
•OH Mineralization of Sulfadiazine during the Treatment of Algae
Bloom Water based on a Drinking Water Treatment System with a
Capacity of 500 M
3
/H
Xianhai Li, Yixuan Yu
*
and Tianjun Sun
Marine Engineering College, Dalian Maritime University, Dalian 116026, China
Keywords:
Algae Bloom, Drinking Water Treatment, Sulfadiazine, •OH Mineralization.
Abstract:
The accumulation of antibiotics in river watersheds and lakes would induce spread of antibiotic resistance
genes in drinking water. For the mineralization of sulfadiazine (SD), •OH equipment was installed in a
drinking water treatment system with a capacity of 500 m
3
/h. The •OH was produced by strong ionization
discharge combined with water jet cavitation. During the transfer of algae bloom water, in only 20 s, a dose
of 1.0 mg/L and 0.5 mg/L •OH completely degraded the SD after coagulation sedimentation and sand
filtration, respectively. All algae bloom was inactivated by disinfection with 0.5 mg/L •OH; the 106 drinking
water quality indexes satisfied the Chinese Standards; and disinfection by-products, such as bromate was not
formed. Based on NaClO disinfection, the total THM content increased to 188 μg/L, which is 2.35 times
higher than the concentration limit regulated by United States Environmental Protection Agency (80 μg/ L).
Advanced •OH oxidation based on strong ionization discharge can be used to completely mineralize
antibiotics during drinking water treatment.
1 INTRODUCTION
Antibiotics which occur and accumulate in varies of
water systems, this condition will generate the
spreading of the antibiotic resistance genes which
affects human health, and as one of the biggest threats
it is taken into consideration by the World Health
Organization. Lately, antibiotic contamination in The
Jiulong River in China has occurred frequently, while
the concentrations have varied from nanograms to
micrograms per liter. Beyond that, due to severe
eutrophication pollution, river basins and lakes often
experience massive blue algal blooms. Nevertheless,
the used techniques of the water treatment, like
coagulation and sedimentation, sand filtration and
chlorine disinfection which cannot continue to play
important roles in the remove of antibiotics from
algal blooms efficiently. To hold back the separation
of antibiotics in humans it is essential to exploit
practical therapeutic techniques before getting the
available potable water.
Advanced Oxidation Technologies (AOT) refer to
the process of generating hydroxyl radical (•OH),
after that a series of chain reactions in •OH are
triggered. and finally degrading organic pollutants
into CO
2
, H
2
O and inorganic salts. In Fenton system,
•OH completely degrades 0.025 mM sulfadiazine
(SD) in glass cells with a diameter of 5.0 cm within
30 minutes, and inactivated 94.7% of Pseudomonas
aeruginosa cells after 5 minutes in a cylindrical
reactor. In the photocatalytic system, •OH degraded
100% pure water SD in 100 mL reactor after 2h., and
100% Microcystis Aeruginosa was inactivated after 4
h in 100 mL reactor. whereas, the present small
laboratory-scale AOT for antibiotic degradation and
algal bloom removal requires a long reaction time.
For this article, •OH mineralization in SD during
algal bloom water treatment was completed in a 500
m
3
/h drinking water treatment system (DWTS)
during algal bloom. Because SD is widely used to
treat some common bacterial infections in humans,
animals and aquatic environments SD was selected to
be the representative to show the effects and
mechanisms in the degradation of •OH. What’s more,
we also studied the influence of the •OH disinfection
on water quality, algae and DBPs which may exist in
the drinking water treatment. The comparation of the
ordinary disinfectant sodium hypochlorite (NaClO).
100
Li, X., Yu, Y. and Sun, T.
•OH Mineralization of Sulfadiazine during the Treatment of Algae Bloom Water based on a Drinking Water Treatment System with a Capacity of 500 M3/H.
DOI: 10.5220/0011186900003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 100-104
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

2 MATERIALS AND METHODS
2.1
Experimental Procedures
During the algae bloom, the total content of algae in
the source water reached 168,000 cells /mL, which
include 92.1% M. Aeruginosa and 1.2%
Pseudanabaena sp. and 0.31% Cyclotella sp., as
shown in Figure 1.
The potable water treatment system consists of
"coagulation sedimentation, sand filtration,
•OH/NaClO disinfection, antibiotic degradation and
a clean water tank" with a capacity of 500 m
3
/h. For
•OH disinfection, part of the sand filter is pumped
into •OH equipment, and •OH solution is generated
through a series of plasma chemical reactions such as
water jet cavitation. After sand filtration, algal bloom
water flows to the clean water tank along the pipe at
a flow rate of 500 m
3
/h. After the injection of
resulting •OH solution to the liquid/liquid mixer, it
will be mixed with water and transferred to the main
pipe. For NaClO disinfection, a peristaltic pump is
used to inject NaClO solution. The treated water
flows through pink pipes to clean tanks. The
treatment lasted for 20 s.
To simulate the severe pollution of antibiotic, part
of algal bloom water was diverted from the main pipe
at a flow rate of 1.0 m
3
/h into a treatment tank. The
prepared SD solution was pumped into the bypass
pipe and then injected with •OH or NaClO solution
for the degradation SD. The reaction time in the by-
pass tube is 20 s.
Figure 1: Images of the total algae and three main algae
species (amplification factor: 400×)
(a) M. aeruginosa (b) Pseudanabaena sp. (c) Cyclotella
sp.
2.2
Analytical Methods
2.2.1 Determination of the Total Reactive
Oxidants and •OH Concentration
•OH and other oxygen radical concentrations as well
as NaClO concentrations were defined as total
reactive oxidants (TRO) concentrations using an
online chlorine analyzer (Hash CL17, USA). As a
free radical probe, 4-hydroxybenzoic acid (4-HBA)
was used for measuring •OH which will form the
hydroxylated derivative 3,4-dihydroxybenzoic acid
(3,4-DHBA). The analysis was performed using a
high-performance liquid chromatograph (HPLC,
Dionex 113 Co. Ltd., USA) equipped with a diode
array detector at 210 nm. When TRO concentration is
1.0 mg/L and 0.5 mg/L, the corresponding •OH
concentration is 6.35 μM and 3.67 μM, respectively.
2.2.2 Determination of SD, DBPs, and
Water Qualities
SD was analyzed by high-performance liquid
chromatograph-mass spectrometry (HPLC-MS/MS,
Agilent 1290-6410 B, USA) on a 3.5 μm C-18
column (2.1 mm × 100 mm, Waters, USA). The flow
rate was 0.6 mL/min. Haloalkanes, formaldehyde and
chloral were analyzed according to EPA methods 556,
524.2 and 551.1 using gas chromatography-mass
spectrometry (QP2020Plus, Japan) and electron
capture detector. Haloacetic acid (HAAs) and rock
salts (including BrO
3
-
, ClO
3
-
, and ClO
2
-
) were used by
ion chromatographs (Thermo 2100, USA), 557 and
300.1, respectively, according to the USEPA method.
Water quality indexes such as total colony, turbidity
and conductivity were measured according to GB-
5750.1-10.
3 RESULTS AND DISCUSSION
3.1
Degradation of Sulfadiazine during
Drinking Water Treatment
Thorough mineralization of antibiotics in drinking
water can prevent the further induction of antibiotic
resistance in humans by residual antibiotics. The
chromatogram of SD degradation by •OH or NaClO
is shown in Figure 2. •OH degraded SD from 68 ng/L
to bellow detection limit (B.D.L) at TRO
concentration of 1.0 mg/L within 20 s after
coagulation, while NaClO degraded SD to 52 ng/L
after 20 s and B.D.L after 2 h. •OH degraded SD from
64 ng/L to B.D.L after 20 s and NaClO degraded SD
•OH Mineralization of Sulfadiazine during the Treatment of Algae Bloom Water based on a Drinking Water Treatment System with a
Capacity of 500 M3/H
101
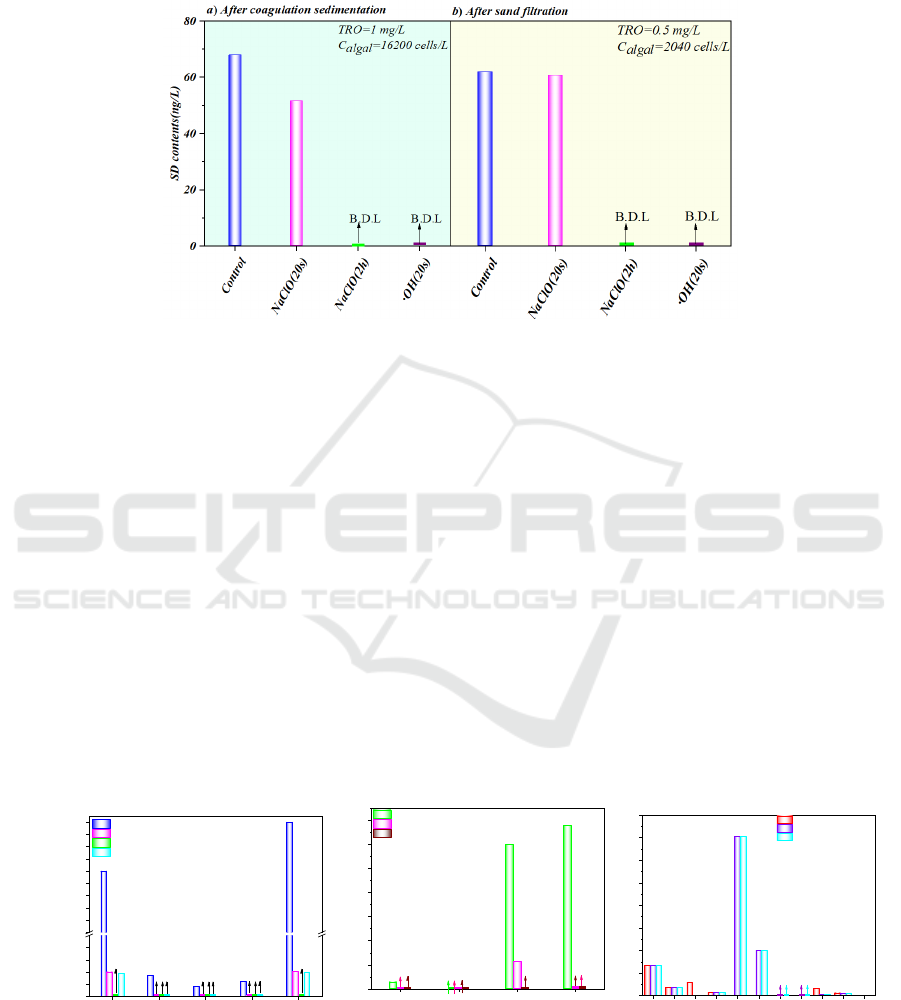
to 62.7 ng/L after 20 s and to B.D.L after 120 minues
after sand filtration with injection of 0.5 mg/L TRO.
Similarly, chlorides produced by electrochemical
methods did not degrade SD until 3 hours later. •OH
produced by strong ionization discharge has the
prospect of large-scale industrial application in
antibiotic degradation.
Figure 2: Degradation of SD by •OH/NaClO (B.D.L =bellow detection limit).
3.2 Effect of •OH Disinfection on Algae
and the Water Quality
•OH disinfection at 500 m
3
/h was performed to
inactivate algae, bacteria, viruse and protozoa to
inhibit their regeneration in the water supply network.
After sand filtration, •OH solution was injected into
the main pipe for disinfection after 20 s reaction. The
results of inactivation of algae and bacteria are shown
in Fig.3. In the source water, the total content of algae
reached 179100 cells/mL. After coagulation and sand
filtration, M. aeruginosa and other algae decreased to
2000 cells/mL and 40 cells/mL, respectively. No live
algae was detected after •OH disinfection.
The heterotrophic plate count in the source water
decreased from 300 cfu/mL to 57 cfu/mL by
coagulation sedimentation and sand filtration, and no
Escherichia coli, heat-resistant coliform group and
total coliform group were detected. No bacteria were
detected after •OH disinfection at 0.5 mg/L. After •
OH disinfection, the COD
Mn
decreased from 1.0
mg/L to 0.9 mg/L which indicaties that •OH
effectively oxidized the reductants and reduced the
relative organic content. •OH enhances
hydrophilicity by oxidizing hydroxyl and carboxyl
groups, reducing turbidity from 0.18 NTU to 0.14
NTU. No obvious change in color, conductivity,
hardness, taste, odour, visible organisms and
ammonia was observed, and 106 indicators of
drinking water quality which could meet the Chinese
Sanitary Standards for Drinking Water (GB5749,
China, 2006).
In consequence, advanced •OH
oxidation technology based on strong ionization
discharge can be used for drinking water treatment
when algal blooms occur.
Figure 3: Data of inactivated algae, bacteria, and water quality indicators in the •OH DWTS (B.D.L =bellow detection limit).
M
.
a
e
r
u
g
i
n
o
s
a
(
c
e
l
l
s
/
m
L
)
P
s
e
u
d
a
n
a
b
a
e
n
a
s
p
.
(
c
e
l
l
s
/
m
L
)
C
y
c
l
o
t
e
l
l
a
s
p
.
(
c
e
l
l
s
/
m
L
)
O
t
h
e
r
s
(
c
e
l
l
s
/
m
L
)
T
o
t
a
l
(
c
e
l
l
s
/
m
L
)
0
1000
2000
3000
4000
170000
171000
172000
173000
174000
175000
176000
177000
178000
179000
B.D.L
Source water
After sand filtration
•OH disinfection (0.5 mg/L ) Alive
•OH disinfection (0.5 mg/L ) Dead
Contens
a) Type of algae
B.D.L
B.D.L
B.D.L
B.D.L
E
s
c
h
e
r
i
c
h
i
a
c
o
l
i
(
c
e
l
l
s
/
m
L
)
T
h
e
r
m
o
t
o
l
e
r
a
n
t
c
o
l
i
f
o
r
m
b
a
c
t
e
r
i
a
(
c
e
l
l
s
/
m
L
)
H
e
t
e
r
o
t
r
o
p
h
i
c
p
l
a
t
e
c
o
u
n
t
(
c
e
l
l
s
/
m
L
)
T
o
t
a
l
c
o
l
i
f
o
r
m
b
a
c
t
e
r
i
a
(
c
e
l
l
s
/
m
L
)
0
50
100
150
200
250
300
350
B.D.L
B.D.L
Source water
After sand filtration
•OH disinfection (0.5 mg/L )
b) Type of bacteria
Contents
B.D.L
B.D.L
W
a
t
e
r
T
e
m
p
.
(
°
C
)
p
H
T
u
r
b
i
d
i
t
y
(
N
T
U
)
C
o
l
o
u
r
(
p
l
a
t
i
n
u
m
-
c
o
b
a
l
t
n
u
m
b
e
r
)
C
o
n
d
u
c
t
i
v
i
t
y
(
μ
s
/
c
m
)
T
a
s
t
e
a
n
d
o
d
o
u
r
V
i
s
i
b
l
e
o
r
g
a
n
i
s
m
s
T
o
t
a
l
o
r
g
a
n
i
c
c
a
r
b
o
n
(
T
O
C
,
m
g
/
L
)
0
20
40
60
80
100
120
140
160
B.D.L
Source water
After sand filtration
•OH disinfection (0.5 mg/L)
c) Water quality indicators
Conents
B.D.L
H
a
r
d
n
e
s
s
(
a
s
C
a
C
O
3
,
m
g
/
L
)
C
h
e
m
i
c
a
l
o
x
y
g
e
n
d
e
m
a
n
d
(
C
O
D
M
n
,
m
g
/
L
)
A
m
m
o
n
i
a
(
N
H
4
,
m
g
/
L
)
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
102

3.3 Analysis of Possible DBPs
In DWTS of 500 m
3
/h, the same dose of •OH and 0.5
mg/L NaClO solution were injected into the main
pipe for disinfection after 20 s of reaction.
Table1.shows that the DBP formed when TRO
dosage (•OH/NaClO) was 0.5 mg/L. No halates, such
as chlorite (ClO
2
-
), chlorate (ClO
3
-
) and BrO
3
-
were
detected during •OH disinfection. When the NaClO
disinfection, ClO
2
-
and BrO
3
-
were not detected, but
the concentration of ClO
3
-
increased to 14 μg/L,
lower than the national standard (GB5749). Notably,
ClO
3
-
in water can be taken up by cells and converted
to more toxic ClO
2
-
by nitrate reductase. According to
the reports, aldehydes are mutagenic in mammalian
cells and cause liver tumors in rodents. In the period
of •OH disinfection, there was no formaldehyde and
chloral detected. While, during NaClO disinfection,
the content of chloral increased to 4.2 μg/L (below the
limit of the Chinese Standard GB5749) because
NaClO oxidizes alcohol functional groups, nitrogen
compounds, and amino acids to form chloral. At
alkaline pH, chloral further decomposes to generate
TCM, which is carcinogenic to human beings.
THM is potentially carcinogenic and genotoxic to
humans. No TCM, DCBM, DBCM, TBM and total
THM were detected during •OH disinfection. During
NaClO disinfection, the contents of
Trichloromethane, DCBM and DBCM reached to
5.2, 4.2 and 2.1 μg/L, respectively, which were all
lower than the national standard (GB5749). The total
THM content was up to 188 μg/L, which was 2.35
times higher than the limit set by EPA (80 μg/L) in
the United States. This is because NaClO oxidizes a
methyl hydrogen atom through a substitution reaction
to form TCM. The OCl
-
could re-oxidize existing Br
-
through electron transfer reaction to form HOBr, and
brominated THMs, such as DBCM and DCBM are
generated by the substitution reaction.
Therefore, •OH did not produce DBPs after
disinfection of algal bloom water, indicating that the
treated drinking water after treatment is safe for
human body.
Table1: Formation of DBPs during •OH/NaClO disinfection.
(TOC = 1.65 mg/L, TRO = 0.5 mg/L, temperature = 26.5 °C, pH = 7.19).
Test items Control (μg/L) •OH disinfection (μg/L) NaClO disinfection (μg/L)
ClO
2
-
B.D.L B.D.L B.D.L
ClO
3
-
B.D.L B.D.L 13±2
BrO
3
-
B.D.L B.D.L B.D.L
Formaldehyde B.D.L B.D.L B.D.L
Chloral B.D.L B.D.L 5±1
Trichloromethane B.D.L B.D.L 5.2±1
Bromodichloro methane B.D.L B.D.L 4.2±0.8
Dibromochloro methane B.D.L B.D.L 2.1±0.5
Tribromomethane B.D.L B.D.L B.D.L
Trihalomethane B.D.L B.D.L 188±3
B.D.L =bellow detection limit
4
CONCLUSIONS
•OH equipment was installed after sand filtration in
500 m
3
/h DWTS during algal bloom outbreaks.
During algal blooms, •OH equipment was installed
after sand filtration in 500 m
3
/h DWTS.
To contrast with common oxidants, the •OH
method possesses great practical application potential
in antibiotic mineralization, algae inactivation,
drinking water disinfection and other aspects. The
main results suggest that:
(1) In the transporting of algal bloom water within
20 s, after coagulation precipitation and sand
filtration by •OH degradation at 1.0 mg/L and 0.5
mg/L there is no SD detected. Compared with it, the
corresponding degradation rates of SD by NaClO
were 24% and 2%, respectively.
(2) In the main pipeline with a treatment capacity
of 500 m
3
/h, •OH disinfection at 0.5 mg/L inactivated
algae from 2040 cells/mL to 0 cells/mL in only 20 s.
The 106 water quality indexes all meet the limit
requirements of China Drinking Water Sanitation
Standard (GB5749, China, 2006).
•OH Mineralization of Sulfadiazine during the Treatment of Algae Bloom Water based on a Drinking Water Treatment System with a
Capacity of 500 M3/H
103

(3) DBPs, for example HAAs, aldehydes, THMs,
and bromate, were not produced in the process of the
•OH disinfection. During the disinfection by NaClO,
the total THMs increased to 188μg/L, 2.35 times
higher than 80 μg/L which is the limit that set by
USEPA standards. This result showed that the •OH
disinfection will lead no harm to human health
potentially.
ACKNOWLEDGMENTS
This work is financially supported by grants from the
National Natural Science Foundation of China (No.
21776266, 22078037) and the Fundamental Research
Funds for the Central Universities (3132021221).
REFERENCES
ASGHAR A, ABDUL RAMAN A A, WAN DAUD W M
A. Advanced oxidation processes for in-situ production
of hydrogen peroxide/hydroxyl radical for textile
wastewater treatment: a review [J]. Journal of Cleaner
Production, 2015, 87(826-38.
BARROTT L. Chloral hydrate: Formation and removal by
drinking water treatment [J]. Journal of Water Supply:
Research and Technology-Aqua, 2004, 53(6): 381-90.
Daniel F B, DeAngelo A B, Stober J A, et al.
Hepatocarcinogenicity of chloral hydrate, 2-
chloroacetaldehyde, and dichloroacetic acid in the male
B6C3F1 mouse[J]. Fundamental and Applied
Toxicology, 1992, 19(2): 159-168.
EPA U S. National primary drinking water regulations:
Stage 2 disinfectants and disinfection byproducts rule.
Agency, USEP[J]. Federal Register, 2006: 387-493.
GB 5749, Standards for drinking water quality, National
Standard of the People’s Republic of China 5749, 2006.
GB 5750.1-10, Standard examination methods for drinking
water, National Standard of the People’s Republic of
China 5750, 2006.
Hautman D P, Munch D J. Method 300.1 Determination of
inorganic anions in drinking water by ion
chromatography[J]. US Environmental Protection
Agency, Cincinnati, OH, 1997.
He J B, Hu A Y, Chen M, et al. Studies on the pollution
levels of antibiotic resistance genes in Jiulong River
estuary and wastewater treatment plants in Xiamen[J].
Microbiology/Weishengwuxue Tongbao, 2012, 39(5):
683-695.
JIA P, ZHOU Y, ZHANG X, et al. Cyanobacterium removal
and control of algal organic matter (AOM) release by
UV/H2O2 pre-oxidation enhanced Fe(II) coagulation
[J]. Water Research, 2018, 131(122-30.
Jojoa-Sierra S D, Silva-Agredo J, Herrera-Calderon E, et al.
Elimination of the antibiotic norfloxacin in municipal
wastewater, urine and seawater by electrochemical
oxidation on IrO2 anodes[J]. Science of The Total
Environment, 2017, 575: 1228-1238.
Munch D J, Hautman D P. Method 551.1: Determination of
chlorination disinfection byproducts, chlorinated
solvents, and halogenated pesticides/herbicides in
drinking water by liquid-liquid extraction and gas
chromatography with electron-capture detection[J].
Methods for the Determination of organic compounds
in drinking water, 1995.
Munch J W, Munch D J, Winslow S D, et al. Method 556:
Determination of carbonyl compounds in drinking
water by pentafluorobenzylhydroxylamine
derivatization and capillary gas chromatography with
electron capture detection[M]. National Exposure
Research Laboratory, Office of Research and
Development, US Environmental Protection Agency,
1998.
ÖZCAN A, ATıLıR ÖZCAN A, DEMIRCI Y. Evaluation of
mineralization kinetics and pathway of norfloxacin
removal from water by electro-Fenton treatment [J].
Chemical Engineering Journal, 2016, 304(518-26.
Stauber J L. Toxicity of chlorate to marine microalgae[J].
Aquatic Toxicology, 1998, 41(3): 213-227.
TANG L, WANG J, ZENG G, et al. Enhanced
photocatalytic degradation of norfloxacin in aqueous
Bi2WO6 dispersions containing nonionic surfactant
under visible light irradiation [J]. Journal of Hazardous
Materials, 2016, 306(295-304.
Ueno H, Nakamuro K, Moto T, et al. Disinfection by-
products in the chlorination of organic nitrogen
compounds: possible pathways for the formation of
disinfection by-products[J]. Water Supply[WATER
SUPPLY]., 1995, 13(3-4).
US EPA Method 524.2, Measurement of purgeable organic
in water by capillary column gas chromatography/mass
spectrometer, Cincinnati, Ohio, 1995.
WANG X, SONG J, SU C, et al. CeOx/TiO2-yFy
nanocomposite: An efficient electron and oxygen
tuning mechanism for photocatalytic inactivation of
water-bloom algae [J]. Ceramics International, 2018,
44(16): 19151-9.
Zaffiro A D, Zimmerman M, Pepich B V, et al. Method 557:
Determination of haloacetic acids, bromate, and
Dalapon in drinking water by ion chromatography
electrospray ionization tandem mass spectrometry (IC-
ESI-MS/MS) [J]. US-EPA, Cincinnati, Ohio, 2009.
ZHANG Y, TIAN Y, ZHANG Z, et al. Experimental and
numerical study of cavitating flow with suction in a
mixing reactor for water treatment [J]. Chemical
Engineering Journal, 2018, 353(796-804.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
104
