
Solid-state Photoluminescent Quantum Dots for Explosive Detection
Federica Mitri
1a
, Andrea De Iacovo
1b
, Serena De Santis
1c
, Carlo Giansante
2d
,
Davide Spirito
3e
, Giovanni Sotgiu
1f
and Lorenzo Colace
1g
1
Department of Engineering, University Roma Tre, Via Vito Volterra 62, 00146, Rome, Italy
2
CNR Nanotec, Istituto di Nanotecnologia, Via Monteroni, Lecce 73100, Italy
3
IHP - Leibniz Institut für innovative Mikroelektronik, Im Technologiepark 25, 15236 Frankfurt (Oder), Germany
carlo.giansante@nanotec.cnr.it, spirito@ihp-microelectronics.com
Keywords: Colloidal Quantum Dots, Photoluminescent Probe, Vapor Explosive Detection.
Abstract: Quantum dots are an emerging class of photoluminescent nanomaterials with peculiar properties arising from
their nanometric size that allows the occurrence of strong quantum confinement effect. In recent years, these
zero-dimensional semiconductor nanoparticles have been attracting increasing attention as luminescent probe
for optical sensing applications. However, to date, almost all quantum dots- based sensors reported in
literature exploit fluorescence from solutions whereas the development of solid-state photoluminescent
quantum dots still remains a challenge. In this paper, we demonstrate the feasibility of exploiting the solid-
state photoluminescence of PbS quantum dots deposited on a silicon substrate for vapor explosive detection,
a worrying priority for homeland security and counter-terrorism applications.
1 INTRODUCTION
Explosive-based terrorism is an ongoing challenge to
governments and societies worldwide due to the
relative ease by which these weapons can be
constructed and deployed (“Trace Chem. Sens.
Explos.,” 2006). Organizations such as JIDO (US
Department of Defense Joint Improvised-Threat
Defeat Organization), AOAV (Action on Armed
Violence), and CPOST (Chicago Project on Security
& Threats) all collect detailed and updated statistics
regarding their use and devastating effects
(Hotchkiss, 2018). The alarming data that emerged,
together with the increasing government regulations
for enhanced security screening, is driving the global
explosive trace detection market growth.
Common trace detection systems rely on
spectroscopic approaches and allow for sensitive,
selective, and fast detection. Such high performance,
a
https://orcid.org/0000-0002-3206-1399
b
https://orcid.org/0000-0001-5006-5505
c
https://orcid.org/0000-0001-9772-2891
d
https://orcid.org/0000-0003-4558-5367
e
https://orcid.org/0000-0002-6074-957X
f
https://orcid.org/0000-0003-2841-9316
g
https://orcid.org/0000-0002-7111-3905
however, comes at the cost of expensive and
cumbersome equipment that can often be operated
only by trained personnel. Moreover, the detection
occurs through the analysis of specifically prepared
specimen, while large environments cannot be
efficiently monitored. Recently, fluorescent gas
sensors have been proposed for the realization of
nitroaromatic compounds (NAC) detectors that can
overcome such limitations (Ma et al., 2015). Among
them, sensors based on quantum dots (QD) have been
realized exploiting their peculiar optical and
electronic properties. These are semiconductor
nanoparticles suspended in the solution phase. QD
were first developed and studied as a promising
material for photodetectors since they are easy to
synthesize and their optical properties can be easily
tuned via chemical approaches (Venettacci et al.,
2019). As all nanocrystals, they are particularly
suitable for gas sensing applications thanks to their
large surface-to-volume ratio that allows outstanding
48
Mitri, F., De Iacovo, A., De Santis, S., Giansante, C., Spirito, D., Sotgiu, G. and Colace, L.
Solid-state Photoluminescent Quantum Dots for Explosive Detection.
DOI: 10.5220/0010870300003121
In Proceedings of the 10th International Conference on Photonics, Optics and Laser Technology (PHOTOPTICS 2022), pages 48-52
ISBN: 978-989-758-554-8; ISSN: 2184-4364
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
reactivity even at room temperature (Galstyan, 2021;
Mitri et al., 2020). Recently, we exploited the peculiar
characteristics of PbS QD to demonstrate a
chemiresistive device for NAC detection (Mitri et al.,
2021). Due to their nanometric size, QD show strong
quantum confinement effect thus exhibiting
interesting optical properties such as intense, narrow
and size-tunable luminescence. In addition, the QD’s
surface can be sensitized with a chemical approach,
allowing selective reaction with specific analyte
molecules. Several authors already demonstrated that
amine treated-QD can be effectively employed as
luminescent probes for the selective detection of
NAC, but the proposed devices cannot detect these
compounds in the vapor phase and still need to be
operated with specific lab equipment (Akhgari et al.,
2015). To date, almost all reported QD-based devices
have involved fluorescence from solutions (Xu et al.,
2020).
Herein, we demonstrate the proof-of-concept on
employing the solid-state photoluminescence (PL)
quenching of PbS QD deposited on a silicon
substrate, as a fluorescent sensing platform for direct
detection of nitrobenzene vapor (NB, as a
representative NAC). We also demonstrate that the
evaluation of the PL quenching can be easily obtained
with low-cost and low-power electronics mounted in
a compact optical chamber. The proposed device
operates in air, at room temperature, and can detect
NB with a concentration as low as 445 ppb.
2 DEVICE FABRICATION
2.1 QD Synthesis
PbS QD were synthesized in a three-neck flask
connected to a standard Schlenk line under oxygen-
and water-free conditions. PbO (450 mg), oleic acid
(9.0 g), and 1-octadacene (3.0 g) were mixed at 80°C.
The mixture was then heated at 100°C until it became
completely transparent. The temperature was raised
to 110°C and 210µL of bis(trimethyl)sulfide diluted
in 2mL of 1-octadacene were rapidly injected in the
solution. Heating was immediately stopped, and the
solution cooled down to room temperature. The
resulting QD were precipitated, purified, and
dispersed in toluene with a 0.5 mM concentration.
The size of the QD (4.7 nm) was determined through
optical absorption measurements.
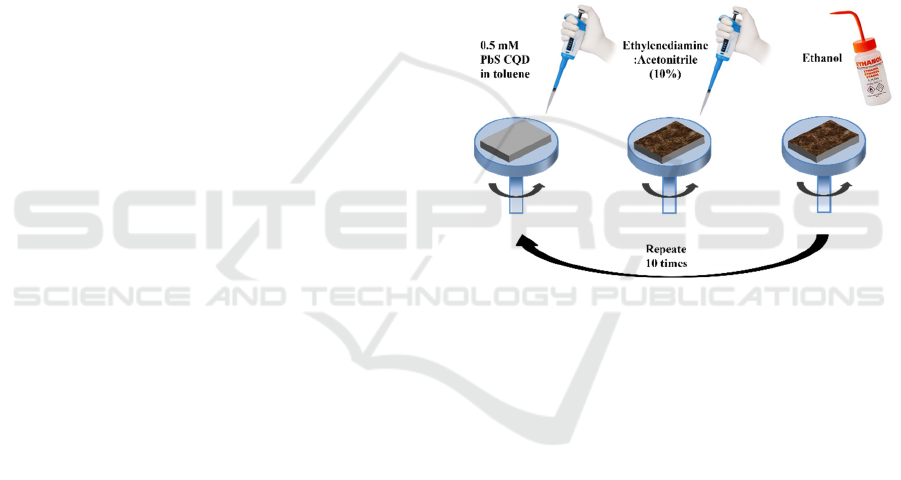
2.2 QD Deposition
Thermally oxidized silicon wafers were cut to a
typical size of 10×10mm and were cleaned in a
NH
4
OH:H
2
O
2
(1:1) solution. After being cleaned, the
substrates were rinsed with deionized water and dried
with dry air before they were used. Devices were
fabricated with a layer-by-layer spin-coating
deposition process. Specifically, a drop of the QD
solution was deposited onto the substrate and spun at
3000 rpm for 30 s. Then, a drop of ethylenediamine
(EDA) solution in acetonitrile (10% vol.) was
deposited on the substrate and left reacting for 30 s,
before spinning at 3000 rpm for 30 s. Finally, the
substrate was washed with pure ethanol. The previous
steps were repeated for 10 times. Figure 1
schematically shows the fabrication process.
Figure 1: Schematic representation of the device fabrication
process.
The resulting QD film was 200 nm thick. The
deposited film was analysed with a scanning electron
microscope (SEM). Figure 2 shows a representative
SEM micrograph. The film appears uniform in
thickness, with several cracks and voids. Such
morphology may increase the total surface of the QD
film, thus allowing for a more efficient interaction
with the NB gas. We characterized the as-deposited
film in terms of PL spectrum. The PL was measured
exciting the film with a 532 nm laser source and
analysing its emission spectrum by means of an IR
spectrometer (Horiba iHR320) equipped with an
extended InGaAs detector and a 600 lines/mm
grating. Several PL spectra were acquired varying the
temperature of the film between -10/+50°C and the
laser power between 0.2/3mW. The resulting spectra
did not show significant variations in terms of central
wavelength, while the PL intensity was proportional
to the pump power over the whole measurement
range. Figure 3 shows a typical PL spectrum of the
QD as deposited on the silicon substrate.
Solid-state Photoluminescent Quantum Dots for Explosive Detection
49
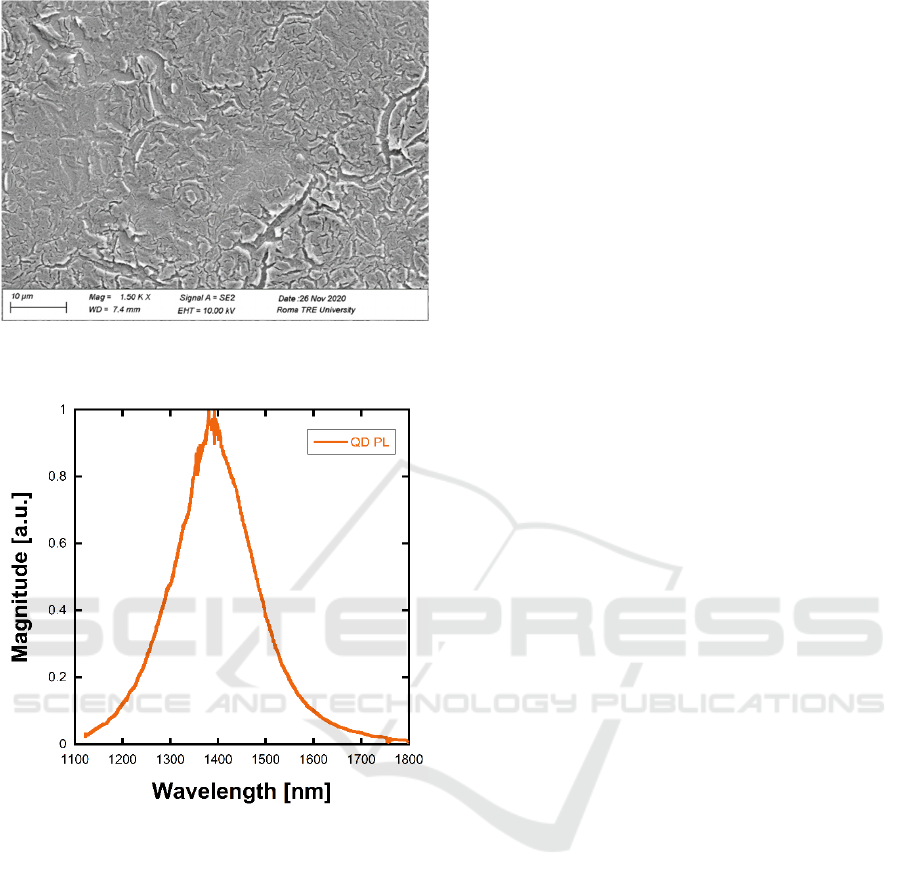
Figure 2: SEM micrograph of the QD film onto the silicon
substrate.
Figure 3: Solid-state QD PL spectrum.
3 SET-UP AND MEASUREMENT
CHAMBER
The device response to NB gas was characterized
with a custom measurement set-up. The device was
kept in a closed 3D-printed measurement chamber,
with in-let and out-let connectors. Ambient air was
fluxed through the container. A closed glass container
was filled with a small amount of NB and left settling
for 1 hour to allow NB gas to reach the equilibrium
vapor concentration. A couple of manual valves
allowed the ambient air to be fluxed through the NB-
filled container before entering the measurement
chamber at a fixed flowrate of 800 mL/min. NB
concentration was varied by heating the glass
container with a water bath. The 10 mL-volume
measurement chamber contained the QD-coated
silicon substrate, a germanium photodiode (PD) and
a blue LED. The device was illuminated through the
blue LED modulated by means of a 520 Hz, 0.5 Vrms
sine oscillator whose mean value could be modulated
through a dc offset. The Ge PD was placed directly
below the device in order to detect the QD’s
fluorescence intensity by generating a proportional
photocurrent then converted to voltage through a 10
kV/A transimpedance amplifier (Thorlabs,
AMP120). Figure 4(a) shows the device’s operating
scheme. The corresponding output was demodulated
by a lock-in amplifier and data were transferred to a
personal computer. As shown in Figure 4(b), the blue
LED (emission peak at λ=465 nm) emits within the
silicon substrate’s low-transmittivity band whereas
QD’s PL (NIR PL peak at λ=1400 nm) falls within
the high-transmittivity band and the Ge photodetector
spectral response. In this way, since the Ge PD is
completely covered by the silicon substrate, just the
NIR radiation reaches the PD that, in turn, generates
a photocurrent proportional to the PL intensity.
Temperature and relative humidity (RH) were
monitored during the measurements and kept
constant at 20°C, 30% RH through room-level air
conditioning.
4 DEVICE
CHARACTERIZATION
Figure 5 shows a typical signal measured during a
complete 1.9 ppm NB gas release and purge cycle.
The device showed complete baseline recovery after
NB gas was purged from the measurement chamber.
The maximum sensor response was obtained after 10
minutes since gas release. The amplitude of the
photovoltage (PV) varied by 9.7% after 10 minutes
since gas release. The phase angle showed the same
behaviour of the amplitude with a decrease during NB
exposure.
The measurements were repeated for different NB
concentrations ranging from 445 ppb to 15.9 ppm.
Figure 6 shows the corresponding real-time
normalized PV amplitude change upon these
increasing concentrations. A nonlinear behaviour is
clearly observed, as the sensor response reaches a
saturation plateau for NB concentrations higher than
5 ppm.
PHOTOPTICS 2022 - 10th International Conference on Photonics, Optics and Laser Technology
50
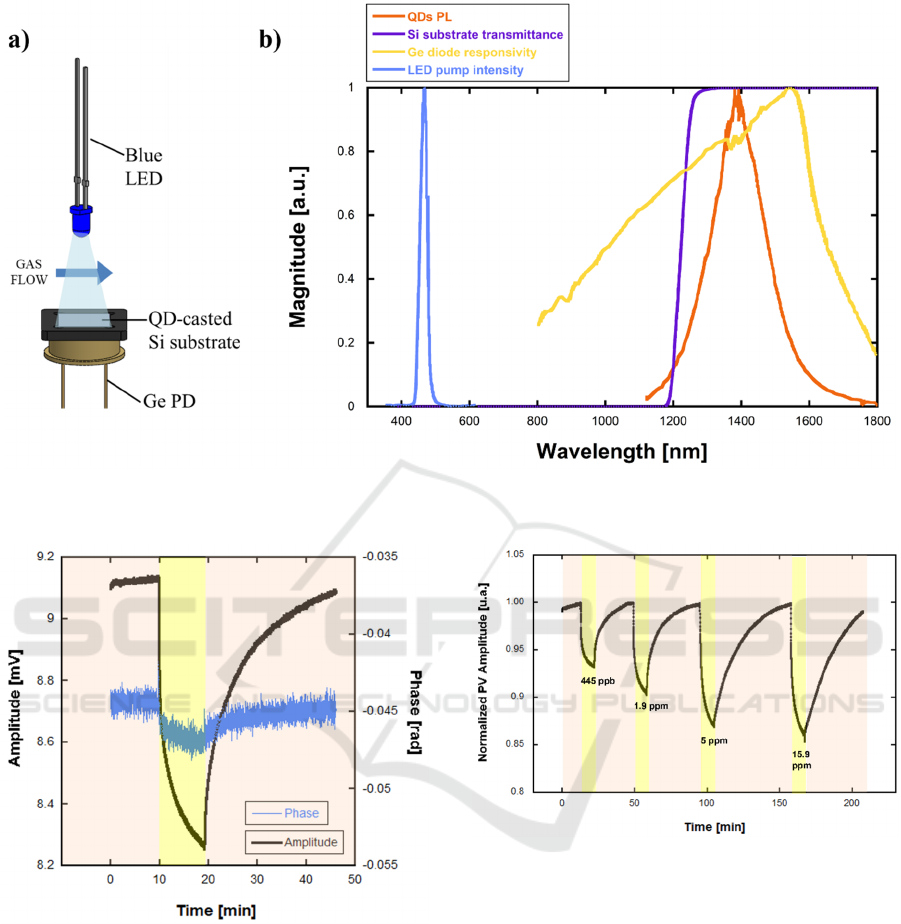
Figure 4: a) Schematic of the optical arrangement; b) QD-film PL spectrum, Si substrate’s transmission, Ge diode’s
responsivity, and LED pump emission spectrum. All curves are normalized to their maximum.
Figure 5: Amplitude and phase angle of the photovoltage
upon sensor exposure to 1.9ppm of NB.
To investigate whether the system sensitivity may
depend on PL emission intensity, a study varying the
mean intensity of LED was performed, as reported in
Figure 7. The PL quenching showed a weak
dependence on the LED pump intensity. An optimal
value of 5.14 mW/cm
2
was identified. Environmental
conditions (temperature and RH) were kept stable
during all the measurements; however, given the
aforementioned stability of the PL signal over a wide
temperature range, we expect that the device can be
effectively operated in environments, as well.
Figure 6: PV amplitude upon repeated exposure to
increasing NB concentrations between 445 ppb and 15.9
ppm.
Concerning the sensing mechanism, amine-
treated QD have been already employed for sensing
NAC, assuming an electronic interaction between the
negatively charged amino-groups and the electron-
poor benzenic ring of the NB molecule, leading to the
formation of Meisenheimer complexes, as shown in
Figure 8 (Tian et al., 2017). Thus, the amine to NB
charge-transfer, with the resonating negative charge
stabilized by the withdrawing nitro group (-NO
2
),
could be responsible for the significant QD PL
quenching observed in the presence of the target gas.
Solid-state Photoluminescent Quantum Dots for Explosive Detection
51
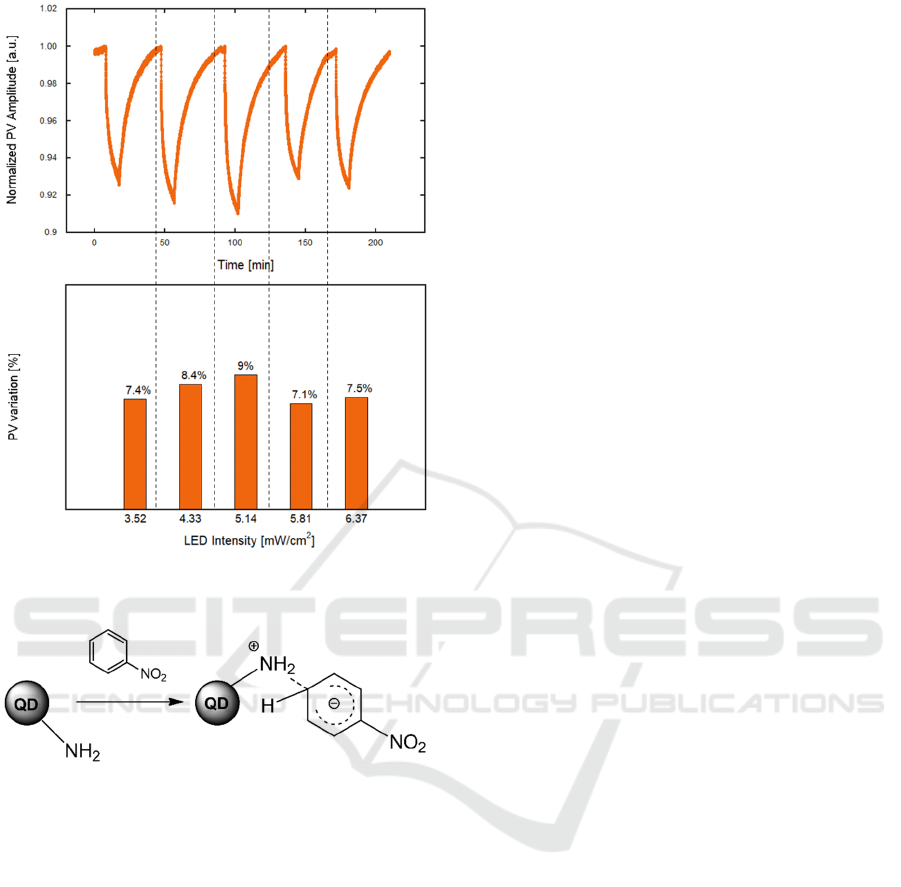
Figure 7: PV amplitude towards NB (1.9 ppm) varying LED
intensity.
Figure 8: Schematic of the Meisenheimer-like amine–NB
complex.
5 CONCLUSIONS
In this paper we demonstrated the feasibility of
exploiting solid-state PL PbS QD as luminescent
device for explosive vapor detection. The device
showed good sensitivity and could detect NB vapor at
room temperature in ambient air. The lowest
measured NB concentration was 445 ppb,
corresponding to a sensor response S = 4.4% after 60
seconds exposure (S = 6.8% after 10 minutes). The
integration of QD luminescent probes into an
appropriate solid support, a silicon chip, is an
important step towards further device optimization
into a portable, miniaturized, and low-cost device for
the detection of explosives in strategic and sensitive
environments.
REFERENCES
Akhgari, F., Fattahi, H., & Oskoei, Y. M. (2015). Recent
advances in nanomaterial-based sensors for detection of
trace nitroaromatic explosives. In Sensors and
Actuators, B: Chemical (Vol. 221). Elsevier B.V.
https://doi.org/10.1016/j.snb.2015.06.146
Galstyan, V. (2021). “Quantum dots: Perspectives in next-
generation chemical gas sensors” ‒ A review. Analytica
Chimica Acta, 1152, 238192. https://doi.org/10.1016/
j.aca.2020.12.067
Hotchkiss, P. J. (2018). Explosive Threats: The Challenges
they Present and Approaches to Countering Them.
Handbook of Security Science, 2016(Dathan 2017), 1–
23. https://doi.org/10.1007/978-3-319-51761-2_19-1
Ma, Y., Wang, S., & Wang, L. (2015). Nanomaterials for
luminescence detection of nitroaromatic explosives.
TrAC - Trends in Analytical Chemistry, 65, 13–21.
https://doi.org/10.1016/j.trac.2014.09.007
Mitri, F., De Iacovo, A., De Luca, M., Pecora, A., & Colace,
L. (2020). Lead sulphide colloidal quantum dots for
room temperature NO2 gas sensors. Scientific Reports,
10(1), 1–9. https://doi.org/10.1038/s41598-020-69478-
x
Mitri, F., De Iacovo, A., De Santis, S., Giansante, C.,
Sotgiu, G., & Colace, L. (2021). Chemiresistive Device
for the Detection of Nitroaromatic Explosives Based on
Colloidal PbS Quantum Dots. ACS Applied Electronic
Materials, 3(7), 3234–3239. https://doi.org/10.1021/
acsaelm.1c00401
Tian, X., Peng, H., Li, Y., Yang, C., Zhou, Z., & Wang, Y.
(2017). Highly sensitive and selective paper sensor
based on carbon quantum dots for visual detection of
TNT residues in groundwater. Sensors and Actuators,
B: Chemical, 243, 1002–1009. https://doi.org/10.1016/
j.snb.2016.12.079
Trace Chemical Sensing of Explosives. (2006). In Trace
Chemical Sensing of Explosives. https://doi.org/10.10
02/0470085207
Venettacci, C., Martin-Garcia, B., Prato, M., Moreels, I., &
De Iacovo, A. (2019). Increasing responsivity and air
stability of PbS colloidal quantum dot photoconductors
with iodine surface ligands. Nanotechnology, 30(40).
https://doi.org/10.1088/1361-6528/ab2f4b
Xu, A., Wang, G., Li, Y., Dong, H., Yang, S., He, P., &
Ding, G. (2020). Carbon-Based Quantum Dots with
Solid-State Photoluminescent: Mechanism,
Implementation, and Application. Small, 16(48), 1–31.
https://doi.org/10.1002/smll.202004621
PHOTOPTICS 2022 - 10th International Conference on Photonics, Optics and Laser Technology
52
