
Classification of Brain Tumour Tissues in Human Patients using
Machine Learning
Françoise Bouvet
1
, Hussein Mehidine
1
, Bertrand Devaux
2,3,4
, Pascale Varlet
2,5,6
and Darine Abi Haidar
1,7,*
1
Université Paris-Saclay, CNRS/IN2P3, IJCLab, 91405 Orsay, France
2
Université de Paris, Faculté de Médecine Paris Descartes, 75006 Paris, France
3
Service de Neurochirurgie, Hôpital Lariboisière, 75010 Paris, France
4
Pôle Neurosciences, GHU-Paris, 75014 Paris, France
5
Department of Neuropathology, GHU Paris-Psychiatrie et Neurosciences, Sainte-Anne Hospital, Paris, France
6
IMA BRAIN, INSERM U894, Centre de Psychiatrie et de Neurosciences, F-75014 Paris, France
7
Université de Paris, IJCLab, 91405 Orsay, France
Keywords: Classification, Endogenous Fluorescence, Machine Learning, Decision Trees.
Abstract: Delineating brain tumor margins as accurately as possible is a challenge faced by the neurosurgeon during
tumor resections. The extent of resection is correlated with the survival rate of the patient while preserving
healthy surrounding tissues is necessary. Real-time analysis of the endogenous fluorescence signal of brain
tissues is a promising technique to answer this problem. Multimodal optical analysis has been proved to be a
powerful tool to discriminate tumor samples of different grade of gliomas and meningiomas from healthy
control samples. In this study, Machine Learning methods are evaluated to improve the accuracy of such
discrimination. Each sample is described by 16 feature given in input to a Decision Tree based model. Once
the learning step is completed, the classifier achieves a 95% correct classification on unknown samples. This
study shows the potential of Machine Learning to discriminate between tumoral and non tumoral tissues based
on optical parameters.
1 INTRODUCTION
Brain and central nervous system cancer is one of the
most lethal cancers that affect humans (Buckner,
2007). Many types of brain tumors exist, which are
classified into different categories and grade
according to their originating cells and pathological
class (Louis, 2016).
Nowadays, total resection is still the primary
therapy for treating the majority of brain tumours and
is considered as the most critical stage in the therapy
procedure of these tumors. The main challenge of the
neurosurgical operations is to obtain a precise
identification of the margins of the tumor in order to
achieve a complete resection (Wilson, 2014). These
margins often contain diffuse isolated tumor cells
outside the solid area that have a visual appearance
similar to adjacent healthy areas, making the surgeon
unable to correctly identify these margins. The
inability to fully visualize these limits results in
*
corresponding author: darine.abihaidar@ijclab.in2p3.fr
incomplete surgical resection, which increases the
risk of recurrence. Similarly, unnecessary removal of
healthy brain tissue that does not contain tumor cells
can lead to major neurological deficits that affect the
patient’s quality of life.
Therefore, and in order to improve diagnosis
information on these margins and to confirm the
success of the operation, biopsy samples are extracted
from these areas for histological analysis, which
involves Haematoxylin and Eosin (H&E) staining,
but the results of this post-operative analysis are
provided a few days later and this information is not
available for the surgeon during surgery.
However, several techniques have been proposed,
developed and transferred to the operation room to
address this problem such as intraoperative-MRI and
ultrasound imaging (Kubben, 2011) (Unsgaard,
2006). The aim of these techniques is to help the
surgeon properly define the limits of the tumor and to
precise spatial information on tumor infiltration at the
Bouvet, F., Mehidine, H., Devaux, B., Varlet, P. and Haidar, D.
Classification of Brain Tumour Tissues in Human Patients using Machine Learning.
DOI: 10.5220/0010909700003121
In Proceedings of the 10th International Conference on Photonics, Optics and Laser Technology (PHOTOPTICS 2022), pages 53-58
ISBN: 978-989-758-554-8; ISSN: 2184-4364
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
53
cellular scale. However, the information provided by
these techniques have not reached the reliability of
the gold-standard histological post-surgery analysis.
To address this challenge, our team at the IJCLab
laboratory is developing a new intraoperative optical
tool that aims to diagnose tumor zones at the cellular
scale in order to obtain fast and accurate information
on the tissue’s nature. This tool consists of a
miniature non-linear multimodal endomicroscope.
This endomicroscope is able to detect both the
quantitative (fluorescence lifetime measurement and
spectral measurement) and qualitative (fluorescence
imaging) response of endogenous fluorescence under
two-photon excitation (TPE) and the detection of the
generation of the second harmonic (SHG) (Ibrahim,
2016) (Sibai, 2018).
However, the development of this tool requires in
parallel the construction of a tissue database that
includes the different imaging modalities that we
want to integrate into our endomicroscope. The
purpose of this database is to characterize and to
discriminate different types of brain tissues, whether
healthy or tumoral, through their specific optical
signatures. Different analysis methods and data
processing will be developed and implemented in our
endomicroscope. The final aim is, based on this
database, to be able to provide the surgeon a fast,
reliable and accurate diagnosis in real time.
In our previous studies, and through different
quantitative optical parameters, we managed to
discriminate, with high specificity and sensitivity,
healthy human brain tissues, from secondary and
primary brain tumors (Poulon, 2018)(Poulon, 2018)
low and high grade glioma (Mehidine, 2019), and
grade I and grade II meningioma (Mehidine, 2021).
The aim of this study is to expand our analysis
towards using Machine Learning (ML) methods to
discriminate healthy from tumor tissues using these
quantitative parameters. ML approach allows to
combine several optical parameters thus combining
the information provided by the different endogenous
fluorescence molecules. As the histological
classification was known, we were able to investigate
supervised methods. Decision Tree is commonly used
for classification and has the benefit of being among
the most explainable ML models. Two studies are
presented, one in the visible excitation domain using
375 and 405 nm, and one in the Deep Ultra-Violet
(DUV) using 275 nm.
2 MATERIALS AND METHODS
2.1 Samples
Samples were obtained from the department of
neurosurgery of Sainte Anne Hospital (Paris) upon
the approval of the Sainte-Anne Hospital – University
Paris Descartes Review Board (CPP Ile de France 3,
S.C.3227). All methods and measurements were
carried out in accordance with the relevant guidelines
and regulations of the cited approval. Informed
consents were obtained also from all patients included
in this study. Each obtained sample was directly sent
after the surgery in a saline solution towards the
neuropathology department in Saint-Anne Hospital
where the visible measurement setup is located. More
details about the Visible measurement setup were
published elsewhere (Poulon, 2017)(Zanello,
2017)(Mehidine, 2018). Afterwards, each collected
sample was stored at −80 °C. Few hours before
cutting, the sample were put at −20 °C, after then it
was cut into 10 μm slices using a cryostat (CM 1950,
Leica Microsystems). The 10 µm slice was then fixed
with 100° ethanol and stored at 4°C until the DUV
measurements. These fixed slices were then used to
realize the spectral measurements on the Deep UV
setup at DISCO Beamline. More details about the
DUV measurements setup were published elsewhere
(Poulon, 2018) (Mehidine, 2021).
2.2 Database
2.2.1 Visible Range
The visible measurements setup uses 375 and 405 nm
as excitation wavelength. Through this wavelengths,
we were able to excite the following endogenous
fluorophores: Nicotinamide adenine dinucleotide
NADH (2 components, Bound NADH and free
NADH), Flavins (FAD), Lipopigments and
Porphyrins I (P1) and II (P2). The samples were
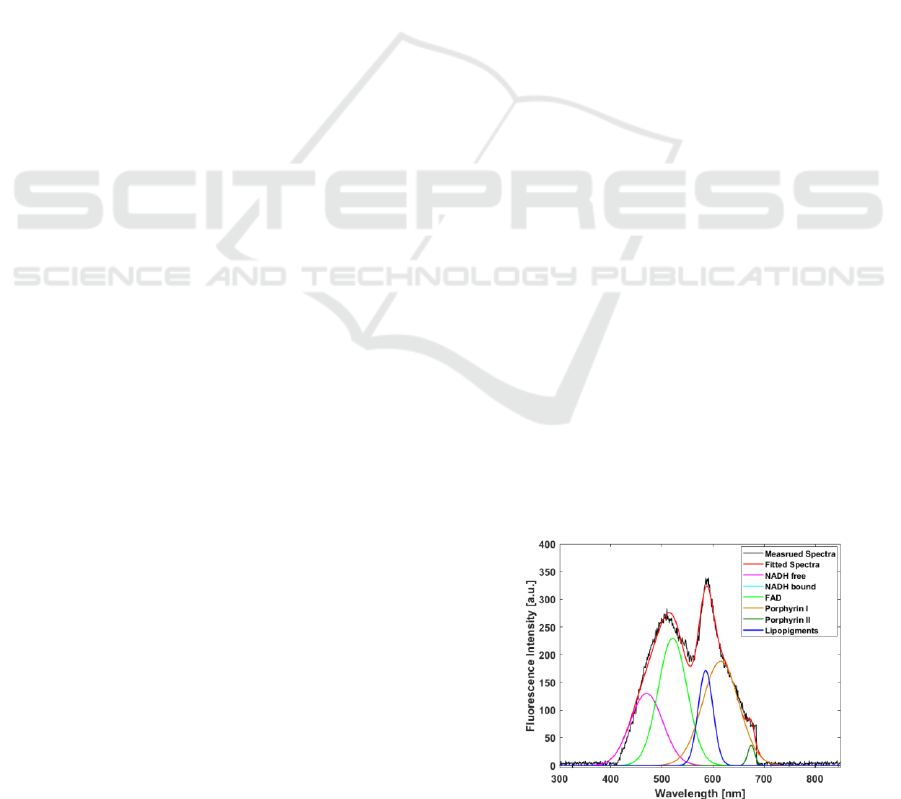
Figure 1: Spectrum fitted by a sum of six Gaussians.
PHOTOPTICS 2022 - 10th International Conference on Photonics, Optics and Laser Technology
54

scanned point by point with a 0.2 mm step along
several parallel lines spaced by 2 mm. The obtained
spectrum at each point is fitted by a sum of six
Gaussians functions, one for each fluorophore. The
integral under the curve and the maximum are
recorded for each Gaussian. Figure 1 illustrates an
acquired spectrum and the six Gaussian fitted curves.
The samples cohort consisted of 21 specimens
relative to four different pathologies: Diffuse Glioma
(DIF), Glioblastoma (GBM), Meningioma (MEN)
and metastasis (MET) and also one control group
(CTR) obtained from epileptic surgeries. Table 1
summarizes the samples cohort used for visible
spectral measurements.
The database in the visible domain totalizes 1701
records. These spectra are those for which data at both
375 and 405 nm are available exactly at the same
position on the same sample.
Table 1: Distribution of the data in the visible domain.
Number of tissue
specimens
Number of
spectrum
CTR
4
685
DIF
5
274
GBM
4
260
MEN
4
310
MET
4
172
Total
21
1701
2.2.2 DUV Range
The DUV measurements setup uses 275 nm as
excitation wavelength. Using this wavelength, we
were able to excite the following fluorophores:
Tyrosine (TYR), Tryptophan (TRY) collagen
crosslinks (COL) and NADH.
In each 10µm slice of each sample, a rectangular area
was chosen. This area was pixelated and spectral
acquisition were performed on each pixel.
Similar to the visible measurements, each spectrum
acquired in the DUV range was fitted by a sum of 4
Gaussians functions, one for each fluorophore, and
the integral under the curve and the maximum are
recorded for each Gaussian.
The samples cohort used in DUV measurements
includes five pathologies and one control group. The
pathologies represented in that group are: High grade
glioma (HGG), Low grade glioma (LGG),
Meningioma grade 2 (GII), Meningioma grade 1 (GI)
and Metastasis (MET) for a total of 38 patients. In
most cases, two slices were collected from each
samples, leading to a total of 67 tissue slices. The
complete DUV database include 129711 records.
Table 2 summarizes the samples cohort used for DUV
spectral measurements.
Table 2: Distribution of the data in the DUV domain.
-
Number of
patients
Number of
tissue
specimens
Number of
spectrum
CTR
6
10
21997
LGG
6
6
32051
HGG
8
15
21807
GI
6
12
19872
GII
6
12
12784
MET
6
12
21200
Total
38
67
129711
2.3 Classification Method
The software was developed in Python. The Scikit-
learn library was used for pre-processing, feature
analysis and ML algorithm.
In a first step, the features were analysed by a pair-
to-plot method in order to roughly evaluate their
discriminating power and to highlight the obvious
correlation between them.
A multivariate analysis was then performed using
a non-parametric supervised ML approach commonly
used for classification problems and based on
Decision Trees (DT). DT are an important type of
algorithm for predictive modelling ML covering both
classification and regression topics. The goal of a DT
is to create a model that predicts the value of a target
variable by learning simple decision rules inferred
from the data features (Gordon, 1984). As the name
suggests, it uses a tree-like model of decisions and
can be used to visually and explicitly represent them.
The structure of the DT is illustrated in figure 2. It is
drawn upside down with its root at the top. The input
subset is successively split into two branches (edges)
according to the condition present in each internal
node. The condition is a threshold on one of the
feature describing the samples. The end of the branch
that does not split anymore is the final decision (leaf)
for that branch. Tuning the model consists in getting
the most homogeneous branches as possible, in other
words branches having groups from similar classes.
The performance of the model is then evaluated on
unknown samples.
The classical DT algorithms have been around for
decades and modern variations like Random Forests
(RF) or Gradient Boosted Decision Trees (DT) are
currently among the most powerful techniques
available.
Classification of Brain Tumour Tissues in Human Patients using Machine Learning
55
Figure 2: Decision Tree structure.
In RF algorithm, several DT are built in order to
decrease the variance thus yielding an overall better
model. The DT are built independently from a
random subset of the input samples and/or from a
random subset of the feature. The final classification
is obtained by averaging the probabilistic prediction
of all the DT (Breiman, 2001).
The GBDT algorithm is an iterative method
(Wolpert, 1992). The DT are built successively by
minimizing a differentiable loss function and a weight
is assigned to each DT for the final classification.
In Random Forest (RF) algorithm, several DT are
built in order to decrease the variance thus yielding an
overall better model. The DT are built independently
from a random subset of the input samples and/or
from a random subset of the feature. The final
classification is obtained by averaging the
probabilistic prediction of all the DT (Breiman,
2001). The Gradient Boosted Decision Trees (GBDT)
algorithm is an iterative method (Wolpert, 1992). The
DT are built successively by minimizing a
differentiable loss function and a weight is assigned
to each DT for the final classification.
3 RESULTS
3.1 Visible Range
Figure 3 shows a typical histogram and a pair-to-pair
plot (log scale) resulting from the preliminary
analysis. Figure 3.a suggests that the illustrated
feature (P2 here) can help discriminate the
pathologies. Figure 3.b clearly highlights that the
integral and the maximum of intensity are highly
correlated. That correlation was observed for each
fluorophore and each wavelength. We therefore only
kept the integral for the analysis.
The features that were taken into account in the
visible domain are the integral under the curve value
for each fluorophore at both 375 and 405 nm.
Previous studies proved that some ratio could also be
a powerful discriminatory feature (Poulon, 2018)
(Poulon, 2018) (Mehidine, 2021). We therefore
included four more parameters, namely the ratio
between integral of NADH-F and NADH-B and the
ratio between integral of P1 and P2 for both
wavelength, leading to a total number of 16 features
for each sample. The model was trained with 1360
samples (80% of the database) and evaluated on the
remaining 341samples (20%). DT achieves a 90%
score, RF 92% and GBDT 95% (Table 3).
We studied the importance of each feature for the
classification. The feature the most useful to build the
GBDT model is the ratio P1/P2 at 375nm. The next
one is integral of NADH-F at 375 nm. Though these
two features are the most useful, training the model
with only one of them or both of them leads to very
poor results (Table 3).
Table 3: Classification on the test database in the visible
domain for 1, 2 and 16 features using Decision Tree,
Random Forest and Gradient Boosting Decision Tree.
P1/P2
NADF_F
& P1/P2
16 features
DT
47%
69%
90%
RF
50%
73%
92%
GBDT
50%
71%
95%
Figure 3: P2 at 375 nm ; histogram of integral under the
curve (a) ; pair-to-pair plot for maximum if intensity versus
integral in a log-scale (b).
PHOTOPTICS 2022 - 10th International Conference on Photonics, Optics and Laser Technology
56

3.2 DUV Range
In the DUV, 8 features were included for each sample
into the model: the integral under the curve of each
of the 4 fluorophores and 4 ratio: TYR/NADH,
COL/NADH, TRY/COL, TYR/TRY. The model was
trained with 90797 samples (80% of the database) and
evaluated on the remaining 38914 samples (20%).
The 3 models achieve very similar score: 87% for
DT, 89% for RF and 88% for GBDT (Table 4).
We also studied the importance of each feature for
the classification. The features the more useful to
build the models are integral of collagene (COL) and
of tryptophane (TRY). Here also, training the model
with only those parameters leads to poor results.
Table 4: Classification on the test database in the DUV for
2 and 16 features using Decision Tree, Random Forest and
Gradient Boosting Decision Tree.
COL & TRY
16 features
DT
49%
87%
RF
42%
89%
GBDT
51%
88%
4 DISCUSSION AND
CONCLUSION
For the first time, we used ML methods on
spectroscopic data from brain tissue samples in order
to discriminate tumoral from non tumoral tissues
using quantitative optical parameters. This
preliminary study suggests that combining several
features into a ML model significantly improve the
classification.
We could not combine DUV and visible data in
the same model because we did not have the exact
position of the spectral records and we could not
establish the correspondence between two samples.
Such combination will be upgraded in the next
database. As the most discriminant features in DUV
and visible don’t come from the same fluorophores,
an improved result can be expected because it can be
assumed that useful information is complementary.
Indeed, though the input number of samples given
to the ML model is very high, they come from a
limited number of histological specimens and it is
necessary to confirm these results on more
specimens.
In this study, we only take into account spectral
data. Work is in progress to take advantage of other
available information such as fluorescence lifetime
and fluorescence and SHG optical images.
ACKNOWLEDGEMENTS
This work is financially supported by ITMO Cancer
AVIESAN (Alliance Nationale pour les Sciences de la Vie
et de la Santé, National Alliance for Life Sciences &
Health) within the framework of the Cancer Plan for
MEVO & IMOP projects, by CNRS with “Dfi
instrumental” grant, by ligue nationale contre le cancer
(LNCC) and the Institut National de Physique Nuclaire et
de Physique des Particules (IN2P3).
We would like to thank Synchrotron SOLEIL for the
accorded beam-time and for all staff members of DISCO
beamline for their help as well their contribution in the
scientific discussion. We would like also to thank the
Delegation for Clinical Research and Innovation (DRCI)
and the Biological Resources Center (CRB) of Sainte-Anne
hospital center for providing the samples.
We would to thank also the neurosurgeons at Sainte-
Anne hospital (M Zanello, E Dezamis, C Benevello, G Zah-
Bi, A Roux) for providing the surgical specimens.
REFERENCES
C. Buckner, et al., (2007) ‘Central Nervous System
Tumors’, Mayo Clin. Proc., vol. 82, no. 10, pp. 1271–
1286.
D. N. Louis et al., (2016), ‘The 2016 World Health
Organization Classification of Tumors of the Central
Nervous System: a summary’, Acta Neuropathol.
(Berl.), vol. 131, no. 6, pp. 803–820.
T. Wilson, M. Karajannis, and D. Harter, (2014),
‘Glioblastoma multiforme: State of the art and future
therapeutics’, Surg. Neurol. Int., vol. 5, no. 1, p. 64.
P. L. Kubben, et al., (2011), ‘Intraoperative MRI-guided
resection of glioblastoma multiforme: a systematic
review’, Lancet Oncol., vol. 12, no. 11, pp. 1062–1070,
G. Unsgaard et al., (2006), ‘Intra-operative 3D ultrasound
in neurosurgery’, Acta Neurochir. (Wien), vol. 148, no.
3, pp. 235–253
A. Ibrahim, et al., ‘Characterization of fiber ultrashort pulse
delivery for nonlinear endomicroscopy’, (2016), Opt.
Express, vol. 24, no. 12, p. 12515.
M. Sibai et al., (2018), ‘The Impact of Compressed
Femtosecond Laser Pulse Durations on Neuronal
Tissue Used for Two-Photon Excitation Through an
Endoscope’, Sci. Rep., vol. 8, no. 1, p. 11124.
F. Poulon et al., (2018), ‘Multimodal Analysis of Central
Nervous System Tumor Tissue Endogenous
Fluorescence With Multiscale Excitation’, Front.
Phys., vol. 6.
F. Poulon et al., (2018), ‘Real-time Brain Tumor imaging
with endogenous fluorophores: a diagnosis proof-of-
concept study on fresh human samples’, Sci. Rep., vol.
8, no. 1, p. 14888.
H. Mehidine et al., (2019), ‘Optical Signatures Derived
From Deep UV to NIR Excitation Discriminates
Healthy Samples From Low and High Grades Glioma’,
Sci. Rep., vol. 9, no. 1, p. 8786.
Classification of Brain Tumour Tissues in Human Patients using Machine Learning
57

H. Mehidine et al., (2021), ‘Molecular changes tracking
through multiscale fluorescence microscopy
differentiate Meningioma grades and non-tumoral brain
tissues’, Sci. Rep., vol. 11, no. 1, p. 3816.
F. Poulon et al., (2017), ‘Optical properties, spectral, and
lifetime measurements of central nervous system
tumors in humans’, Sci. Rep., vol. 7, no. 1.
M. Zanello et al., (2017), ‘Multimodal optical analysis of
meningioma and comparison with histopathology’, J.
Biophotonics, vol. 10, no. 2, pp. 253–263.
H. Mehidine et al., (2018), ‘Multimodal imaging to explore
endogenous fluorescence of fresh and fixed human
healthy and tumor brain tissues’, J. Biophotonics, p.
e201800178.
A. D. Gordon, et al., (1984), ‘Classification and Regression
Trees.’ Biometrics, vol. 40, no. 3, p. 874.
L. Breiman, (2001), Mach. Learn., vol. 45, no. 1, pp. 5–32.
D. H. Wolpert, (1992), ‘Stacked generalization’, Neural
Netw., vol. 5, no. 2, pp. 241–259.
PHOTOPTICS 2022 - 10th International Conference on Photonics, Optics and Laser Technology
58
