0.05) the levels of lactate, urea nitrogen and
malondialdehyde in serum, increased (p<0.05) the levels of non-esterfied fatty acid in serum, as well as the
glycogen levels in liver and muscle. In conclusion, PMe has the anti-fatigue effects and its mechanisms
might be related to the fact that PMe could reduce the production of metabolites or delay the accumulation
of metabolites, attenuate protein and amino acid metabolism, and enhance fat metabolism, reduce oxidative
stress and protect oxidative damage induced by exercise, and improve the energy substance storage or
reduce energy substance consumption.
1 INTRODUCTION
1
Morchella esculenta (L.) Pers. (M. esculenta)
belongs to the family of Ascomycota of the order
Discomycetes, which grows in temperate regions of
Asia, Europe and Americas. It is a class of rare
edible and medicinal mushrooms with rich nutrition
and delicious taste (Nitha, Fijesh, Janardhanan,
2013). As early as 2000 years ago, M. esculenta was
used in Traditional Chinese medicine (TCM) for the
treatment of spleen and stomach weakness,
indigestion, phlegm and shortness of breath,
dizziness, insomnia and other diseases (Cui, Chen,
Wang, Kai, Fang, 2011). Various bioactive
ingredients from M. esculenta have been isolated
and reported, such as polysaccharides, tocopherol,
carotenoids, organic acids and polyphenols (Yang,
Yin, Zhang, 2015). Modern pharmacological studies
have demonstrated that polysaccharides is one of the
most important bioactive ingredients of M. esculenta
a
https://orcid.org/0000-0001-6749-4912
b
https://orcid.org/0000-0003-1498-3864
and have a variety of pharmacological actions,
including immunomodulatory, antioxidation,
antibacterial, anti-viral, antimicrobial, antitumor,
anti-proliferation, anti-inflammatory,
hepatoprotective, and many other effects (Liu, Pan,
2016). However, little is known about anti-fatigue
activity of polysaccharides from M. esculenta
(PMe). The aim of present work was to evaluate the
effects of PMe on physical fatigue by using a
swimming exercise animal model. Further, its
possible mechanism of anti-fatigue effects also will
be investigated, which will provide a scientific basis
for the use of this active ingredient
2 MATERIALS AND METHODS
2.1 Materials and Chemicals
Dry M. esculenta mycelium was obtained from
ZhongZhiKang Mushroom Science & Technology
Development Co., Ltd. (Hangzhou, China). Assay
kits for determination of lactate (LA) and glycogen
132
Yin, F. and Liu, L.
Anti-fatigue Effects of Polysaccharides from Morchella esculenta.
DOI: 10.5220/0011191100003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 132-138
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

were provided by the LeiGen Biotechnology Co.
(Beijing, China). Assay kits for determination of
urea nitrogen (UN) and non-esterfied fatty acid
(NEFA) were provided by the HuiLi Biochemical
Reagents Co. (Changchun, China). Assay kits for
determination of malondialdehyde (MDA) were
provided by the JianCheng Biotechnology Co.
(Nanjing, China). All the other chemicals and
reagents used in this study were of analytical grade
and were provided by the commercial channels. All
solutions were prepared with deionized water to
eliminate metal ion contamination.
2.2 Polysaccharides from M. Esculenta
Preparation
PMe was extracted according to the pre-described
method by published literature and subjected to
small modification (Cui, Chen, Wang, Kai, Fang,
2011). The dry M. esculenta mycelium were further
dried to constant weight at 45°C, and ground into
fine powder (100 mesh) using a shredder. Then, the
powder was extracted by refluxing in 80% ethanol
for 7 h to remove the ethanol-soluble materials,
including colored substances, small molecule
substances, monosaccharides, and oligosaccharides.
The ethanol was volatilized and the pretreaed dry
powder was obtained. Pretreated powder was
extracted with 10 volumes of distilled water at 90°C
for 3 h. After centrifugation (2432 × g, 15 min), the
residue was extracted for another 3 h at 90ºC. All
supernatants were combined and concentrated in a
rotary evaporator under low pressure. The
concentrated supernatants were mixed with 3
volumes of 95% ethanol at 4°C for 24 h. After
centrifugation (2432 × g, 15 min), the precipitate
was washed twice with 100% ethanol and acetone,
subsequently re-dissolved in distilled water, and
again precipitated with 95% ethanol. The resulting
precipitate was collected by centrifugation (2432 ×
g, 15 min) and the proteins were removed by Sevag
method. Finally, the supernatant was collected and
lyophilized to obtain PMe.
2.3 Experimental Animals and Care
Conditions
Healthy male Kunming mice weighting between 18
and 22g were housed in standard conditions with
maintained relative humidity (50 ± 10%), controlled
temperature (22 ± 1°C), and an artificial 12-h
light/dark cycle (lights on 07:30-19:30 h). The mice
were allowed free access to standard food particles
(unless otherwise stated) and tap water ad libitum
throughout the experimental period. All animals are
subjected to humanitarian care in compliance with
the "Measures for the Administration of Laboratory
Animals in Hunan Province", which strictly comply
with the orders issued by the Ministry of Science
and Technology of China in 1988. The experimental
protocol was approved by the Ethics Committee of
Central South University
2.4 Experimental Design
After 7 days of adaptation to the feeding
environment, the mice were randomly assigned into
the following four groups (10 mice per group).
Group 1: normal control (NC) group, the mice were
treated with distilled water. Group 2: low dose PMe
treatment (LPT) group, the mice were treated with
100 mg/kg of PMe. Group 3: middle dose PMe
treatment (MPT) group, the mice were treated with
200 mg/kg of PMe. Group 4: high dose PMe
treatment (HPT) group, the mice were treated with
400 mg/kg of PMe. PMe was dissolved in distilled
water. The treatment groups received different doses
of PMe through gastric gavage once per day for 4
weeks, and NC group received the same volume of
distilled water.
On the last day of the experiment, 30 min after
the last treatment, the forced swimming test was
performed as previously reported. The apparatus
used was a plastic water tank (length: 65 cm, width:
50 cm, depth: 50 cm) filled with water of 30 cm
deep at room temperature (25 ± 1°C). Each mouse
tail tied a wire bundle (equivalent to 10% body
weight) to swim in order to shorten the test time.
Mice are considered exhausted when the animal sink
into the water and can not float on the water surface
within 10 s (Zhang, 2015), and their swimming time
to exhaustion was immediately recorded.
2.5 Biochemical Analysis
After the forced swimming test, the mice were
anesthetized by intraperitoneal injection of 10%
(w/v) chloral hydrate (350 mg/kg body weight) and
sacrificed via decapitation. Blood samples were
collected and serum were prepared by centrifugation
(1800 × g, 15 min) at 4°C for the estimations of
levels of LA, UN, NFFA, and MDA. Then the liver
and quadriceps femoris muscle were quickly
resected, washed with physiological saline, and
stored in liquid nitrogen at -80°C for the estimations
of glycogen contents. All biochemical parameters
were measured using the corresponding commercial
Anti-fatigue Effects of Polysaccharides from Morchella esculenta
133
assay kits according to the manufacturer's
recommended instructions.
2.6 Statistical Analysis
Values are presented as Mean ± standard deviation
(SD). Statistical analysis was performed using SPSS
data analysis software (version 18.0, Chicago,
USA). Statistical significance was done using one
way analysis of variance (ANOVA) test and then by
Dunnett's test. p<0.05 was considered significant.
3 RESULTS
3.1 Effect of PMe on the Swimming
Time to Exhaustion of Mice
As shown in Fig. 1, the swimming time to
exhaustion of mice in the LPT, MPT, and HPT
groups (7.94 ± 1.04, 8.21 ± 0.86, and 8.98 ± 1.15
min, respectively) were significantly longer
(p<0.05) than that in the NC group (6.87 ± 0.97
min).
Figure 1: Effect of PMe on the swimming time to exhaustion of mice. Values are presented as Mean ±SD. ap<0.05
compared to the NC group.
3.2 Effect of PMe on the LA and UN in
Serum of Mice
As shown in Fig. 2, the serum LA levels of mice in
the LPT, MPT and HPT groups (13.49 ± 1.84, 12.16
± 2.23, and 10.83 ± 1.78 mmol/L, respectively) were
significantly lower (p<0.05) than that in the NC
group (15.21 ± 2.16 mmol/L). Meanwhile, the
serum UN levels of mice in the MPT and HPT
groups (8.74 ± 0.75 and 8.21 ± 0.97 mmol/L) were
significantly lower (p<0.05) than that in the NC
group (9.85 ± 1.16 mmol/L).
Figure 2: Effect of PMe on the LA and UN in serum of mice. Values are presented as Mean ±SD. ap<0.05 compared to the
NC group.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
134
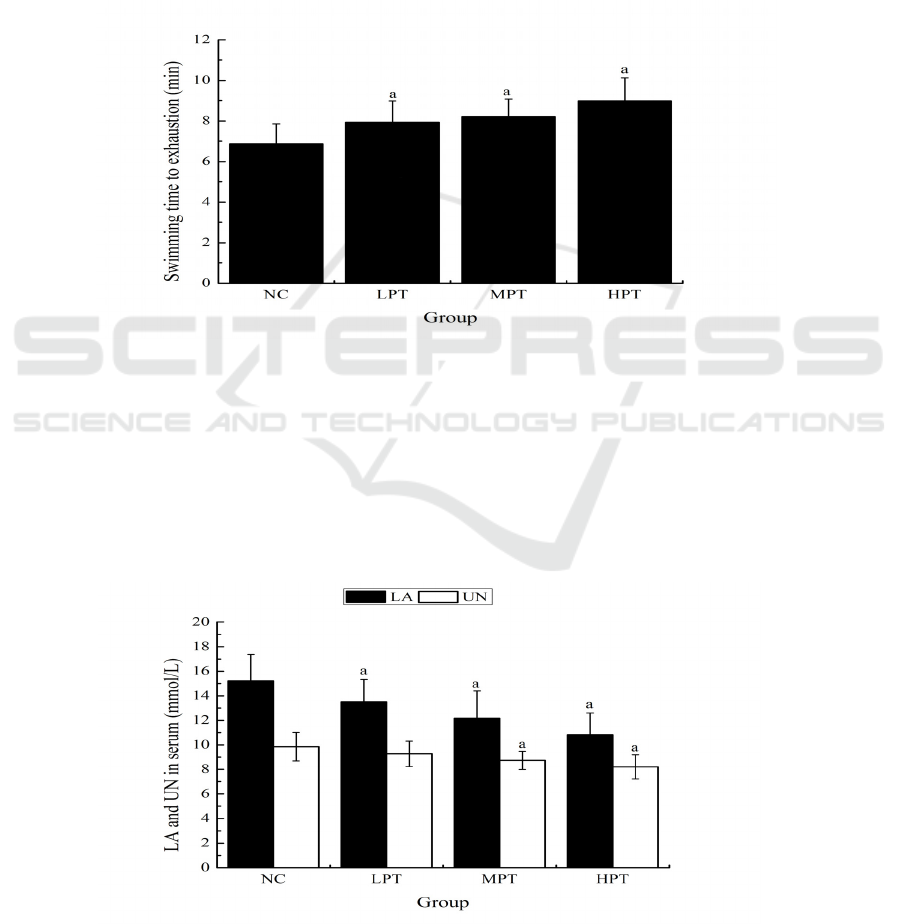
3.3 Effect of PMe on the Serum NEFA
of Mice
As shown in Fig. 3, the serum NEFA levels of mice
in the MPT and HPT groups (39.47 ± 2.06 and
46.23 ± 6.12 umol/L) were significantly higher
(p<0.05) than that in the NC group (34.56 ± 5.13
umol/L).
Figure 3: Effect of PMe on the serum NEFA of mice. Values are presented as Mean ±SD. ap<0.05 compared to the NC
group.
3.4 Effect of PMe on the Serum MDA
of Mice
As shown in Fig. 4, the serum MDA levels of mice
in the MPT and HPT groups (10.34 ± 1.40 and 8.15
± 1.03 nmol/mL) were significantly lower (p<0.05)
than that in the NC group (12.51 ± 1.47 nmol/mL).
Figure 4: Effect of PMe on the serum MDA of mice. Values are presented as Mean ±SD. ap<0.05 compared to the NC
group.
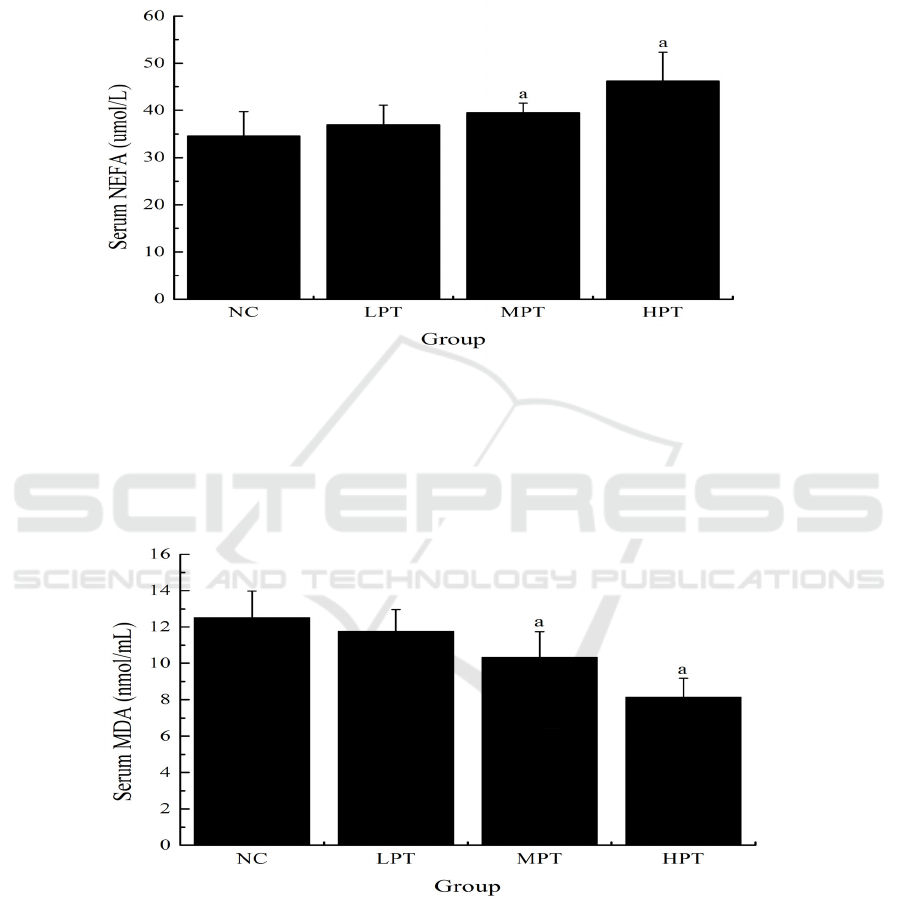
3.5 Effect of PMe on the Glycogen in
Liver and Muscle of Mice
As shown in fig. 6, the liver glycogen levels of mice
in the LPT, MPT and HPT groups (8.86 ± 0.94, 9.47
± 1.16, and 11.32 ± 1.03 mg/g, respectively) were
significantly higher (p<0.05) than that in the NC
group (7.69 ± 0.89 mg/g). Similarly, the muscle
glycogen levels of mice in the MPT and HPT groups
(1.75 ± 0.27 and 1.94 ± 0.22 mg/g) were
significantly higher (p<0.05) than that in the NC
group (1.43 ± 0.21 mg/g).
Anti-fatigue Effects of Polysaccharides from Morchella esculenta
135
Figure 5: Effect of PMe on the glycogen in liver and muscle of mice. Values are presented as Mean ±SD. ap<0.05
compared to the NC group.
4 DISCUSSIONS
Exercise tolerance is the most direct and important
indicators to reflect the physical fatigue. Enhanced
exercise tolerance in an exercise test means a
lessening of fatigue (Xu, Zhang, 2013). The animal
model for evaluating exercise tolerance mainly
includes forced wheel running test, forced treadmill
running test, forced climbing test and forced
swimming test and so on. Forced swimming test
rather than other forced exercise tests in this study
was chosen as an animal model because it can cause
minimal damage to animals, and has a high
reproducibility (Jin, Wei, 2011). The lengths of
swimming time to exhaustion can reflect the degree
of exercise tolerance. The data obtained from this
study show that different dose of PMe significantly
prolonged the swimming time to exhaustion of
mice, which indicated that PMe could improve
exercise tolerance and had the anti-fatigue effects.
Energy supply from the glycolysis is the main
energy source of strenuous exercise (Yan, Hao,
2016). The increase in muscle oxygen consumption
would lead to hypoxia of body, resulting in
accelerated glycolysis reaction, and produce a lot of
LA during strenuous exercise. The accumulation of
serum LA could cause the cell pH value to decrease,
leading to a series of biochemical changes, which
finally lead to fatigue. Therefore, the accumulation
of serum LA could show the speed and extent of
fatigue development. UN is a product of protein and
amino acid catabolic metabolism. During strenuous
exercise, protein and amino acid catabolic
metabolism would be strengthened when for a long
time body cannot get enough energy by means of
sugar and fat catabolic metabolism (Lin, Liu, 2014).
Meanwhile, nucleotides metabolism would also be
quickened. These two metabolic pathways
eventually form UN. Less produced serum UN
indicated stronger exercise tolerance and bearing
capability of body (Liu, Ji, Li, 2013). So, serum UN
is another sensitive indicator of fatigue. In this
study, middle and high dose PMe significantly
decreased the LA and UN levels in serum of mice,
which indicated that PMe could reduce the
production of serum LA or delay the accumulation
of serum LA, decrease serum UN levels by
attenuating protein and amino acid metabolism,
thereby delaying fatigue.
Enhancing the proportion of energy supply from
fat catabolic metabolism during strenuous exercise
could save the glycogen consumption of body,
keeping the blood glucose in a physiological range
in order to meet the needs of the brain central
nervous system (Xu, 2012). This could improve
exercise tolerance and delay the occurrence of
fatigue. It is reported that the increased availability
of NEFA results in greater fat metabolism in the
muscle. In this study, middle and high dose PMe
significantly increased the serum NEFA levels of
mice, which indicated that PMe could improve
exercise tolerance might due to enhanced fat
metabolism by increasing availability of NEFA.
Strenuous exercise increases the production of
free radicals and ROS, which thus attacks the
membrane lipid and causes the lipid peroxidation
product to form. In turn, the formation and
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
136

accumulation of lipid peroxidation will damage the
cells, especially membrane structure and genetic
material changes, to further cause the body's
oxidative damage, accelerating the development of
fatigue (Yan, Hao, 2016). MDA, one of the
degradation products from lipid peroxidation, is
known to be the most sensitive parameter reflecting
oxidative damage (Chen, Li, Wang, Zhang, 2013).
In this study, middle and high dose PMe
significantly decreased the serum MDA levels of
mice, which indicated that the anti-fatigue effects of
PMe might be due to protecting oxidative damage
induced by strenuous exercise through reducing
lipid peroxidation.
Exercise energy is originally derived from the
decomposition of glycogen, which can supplement
blood glucose consumption, and maintain blood
glucose levels stable in the physiological range (Yu,
Huang, 2012). The increase in muscle glycogen
consumption in strenuous exercise will promote the
liver glycogen decomposition of glucose to speed up
to maintain blood glucose levels stable. Glycogen
storage directly affects exercise endurance. Thus,
the glycogen is another important indicator related
to fatigue. In this study, middle and high dose PMe
significantly increased the glycogen levels in liver
and muscle of mice, which indicated that anti-
fatigue effects of PMe might be due, at least in part,
to improving glycogen storage, or reducing
glycogen consumption during strenuous exercise.
In recent years, a series of mechanisms on
physical fatigue have been explored, such as free
radical theory, exhaustion theory, metabolic matter
accumulation theory, internal environmental
imbalance theory, mutation theory, protective
inhibition theory and so on (Wang, Xing, 2014). In
this study, we reveal the anti-fatigue mechanisms of
PMe from three aspects of energy metabolism and
storages, metabolite accumulation, and free radical
induced oxidative stress.
5 CONCLUSION
Based on the above tests and analysis, it can be
concluded that PMe has the anti-fatigue effects as
evidenced by prolonging the swimming time to
exhaustion of mice, reducing the levels of LA, UN
and MDA in serum, and increasing the levels of
NEFA in serum, as well as the glycogen levels in
liver and muscle. The anti-fatigue mechanisms of
PMe might be through the following pathways.
(1) PMe could reduce the production of
metabolites or delay the accumulation of
metabolites.
(2) PMe could attenuate protein and amino acid
metabolism, and enhance fat metabolism.
(3) PMe could reduce oxidative stress, and
protect oxidative damage induced by exercise.
(4) PMe could improve the energy substance
storage or reduce energy substance consumption.
Further research is needed to clarify the detailed
mechanism of PMe's anti-fatigue effects.
REFERENCES
Cui. H.L., Chen, Y., Wang, S.S., Kai, G.Q., Fang, Y.M.
(2011). Isolation, partial characterisation and
immunomodulatory activities of polysaccharide from
Morchella esculenta. J. Sci. Food Agric., 91: 2180-
2185
.
Chen, Z., Li, S., Wang, X., Zhang, C.L. (2013). Protective
effects of Radix pseudostellariae polysaccharides
against exercise-induced oxidative stress in male rats.
Exp. Ther. Med., 5: 1089-1092
.
Jin, H.M., Wei, P. (2011). Anti-fatigue properties of
tartary buckwheat extracts in mice. Int. J. Mol. Sci.,
12: 4770-4780
.
Liu, W., Pan, H., Zhang, C., Zhao, L, Zhao, R., Zhu, Y.,
Pan, W. (2016). Developments in methods for
measuring the intestinal absorption of nanoparticle-
bound drugs. Int. J. Mol. Sci., 17: E1171
.
Lin, Y., Liu, H.L., Fang, J., Yu, C.H., Xiong, Y.K., Yuan,
K. (2014). Anti-fatigue and vasoprotective effects of
quercetin-3-O-gentiobiose on oxidative stress and
vascular endothelial dysfunction induced by
endurance swimming in rats. Food Chem. Toxicol.,
68: 290-296
.
Liu, D.D., Ji, X.W., Li, R.W. (2013). Effects of siraitia
grosvenorii fruits extracts on physical fatigue in mice.
Iran. J. Pharm. Res., 12:.115-121
.
Nitha, B., Fijesh, P.V., Janardhanan, K.K. (2013).
Hepatoprotective activity of cultured mycelium of
morel mushroom, Morchella esculenta. Exp. Toxicol.
Pathol., 65: 105-112
.
Xu, Y.X., Zhang, J.J. (2013). Evaluation of anti-fatigue
activity of total saponins of Radix notoginseng. Indian
J. Med. Res., 137: 151-155
.
Xu, C., Lv, J, Lo, Y.M., Cui, S.W., Hu, X., Fan M.
(2012). Effects of oat β-glucan on endurance exercise
and its anti-fatigue properties in trained rats.
Carbohydr. Polym., 92: 1159-1165
.
Yang, H., Yin, T.T., Zhang, S.T. (2015). Isolation,
purification, and characterization of polysaccharides
from wide Morchella esculenta (L.) Pers. Int. J. Food
Prop., 18: 1385-1390
.
Yan, F., Hao, H. (2016). Effects of Laminaria japonica
polysaccharides on exercise endurance and oxidative
Anti-fatigue Effects of Polysaccharides from Morchella esculenta
137

stress in forced swimming mouse model. J. Biol. Res.,
(Thessalon) 23: 7
.
Yu, S.H., Huang, H.Y., Korivi, M., Hsu, M.F., Huang,
C.Y., Hou, C.W., Chen, C.Y., Kao, C.L., Lee, R.P.,
Lee, S.D., Kuo, C.H. (2012). Oral Rg1
supplementation strengthens antioxidant defense
system against exercise-induced oxidative stress in rat
skeletal muscles. J. Int. Soc. Sports Nutr., 9: 23
.
Wang, X., Xing, R., Chen, Z., Yu, H., Li, R., Li, P.
(2014). Effect and mechanism of mackerel
(Pneumatophorus japonicus) peptides for anti-fatigue.
Food Funct., 5: 2113-2119
.
Zhang, L. (2015). Free Radical scavenging properties and
anti-fatigue activities of Angelica sinensis
polysaccharides. Adv. Mater. Res., 1092-1093: 1538-
1542
.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
138
