
Removal of Nitrate from Water by Reed Straw Biochar with
Different FeCl
3
Modification Method
Zhongwei Zhang
1,2 a
, Peijing Kuang
1,2,* b
, Junwen Ma
1c
, Yubo Cui
1,2 d
and Zhaobo Chen
1,2 e
1
Key Laboratory of Biotechnology and Bioresources Utilization, Ministry of Education, Dalian Minzu University,
Dalian, China
2
College of Environment and Resources, Dalian Minzu University, Dalian 116600, China
*
Corresponding author
Keywords: Reed Straw, Biochar, Nitrate Nitrogen, Modification.
Abstract: Nitrate contamination became an ever-increasing serious environmental problem and some conventional
methods had the higher operating costs and the lower efficiency. In order to develop low-cost technology for
aqueous nitrate treatment, various agro-forest residuals have attracted a great deal of research attention to
preparing biochar adsorbents for nitrate adsorption. In our study, reed straw biochar was prepared from
wetland plant residuals at different pyrolysis temperatures (300ºC, 400ºC, 500ºC, 600ºC and 700ºC).
Meanwhile, biochar was modified by FeCl
3
with different methods to optimize their adsorbent performance.
The results show that the optimal preparation conditions could achieve at carbonization temperature of 600ºC
for 2 h, followed by soaking in FeCl
3
solution for 24 hours in alkaline condition, for which the highest
adsorption capacity could reach at 1.97 mg/g after modification. SEM images show that a large amount of
iron oxides were loaded on the surface of biochar, as well as in the pores of biochar, promoting the removal
of nitrate. This study can provide a theoretical basis for the comprehensive utilization of agro-forest waste
and nitrate removal.
1 INTRODUCTION
1
Unreasonable discharge of industrial nitrogen-
containing wastewater, random stacking of solid
wastes such as domestic waste and nitrogen-
containing waste residue, unreasonable use of
nitrogen-containing pesticides and fertilizers will
lead to a large number of NO
3
-N into the water
environment, resulting in NO
3
-N pollution in water
environment (JesúsGarcía-Fernández, 2018). Due to
the limited self-purification capacity of water and the
natural degradation capacity of microorganisms,
NO
3
-N gradually accumulates in the environment,
and the concentration continues to increase (Liu
2021). Enriched NO
3
-N makes the water
eutrophication, a large number of algae cover the
water surface so that sunlight can not be transmitted,
a
https://orcid.org/0000-0001-5413-554X
b
https://orcid.org/0000-0003-1838-4252
c
https://orcid.org/0000-0001-5716-9446
d
https://orcid.org/0000-0001-8950-5889
e
https://orcid.org/0000-0003-2786-6173
the photosynthesis of aquatic plants is weakened, and
the respiration is enhanced, resulting in the death of a
large number of aquatic organisms such as fish, and
the deterioration of water quality (Huang 2017). In
order to reduce the damage of NO
3
-N pollution to the
water environment and human health, it is of great
significance to seek economical and effective
technology to control the concentration of NO
3
-N in
water.
In recent years, the use of low-cost and effective
adsorbents to adsorb pollutants is the focus of
scholars (Cao 2019). As a new type of environment-
friendly adsorbent, biochar has strong adsorption
capacity for heavy metals and organic pollutants
because of its easily available raw materials and rich
surface functional groups. Biochar has become one of
the main ways of resource utilization of agricultural
26
Zhang, Z., Kuang, P., Ma, J., Cui, Y. and Chen, Z.
Removal of Nitrate from Water by Reed Straw Biochar with Different FeCl3 Modification Method.
DOI: 10.5220/0011176000003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 26-30
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

waste (HussainLAHORI 2017). The adsorption
performance of biochar can be improved by
modifying biochar
(Chen 2014). Ordinary biochar has
large specific surface area and high porosity. Biochar
can be modified to change its surface structure
(Zhu
2017), increase surface functional groups, enhance
the adsorption capacity of biochar to NO
3
-N and
improve the removal rate of NO
3
-N. Some studies
have shown that iron oxides have high affinity and
adsorption selectivity for oxygen-containing anions
in water (Namasivayam 2005). Wheat straw biochar
was prepared by Li et al and modified with FeCl
3
. The
results showed that when the mass ratio of Fe/C was
0.7, the maximum NO
3
-N adsorption capacity of iron
modified biochar was 2.47 mg/g fitted by Langmuir
isothermal adsorption model, while that of
unmodified biochar was only 0.13 mg/g (Li 2015).
Dewage et al prepared Douglas fir biochar and
modified it with FeCl
3
. It was found that Fe particles
in the form of α-Fe
2
O
3
and Fe
3
O
4
could lead to
magnetization of biochar and produce a large number
of adsorption sites
(Dewage 2018). However, few
people have studied the treatment of pollutants in
water by FeCl
3
modified reed straw biochar.
As a kind of wetland aquatic plant, reed straw was
made into biochar to explore its adsorption capacity
to nitrate nitrogen in water environment. reed straw
biochar was modified by FeCl
3
to determine the
modification conditions with the best adsorption
capacity. The related properties of biochar (modified
biochar) were analyzed by scanning electron
microscope (SEM) and X-Ray diffraction (XRD).
The purpose of this study is to provide a scientific
basis for the resource utilization of aquatic plants in
wetlands and the pollution control of nitrate nitrogen
in water.
2 MATERIALS AND METHODS
2.1 Preparation of Biochar from Reed
Straw
In this study, the reed was defoliated, washed and
crushed into powder with a powder mesh of about 60-
80 mesh. The reed straw powder was washed with a
large amount of deionization and dried in an oven at
105°C. Under the conditions of 10°C/min heating
gradient, 120 min combustion residence time and
oxygen limitation, reed powder was prepared at five
operating temperatures of 300ºC, 400ºC, 500ºC, 600
ºC, 700 ºC respectively, and BC300, BC400, BC500,
BC600 and BC700 were obtained.
2.2 Preparation of Biochar from
Different Modified Reed Straw
Different preparation methods are as follows:
(a) Reed straw was pre-modified with FeCl
3
solution and rotated in shaker for 24 hours, then
carbonized at 600°C for 2 hours in muffle furnace
under oxygen-limited condition;
(b) Reed straw was carbonized at 500°C for 1
h, soaked in FeCl
3
solution for 24 h, then washed with
deionized water for five times (filtrate Ph was about
4.3), then dried;
(c) Reed straw was carbonized at 600°C for 2
h, soaked in FeCl
3
solution, adjusted to alkaline
solution, soaked 24 h, washed with deionized water
for five times (filtrate Ph was about 4.5);
(d) Reed straw was pre-soaked with Hcl
solution for 24h, then rinsed with deionized water for
five times (filtrate Ph was about 4), carbonized at
600°C in muffle furnace under oxygen limitation for
2 h, removed and soaked with FeCl
3
for 12 h, then
dried;
(e) Reed straw was carbonized at 600°C for 2
hours, soaked in Hcl solution for 24 hours, then
washed with deionized water for five times (filtrate
Ph = 5.5), then dried, soaked in FeCl
3
solution for 12
hours, and dried.
2.3 Preparation of Nitrate Solution
Accurately weigh KNO
3
(analytical purity) 0.14436 g
in the beaker, add appropriate amount of deionized
water to dissolve, fix the volume to 1 L, obtain the
NO
3
-N reserve solution of 20 mg/L, avoid light and
store at 4°C.
2.4 Experiment on Determination of
Adsorption Effect of NO
3
-N
Configure the KNO
3
solution of 20 mg/L, adjust the
solution pH to about 7, weigh 0.2 g reed straw biochar
(modified straw biochar), add it to the 50 ml capacity
flask of KNO3 solution containing 20 mg/L, shake in
the shaker for 24 hours, and then use 0.22 μm filter
membrane to determine the concentration of NO
3
-N
in the supernatant. Thus, the adsorption effect of reed
straw biochar (modified straw biochar) on NO
3
-N
after 24 h adsorption was calculated. NO
3
-N was
detected by CleverChem Anna automatic
discontinuous chemical analyser, and the SEM
characterization of reed straw biochar was measured.
X-Ray powder diffraction (XRD) is used to scan the
crystal structure of the material in the range of 10-60
Removal of Nitrate from Water by Reed Straw Biochar with Different FeCl3 Modification Method
27

degrees using Bruker D4 Endeavor Powder X-Ray
diffractometer.
2.5
Analytical Methods
The adsorption capacity of biochar to NO
3
-N is
calculated by formula (1):
q
e
= V/M(C
0
-C
e
)
(1)
Among them, q
e
(mg/g) is the equilibrium
adsorption capacity; C
0
(mg/L) is the initial
concentration of NO
3
-N in the water sample; C
e
(mg/L) is the concentration of NO
3
-N in the
adsorption equilibrium; M (g) is the amount of
biochar added; V (L) is the volume of the water
sample.
The experimental data were plotted using Excel
2016 and Origin 2018 software.
3 RESULTS AND DISCUSSION
3.1
Effect of Biochar at Different
Preparation Temperatures on the
Adsorption Properties of Nitrate
Nitrogen
It can be seen from figure 1 that for the solution with
the same NO
3
-N concentration (20.381mg/L), the
adsorption capacity of reed straw biochar is in the
order of BC600 > BC700 > BC300 > BC500 > BC400
under the preparation conditions of 300°C, 400°C,
500°C, 600°C and 700°C. The three kinds of biochar
BC300, BC400 and BC500 had negative adsorption
effect on NO
3
-N, while under the preparation
conditions of 600°C and 700°C, the adsorption effect
of reed straw biochar on NO
3
-N was close to that of
reed straw biochar.
Figure 1: Adsorption effect of reed straw biochar prepared
at different temperatures for NO
3
-N.
As shown in figure 2, with the increase of the
pyrolysis temperature of straw biochar material, the
concentration of NO
3
-N in the target solution
decreases gradually. The higher the pyrolysis
temperature, the higher the adsorption capacity of
straw biochar to NO
3
-N, and the adsorption capacity
of BC600 and BC700 to NO
3
-N is the largest
compared with other biochar. This should be due to
the fact that the surface of biochar prepared by high
temperature pyrolysis has more pore structure than
that of biochar prepared at 300°C ~ 500°C. With the
increase of pyrolysis temperature, the porosity and
specific surface area of biochar are increasing, and the
porosity is becoming more and more perfect. In the
SEM diagram of the original reed, due to the crushing
of the reed straw, the surface of the reed straw is
rough and the electrical conductivity is poor. In the
SEM diagram of BC300, biochar is not carbonized
completely and does not form complete porosity,
which will lead to poor electrical conductivity of
biochar. In the SEM diagram of BC400 and BC500,
the porosity of biochar has been gradually formed. In
the SEM diagram of BC600, the porosity of reed
carbon is relatively complete, and the voids are larger,
and there are many small holes in the voids, which
may cause NO
3
-N to adhere to the voids more easily.
In the SEM diagram of BC700, the porosity of reed
carbon has been completely formed, and the
arrangement is relatively neat, and the inner surface
of the pore is smooth.
Figure 2: Micro-morphology pictures of SEM (10 μm)
prepared by biochar at different temperatures.
3.2
Effect of Biochar Modified by
Different Methods on Adsorption
Properties of NO3-N
As shown in figure 3, the effect of (c) method is the
best among the five modification methods, reaching
1.97 mg /g. In (c) method, Ph, is adjusted to FeCl
3
solution to make it alkaline. NaOH can interact with
carbon matrix to increase the porosity and specific
surface area of biochar. At the same time, it can also
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
28
increase the amount of -OH, -COOH and cation
exchange capacity. FeCl
3
is easy to form iron
hydroxide under alkaline conditions. Iron hydroxide
is easier to combine with the surface functional
groups in biochar to form iron oxide, and it is easy to
be loaded in biochar. The effect of (b) is not as good
as that of (c), which may be due to the fact that the
porosity is not as good as that of (c) due to the lack of
carbonization time. Although the adsorption effect of
(d) and (e) is not as good as that of (c), they also have
better adsorption capacity, which may be due to the
effect of HCI solution, because the surface elemental
composition of biochar changes when soaked in acid
solution: on the one hand, the C content decreases and
the O content increases due to the loss of organic
carbon in biochar, on the other hand, the relative
content of C increases due to the decrease of ash
content in biochar. In addition, the increase of
oxygen-containing functional groups on the surface
of biochar can also increase its O content. Therefore,
acid modification can effectively increase the number
and variety of oxygen-containing functional groups
on the surface of biochar, and greatly increase the
porosity of the materials. In (d) method and (e)
method, the modification order of HCl solution is
different, and the reed straw treated with HCI solution
does not play a key role in carbonization. On the
contrary, the carbonized reed carbon produces a large
amount of CO and H
2
O due to soaking in HCI
solution, which makes the pore structure of biochar
more developed, increases the specific surface area
and reduces the crystallinity of cellulose in the
process of biomass carbonization. The improvement
of its pore structure leads to the increase of the
porosity of biochar, which makes the oxide of Fe
more easily attached to it.
Figure 3: Adsorption effect of NO
3
-N on biochar under
different modified methods.
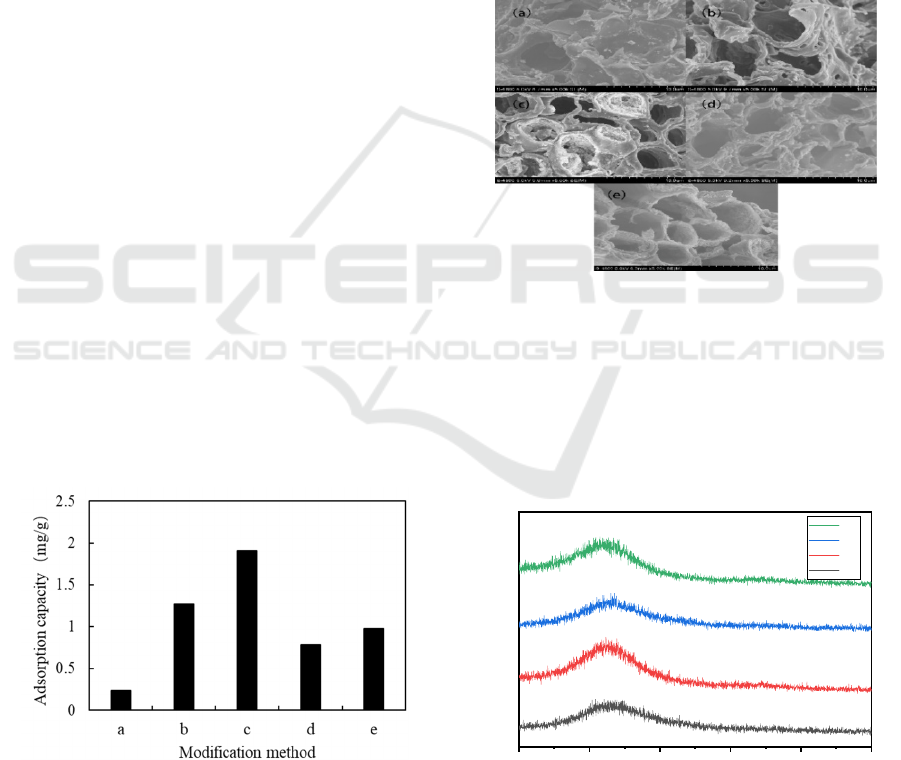
As shown in figure 4, most of the biochar has a
porous structure in the (a) ~ (e) modification method.
In (a) modification method, the porous structure of
biochar and very few Fe oxide particles attached to the
surface of biochar have large and small pore structure,
which may be caused by carbonization time of only 1
h. In (c) method, the crystallization of oxides
containing a large amount of iron in the pores can be
observed, which may indicate that FeCl
3
solution
modification is more successful than the previous
methods. Due to the pre-treatment of reed straw by
HCl immersion in (d) method, the solution is slightly
acidic after washing, and the pores are destroyed and
not round, which may make the adsorption effect not
as good as that of (e) method. In the SEM diagram of
(e) method, it is obvious that the iron oxides have been
attached to the pores, which is because the carbonized
pores are amplified by HCl solution.
Figure 4: SEM (10 μm) micro-morphology pictures
prepared by different modification methods of biochar.
Figure 5 shows the XRD pattern of biochar
modified by FeCl
3
solution, which does not show the
characteristic diffraction peak of iron, indicating that
the two kinds of modified biochar are amorphous. Hu
et al also reached a similar conclusion
(Hu 2016). The
iron-bearing particles are amorphous, which may
have a good adsorption effect on NO
3
-N.
Figure 5: XRD pattern of biochar modified by FeCl
3
solution.
10 20 30 40 50 60
Intensity(a.u)
Two theta
(
de
g
ree
)
(e)
(d)
(c)
(b)
Removal of Nitrate from Water by Reed Straw Biochar with Different FeCl3 Modification Method
29

4 CONCLUSIONS
The main results are as follows:
(1) The biochar of reed straw loaded with iron
oxide was prepared by the activation of FeCl
3
, and the
preparation conditions with the best adsorption
capacity of NO
3
-N were obtained, that is,
carbonization at 600ºC for 2 h and soaking in FeCl
3
solution (alkaline) for 24 h. The adsorption capacity
under the optimal conditions is 1.97 mg/g .
(2) The porosity of reed straw biochar prepared
under the optimal preparation conditions is higher
than that of biochar prepared by other methods;
biochar has a porous structure and loaded with a
variety of iron oxide components.
(3) Acid modification reagents can change the
specific surface area and pore structure of biochar,
and increase the porosity of biochar; Appropriate
amount of alkali modification not only increases the
specific surface area and porosity of biochar, but also
increases the number of -OH and -COOH of biochar.
ACKNOWLEDGEMENTS
The research was financed by the Natural Science
Foundation of Liaoning, China (2020-MZLH-02) and
Science and Technology Innovation Foundation of
Dalian, China (2018J12SN080).
REFERENCES
C. Namasivayam, K. Prathap. Journal of Hazardous
Materials. Recycling Fe(III)/Cr(III) Hydroxide, an
Industrial Solid Waste for the Removal of Phosphate
from Water, 1, 123 (2015).
Dewage N B, Liyanage A S, Pittman C U, et al.
BioresourceTechnology. Fast Nitrate and Fluoride
Adsorption and Magnetic Separation from Water on α-
Fe
2
O
3
and Fe
3
O
4
Dispersed on Douglas Fir Biochar,
263, 258-265 (2015).
Garcia M J, Pastor M M, Epron F, et al. Applied Catalysis
B Environmental. Proposed Mechanisms for the
Removal of Nitrate from Water Byplatinum Catalysts
Supported on Polyaniline and Polypyrrole, 225, 162-
171 (2018).
J.H. Li. Chinese Academy of Agricultural Sciences
Dissertation. Adsorption of Nitrate and Phosphate by
Modifned Biochar, (2015).
J.H. Cao, L.Q. Liu, Y.J. Huang, et al. Chemical Industry
Progress. Effects of Feedstock Type and Pyrolysis
Temperature on Cd
2+
Adsorption by Biochar, 38, 4183-
4190 (2019).
Lahori A H, Z.Y. Guo, Z.Q. Zhang, et al. Pedosphere. Use
of Biochar as an Amendment for Remediation of Heavy
Metal-contaminated Soils: Prospects Andchallenges,
27, 991-1014 (2017).
Q. Hu, N. Chen, C. Feng, et al. Journal of the Taiwan
Institute of Chemical Engineers. Nitrate Removal from
Aqueous Solution Using Granular Chitosan-Fe(III)-
Al(III) Complex: Kinetic, Isotherm and Regeneration
Studies, 63, 216-225 (2016)
S.H. Zhu, J.J. Zhao, L.G. Chu, et al. Journal of Agro-
Environment Science. Comparison of Copper
Adsorption onto Unmodified and Nano-
hydroxyapatite-Modified Wheat Straw Biochar, 36,
2092-2098 (2017).
W.L. Liu, Y.P. Yuan, Maxwell Bryan. Science of the Total
Environment. Letter to the Editor: Comments on
“Springs Drive Downstream Nitrate Export from
Artificially-drained Agricultural Headwater
Catchments” by Goeller et al, Part B, 783 (2018).
W.F. Chen, F. Wen, W.M. Zhang, J. Meng. Journal of
Agro-Environment Science. Biochar and
Agroecological Environment: Review and Prospect, 33,
821-828 (2014).
Y. Huang, X. Chen, P. Li, et al. Bioresour Technol.
Pressurized Microcystis Can Help to Remove Nitrate
from Eutrophicwater, 248, 140-145 (2018).
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
30
