
A Natural Interaction Paradigm to Facilitate Cardiac Anatomy
Education using Augmented Reality and a Surgical Metaphor
Dmitry Resnyansky
1 a
, Nurullah Okumus
2 b
, Mark Billinghurst
1 c
, Emin Ibili
3 d
,
Tolga Ertekin
4 e
, Düriye Öztürk
5 f
and Taha Erdogan
5 g
1
School of Information Technology and Mathematical Sciences, University of South Australia, Mawson Lakes, Australia
2
Department of Child Health and Diseases, Afyonkarahisar Health Sciences University, Afyonkarahisar, Turkey
3
Department of Healthcare Management, Afyonkarahisar Health Sciences University, Afyonkarahisar, Turkey
4
Department of Anatomy, Afyonkarahisar Health Sciences University, Afyonkarahisar, Turkey
5
Department of Radiation Oncology, Afyonkarahisar Health Sciences University, Afyonkarahisar, Turkey
emin.ibili@afsu.edu.tr, tolga.ertekin@afsu.edu.tr, duriye.ozturk@afsu.edu.tr, taha.erdogan@afsu.edu.tr
Keywords: Augmented Reality, Visualisation, Cardiac Anatomy Learning, Interactivity, HMD.
Abstract: This paper presents a design approach to creating a learning experience for cardiac anatomy by providing an
interactive visualisation environment that uses Head Mounted Display (HMD)-based Augmented Reality
(AR). Computed tomography imaging techniques were used to obtain accurate model geometry that was
optimised in a 3D modelling software package, followed by photo-realistic texture mapping using 3D painting
software. This method simplifies the process of modelling complex, organic geometry. Animation, rendering
techniques, and AR capability were added using the Unity game engine. The system’s design and
development maximises immersion, supports natural gesture interaction within a real-world learning setting,
and represents complex learning content. Hand input was used with a surgical-dissection metaphor to show
cross-section rendering in AR in an intuitive manner. Lessons learned from the modelling process are
discussed as well as directions for future research.
1 INTRODUCTION
Understanding the anatomical composition of the
heart is a difficult task for medical students due to the
organ’s complex arrangement of individual elements
and multi-chambered structures (Maresky et al.,
2019). Traditional anatomy learning methods are
based on 2D images, slides, and plastic models, and
can be less effective due to lack of interactivity, and
difficulties with understanding 3D structure from 2D
materials (Kurniawan et al., 2018; Rosni et al. 2020).
Augmented Reality (AR) and Virtual Reality (VR)
can enhance anatomy learning by addressing these
a
https://orcid.org/0000-0003-3897-7864
b
https://orcid.org/0000-0001-6082-0818
c
https://orcid.org/0000-0003-4172-6759
d
https://orcid.org/0000-0002-6186-3710
e
https://orcid.org/0000-0003-1756-4366
f
https://orcid.org/0000-0002-3265-2797
g
https://orcid.org/0000-0002-3559-8933
problems and creating a more interesting, active and
flexible learning experience (Bork et al., 2019).
This paper is part of a wider educational project
that aims to support effective knowledge transfer of
cardiac anatomy and physiology for medical students
through innovative technologies. A Head-Mounted
Display (HMD)-based interactive Augmented Reality
Learning Environment (ARLE) prototype is currently
being developed with the aim of communicating the
multitude of the heart’s inner and outer structure, as
well as basic aspects of its circulatory system. This
has been designed with the focus of creating a
realistic interactive augmented reference medium for
204
Resnyansky, D., Okumus, N., Billinghurst, M., Ibili, E., Ertekin, T., Öztürk, D. and Erdogan, T.
A Natural Interaction Paradigm to Facilitate Cardiac Anatomy Education using Augmented Reality and a Surgical Metaphor.
DOI: 10.5220/0010835400003124
In Proceedings of the 17th International Joint Conference on Computer Vision, Imaging and Computer Graphics Theory and Applications (VISIGRAPP 2022) - Volume 1: GRAPP, pages
204-211
ISBN: 978-989-758-555-5; ISSN: 2184-4321
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

students to compare to textbooks, physical models,
dissections, and surgical observation sessions.
The intended design goals of the prototype ARLE
are: (1) improving the production pipeline of
complex, organic models with Computed
Tomography- (CT) extracted 3D scan data; (2)
increasing learner immersion when interacting with
visualisations by facilitating user stereopsis with the
aid of a HMD; (3) supporting natural interaction with
AR visualisations without the need for marker-based
tracking, mobile devices, or physical peripherals; and
(4) representing information in a realistic and
engaging manner with the aid of graphic and
animated content.
This paper focuses on the design of the prototype
system and highlights the importance of adopting a
multi-layered approach to the creation of visual
learning content, and to supporting natural interaction
(Piumsomboon et al., 2014). This work deals with a
limited subset of the content necessary for the
teaching of the anatomical structure of the human
heart: in this case, the primary heart aorta, aortic
valve, and aortic circulation system.
The rest of the paper is organised as follows:
In section 2, the relevant literature is outlined.
Section 3 describes our prototype system and its
associated interaction metaphors and a brief rationale
for the design decisions made. Section 4 describes an
approach to development of visualisations deriving
from medical imaging data obtained from CT scans,
including 3D asset creation and preparation inside a
game engine for adding functionality. Finally, section
5 concludes the paper by reiterating the aim,
formulating insights gained, and future work.
2 RELATED WORK
The main advantages of using AR or VR in anatomy
learning is that these technologies can help students
with lower visual-spatial abilities (Bogomolova et al.,
2021) and enable more active, exploratory, and
embodied learning, which improves learning
efficiency and memory retention (Cakmak et al.,
2020).
There is significant research on virtual learning
for anatomy students using different delivery
modalities, such as highly immersive (stereoscopic;
3D VR) vs. less immersive (desktop). For instance,
Kurul et al. (2020) suggest that a 3D immersive VR
system can provide a useful alternative to
conventional anatomy training methods. In Wainman
et al. (2020), VR and AR representations of pelvic
anatomy were tested in comparison to photographs
and physical models. They found that VR and AR had
fewer advantages for learning than physical models,
and that “true stereopsis is critical in learning
anatomy” (p. 401). In a pilot study (Birbara et al.,
2020), a skull anatomy virtual learning resource was
developed using Unity, and was made available in
different delivery modalities (more immersive and
less immersive). The stereoscopic delivery was found
to be more mentally demanding and a greater degree
of physical discomfort and disorientation was
reported by some participants. Moro et al. (2021)
compared the effectiveness of delivering human
physiology content in AR through the HoloLens
HMD and in non-AR on a tablet. The study did not
find any significant differences in test scores, and
provided evidence that both modes could be effective
for learning, although dizziness when using the
HoloLens was reported by some participants.
However, other studies suggest that anatomical
learning could benefit from the various affordances of
AR/VR such as immersive interactivity and
visualisation (Samosky et al., 2012; Venkatesan et al.,
2021). For example, Gloy et al. (2021) provide an
example of this in their development of an immersive
anatomy atlas that enables virtual dissection as a way
to interact with human anatomical structures. The
application (using a HMD and bi-manual controllers)
allows users to explore anatomy by modifying organ
transparency, toggling overall visibility within a
specific radius with an interactive manipulation tool,
or revealing and concealing selected geometry by
offering a cross-section capability. Higher retention
rate was demonstrated by students using the
interactive atlas compared to those using traditional
anatomical textbooks. Bakar et al. (2021) describe
GAAR (Gross Anatomy Augmented Reality) – a
mobile AR learning tool. By interacting with 3D
objects, video, and information while using the tool,
users can experience the perception of operating on a
“real” organ (p. 162).
In a review of mobile AR applications for learning
biology and anatomy (Kalana et al., 2020), four
interactive AR tools have been reviewed: APPLearn
(Ba et al., 2019), to support learning biology in
secondary schools; a Magic Book application for
medical students to learn neuroanatomy (Küçük et al.,
2016); an application that combines 3D modelling
and AR for learning molecular biology (Safadel &
White, 2018); and Human Anatomy in Mobile-
Augmented Reality (HuMAR) (Jamali et al., 2015)
which focuses on 3D visualisation of selected bones.
Kalana et al. (2020) emphasise AR’s potential for
visualisation of complex information and enabling
active learning. They also review commercial AR
A Natural Interaction Paradigm to Facilitate Cardiac Anatomy Education using Augmented Reality and a Surgical Metaphor
205

applications for learning anatomy, highlighting
features that enable interaction between the user and
the 3D models, such as using the “Pinch In-Pinch
Out” gesture, and controllers for moving 3D models
in four directions, rotating and scaling, or “peeling
off” the layers of an organ (pp. 582-583).
Romli and Wazir (2021) assess and compare six
applications: Web based AR for Human Body
Anatomy Learning (Layona et al., 2018); Human
Anatomy Learning Systems Using AR on Mobile
Application (Kurniawan et al., 2018); AR for the
Study of Human Heart Anatomy (Kiourexidou et al.,
2015); an AR application for smartphones and tablets
for cardiac physiology learning (Gonzalez et al.,
2020); a human heart teaching tool (Nuanmeesri,
2018); and a mobile AR application for teaching heart
anatomy (Celik et al., 2020). Romli and Wazir (2021)
maintain that marker-based, mobile AR systems
enable users to scan physical images in the real world,
and display 3D visualisations that may be acted upon
with the mobile device.
To summarise, a major advantage of immersive
HMD-based technologies such as AR/VR to deliver
anatomy-related learning content is that it enables
unique interaction modalities innate to the hardware.
Natural interaction is afforded more readily in the
form of simulated dissection and transparency
techniques. However, less immersive approaches
(specifically, mobile AR with touch-based GUIs) that
afford less natural interaction relative to a HMD tend
to be more widely used for teaching anatomy. One of
the problems that need to be addressed is creation of
the anatomy and the visualisation of cardiac blood
circulation. For developers, simulation of dynamic
fluids for representing physiological processes
involving human circulation is one specific challenge
(Rosni et al., 2020). Such dynamic visualisation
requires the physics processing capabilities of a game
engine in order to avoid expensive processing of pre-
cached particle animations. The difficulty is
compounded by the subsequent limitations of
common game engines in rendering liquids in real
time at an adequate level of realism.
3 SYSTEM DESCRIPTION
The prototype ARLE affords interaction in three
dimensions for engaging with 3D visualisations.
Compared to a 2D interaction approach, which uses
contact-based touch and swipe gestures to interact
with virtual objects, 3D interaction offers a method of
natural interaction using depth-tracking hand gestures
in 3D space (Mandalika et al., 2017).
The main features of the ARLE are: (1)
markerless AR interaction supported by a HMD to
provide six degrees of freedom (6DOF) of movement
and vision for users in and around the anatomical
visualisation; (2) hand-based manipulation of 3D
anatomy models, allowing rotation and scaling of the
cardiac system; (3) hand-based interaction that uses a
cross-section tool in 3D space to dissect anatomy.
A HMD can readily provide stereopsis capability
to users, enabling immersive and engaging
representations of visual information by displaying
anatomy models in actual 3D space. In addition, the
depth perception affordances of the HoloLens2
encourage natural interaction using the hands as well
the entire body by facilitating a “walkthrough”
experience where the user can walk around the AR
view (Billinghurst & Henrysson, 2009). This allows
learners the freedom to study the organic structure of
complex organs through a “hands-on, feet-first”
approach by exploring a heart model in physical
space with their own hands as well as on foot.
The prototype ARLE uses interactive scale
manipulation to represent visual information in a
broader context. This contrasts with mobile AR
techniques where zoom controls (e.g. sliders, finger
swiping) or (physical) device proximity allows users
to observe detailed information for a specific object,
but seeing “the bigger picture” becomes a challenge.
Restricted magnification of an individual object is
thus prioritised over the ability to view and
understand its global relationship to its neighbours.
Object rotation can be achieved through a similar 3D
interaction approach. Users can expand and contract
the virtual representation by manipulating anchor
points on a bounding box that surrounds the heart
model. A surgical dissection tool metaphor is
included in the natural interaction paradigm by
enabling the user to “dissect” models in 3D space by
way of a hand-manipulated virtual tool.
4 DESIGN: APPROACH AND
OBSERVATIONS
4.1 Equipment and Interaction
The application uses a Microsoft HoloLens2 see-
through AR HMD that provides stereo viewing of
virtual content, inside-out head tracking, and support
for natural two-handed gesture input. The HMD
enables viewing of virtual anatomy on top of the
user’s physical environment. A 3D model of the
primary heart aorta is chosen as the basis of the
GRAPP 2022 - 17th International Conference on Computer Graphics Theory and Applications
206
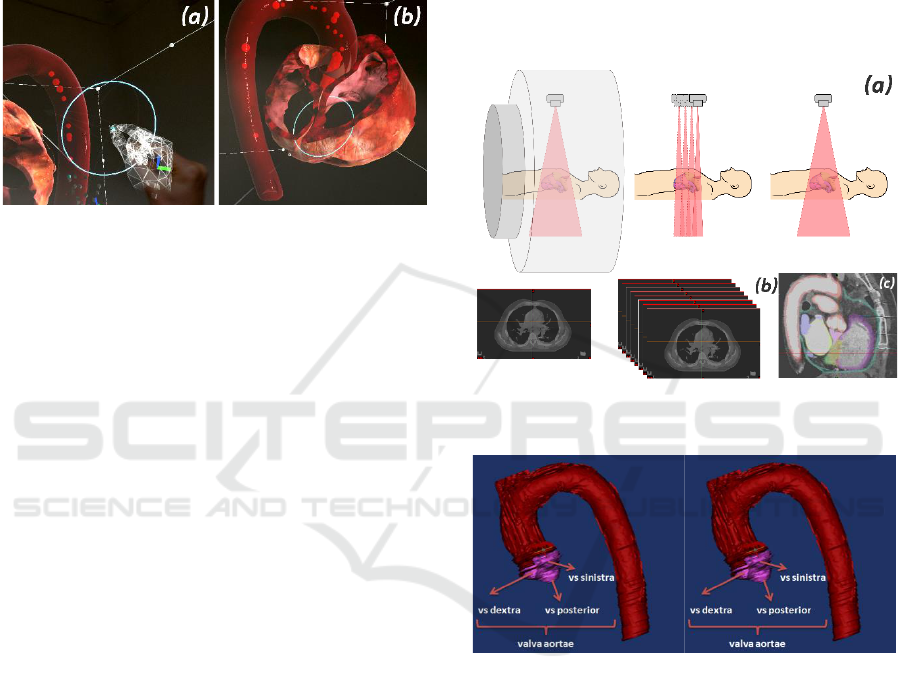
visualisation, along with an aortic valve animation
and animated blood flow simulation. The AR
component may be interacted with by gesture-based
input, thereby supporting exploration of the
anatomical model through scale and rotation controls.
Fig. 1 indicates the user-manipulated AR articulated
hand controller, and the gesture-based cross-section
tool that shows the inner structure of the aorta.
Figure 1: Articulated hand controller operated by user’s
hand input for operating the cross-sectioning tool (a) and
cross-section tool in a passive state of dissection over the
left and right atriums (b).
4.2 Production Process
The amended development process of the prototype
ARLE comprises the following stages:
1. Scanning: image extraction; image layer
splicing; creation of 3D point cloud data sets; and
conversion of scan data to 3D geometry.
2. Modelling: conversion of individual
polygon arrays to a unified 3D mesh inside a 3D
modelling package; cleaning stray polygons and
vertices resulting from data point scatter; mesh repair
of missing faces and topology; model optimisation
and export to game engine; additional modelling and
export of aortic valve anatomy to game engine.
3. Texturing: creation of reference textures in
a 2D image editor; painting of texture maps for each
organ model in 3D painting software; export of maps
as Unity-compatible textures.
4. Animation: animation of aortic valve in a
3D modelling package; path construction and particle
simulation in a game engine for aorta blood flow
visualisation.
5. Rendering: setup of composite materials
using painted textures and cross-section shader.
6. Interaction: reconstruction of heart
structure using imported anatomical models; addition
of hand-based scale/angle manipulation; creation of
dissection tool with hand interaction.
4.2.1 Modeling, Animation & Particle
Simulation
Information about the patient’s anatomy can be
transferred to digital media with medical imaging
techniques. The most used imaging methods today
are Computed Tomography (CT) and Magnetic
Resonance Imaging (MRI) (Eichinger et al., 2010).
CT is a radiological method that creates a cross-
sectional image of the examined area of the body with
X-rays (Fig. 2(a)).
Figure 2: Obtaining a cross-sectional image from CT scan
data in MIMICS.
Figure 3: Anatomical structures resulting from 3D scan
belonging to the aorta anatomy.
After taking cross-sectional images (Fig. 2(a, b)),
the contouring of all anatomical structures of the aorta
was started on transverse sections (Fig. 2(c)). These
cross-sectional images were modelled by the
MIMICS image processing software for 3D design
and modelling (Fig. 3). Modelling is the conversion
of anatomy structures into three-dimensional form
using CT scan data. This procedure requires serious
anatomical knowledge, because the image obtained
from the anatomy can become quite complex due to
the low resolution and patient variations. The
geometric mesh surfaces of the primary heart aorta
and additional structures were built by converting
medical scan data from a CT image to AutoCAD
A Natural Interaction Paradigm to Facilitate Cardiac Anatomy Education using Augmented Reality and a Surgical Metaphor
207
DXF format for import into a 3D modelling and
animation package.
AutoDesk 3DStudio Max was used to edit
individual imported DXF polygon object arrays and
carry out a conversion, retopology, and mesh repair
process for each organ structure. Models were then
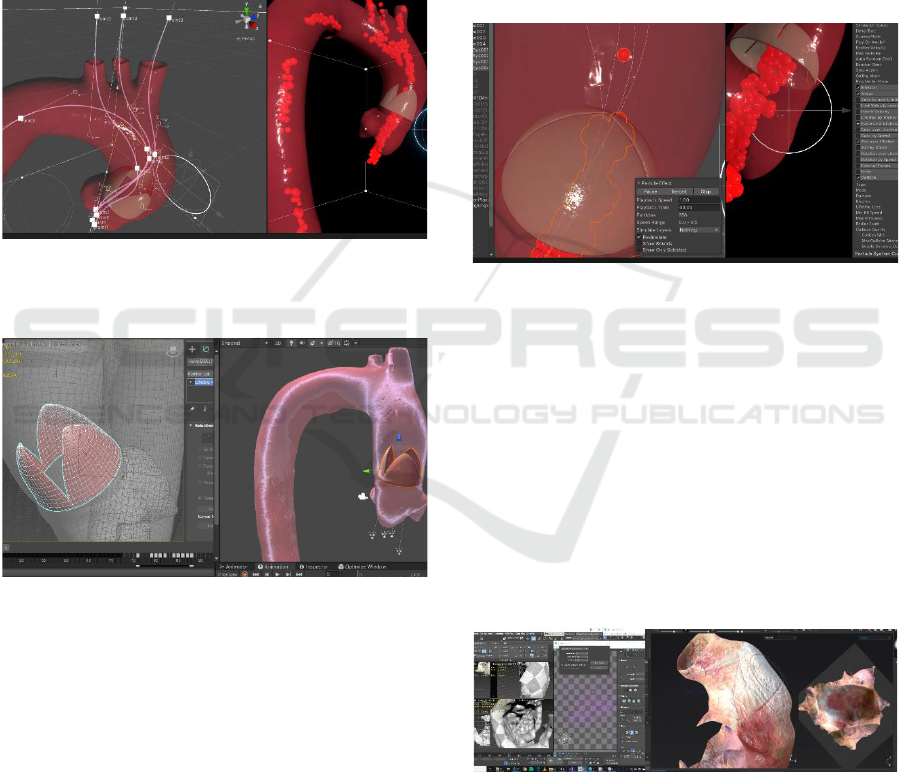
imported into Unity, where four splines were
constructed and positioned inside the aorta to serve as
paths for the blood flow simulation. This method
allowed for additional control over the paths of each
particle simulation by parenting each particle array to
the splines (Fig. 4).
Figure 4: Reconstructed particle animation in Unity’s
physics engine with newly-constructed splines for directing
blood flows.
Figure 5: Animation process of aortic valve using Object-
Space Modifers in 3DS Max and final looped animation
sequence in Unity.
A newly created aortic valve was modelled and
animated in 3DS Max (Fig. 5). A cloth modifier was
used in the valve model to allow for faster simulation
of symmetrical geometry. This provides the ability to
animate without a bone-skin-Inverse Kinematics (IK)
system. However, export issues arose when
converting the valve animation to Unity-compatible
formats, none of which presently support Object-
Space Modifiers.
Various approaches involving vertex animation-
baking yielded a solution in the form of a third-party
mesh baker plug-in that was able to pre-cache the
animation as an OBJ mesh sequence. When combined
with an additional third-party plug-in to import the
baked data into Unity, this was able to output the cloth
simulation correctly. The animation process was
finalised by looping the sequence in Unity’s
Mecanim, then parenting the new valve to the aorta
model, thus allowing for the valve to transition in
together with the aorta and blood flow particle
animations. Unity’s particle physics engine was used
to provide a more accurate representation of blood
flow by using particle collision in conjunction with a
pre-cached valve animation converted to a rigid body
(Fig. 6).
Figure 6: Unity’s physics engine was used to provide
accurate particle simulation for blood flow animation.
4.2.2 Texturing Mapping and Render
Process
The challenges presented by manually unwrapping,
stitching and painting textures on complex geometry
were overcome with the aid of the Substance Painter
software package. This enabled instantaneous,
automated UV unwrapping of geometry that included
automatic stitching of individual UV maps into a
single pelt map. The process was further simplified
due to using the same virtual map in 3D space for
direct-to-surface painting.
Figure 7: Early UV mapping process in 3DS Max,
compared to texture map creation in Substance Painter.
This was achieved by baking the mesh geometry
into textures, which allowed for automatic alignment
of painted sections and a fully-rendered output of the
final texture at all times. This approach was used to
dynamically paint and edit custom texture channels
GRAPP 2022 - 17th International Conference on Computer Graphics Theory and Applications
208

on top of a 3D object, then export them as 2D maps,
reversing the traditional workflow of 2D to 3D to 2D
software package (Fig. 7).
Most traditional workflows do not afford dynamic
evaluation of the final result during map editing, and
instead rely on the user’s imagination, high-end,
expensive 2D graphics packages, and third-party
tools to produce texture channels. A variety of
customised brushes and textures were created by
referencing open-source anatomical material in the
form of photographs and realistic CGI renders.
Figure 8: Texture maps created in Substance Painter as
viewed on HoloLens2 in physical environment.
Once texture sets for each organ structure were
imported into Unity, a collection of third-party cross-
section shaders with specular shading and normals-
rendering support were configured to render the
organic material properties of the structures (Fig. 8).
Figure 9: Planar cross-section gizmo configured with
compatible shaders and Substance Painter textures applied
to anatomy in Unity.
Finally, the cross-section manipulation tool was
created in Unity using third-party cross-section
shaders and a planar gizmo to adhere to a natural
surgical-dissection metaphor inside the AR
environment. A simple visual cue was used to denote
the tool’s position and facing direction to aid
usability. Gesture input capability was configured
within the tool to allow users to have dynamic control
over the dissection process (Fig. 9).
4.3 Observations on Technical Practice
and Workflow
One of the first challenges was accurate conversion
of CT imaging data into three-dimensional data for
the visualisation assets. In order to accurately model
the heart aorta, chambers, and its associated
anatomical structures, it was necessary to extract
image slices from CT data, followed by preparation
through combining slices into a 3D AutoCAD object
suitable for modelling software. The scatter of
physical data points resulting from the scanning
process created a challenge during the modelling
stage, and required flexible methods for optimising
model geometry for texturing and animation.
Artefacts from the initial scan data shifted the
workload to optimisation of the complex organic
objects in a modelling software package. Since
accurate conversion of individual polygons into unified
model follows “garbage in, garbage out,” it requires
initial CT scan data capture with high enough precision
to capture the maximum amount of physical geometry
of fine and complex organ structures and avoid missing
geometry during the conversion (Fig. 10).
Figure 10: 3D point cloud data during polygon unification
process, depicting multiple stray artefacts and missing
geometry.
Relatively simple and optimised geometry still
required lengthy editing of UV coordinate maps for
texturing complex organic structures. This was done
using the Adobe Substance Painter 3D painting
application that allowed for procedurally generated
texturing and auto UV mapping and dramatically
decreased the time and effort expended on creation of
realistic anatomy assets.
Contrary to traditional methods of using a 3D
modelling and animation package to export
visualisations, the full range of rendering techniques
provided by Unity affords richer experiences that
support active learning and the AR medium by using
shaders to reveal and conceal interior contents of
anatomical structures.
A Natural Interaction Paradigm to Facilitate Cardiac Anatomy Education using Augmented Reality and a Surgical Metaphor
209

Particle simulation for visualising dynamic
processes such as blood flow in an aorta should
follow a workflow that prioritises the game engine
used for building the ARLE and not 3D animation
software. Despite the capability of 3DS Max to
produce realistic liquids and synchronise blood pump
sequences easily to other animated organs, export
compatibility issues and severe memory loads both
for exported particle animation caches and Unity’s
rendering engine make cross-package workflows a
challenge. Game engines offer faster computation and
rendering of particles, as well as versatility in
controlling animation. Physics-based animation is
achieved easily, with minimum memory overhead,
allowing particles to correspond accurately to
imported model geometry and animation.
5 CONCLUSION AND FUTURE
WORK
This paper has outlined techniques for creating a
photo-realistic cardiac anatomy and physiology
visualisation in an AR HMD. This approach enables
the learner to observe anatomical content in a real-
world setting, while avoiding hands-on interaction
with the environment.
The development of the prototype ARLE
contributes to AR design practice, particularly to
design and development pipelines that focus on HMD-
supported AR visualisation. A range of technical
challenges related to data visualisation, 3D asset
creation, and AR environment design were identified
and addressed. These serve as the impetus for some
general conclusions: (1) an AR environment that uses
HMDs for presenting anatomical visualisations can
support immersion and improved usability due to
presenting models in three dimensions; (2) anatomical
visualisations, particularly those involving compound
organ structures of complex shapes and configurations,
viewed in an AR HMD may benefit from a dissection
metaphor; (3) a walkthrough modality for viewing
anatomy visualisations could further shift cognitive
load away from manual manipulation actions through
hand interaction by allowing users to physically travel
around the heart model and interactively study its
structure.
These conclusions suggest grounds for evaluation
using metrics such as users’ dissection frequency and
position during interaction, or frequency of physical
traversal within the AR space.
Future plans to develop the system include a more
precise extraction of CT scans. This should address the
problem of missing geometry in complex and thin
sections of organic structures, caused by limited
scanning resolution. Once obtained, subsequent
remodelling and retexturing of structures will take
place, creating a more expansive AR representation of
the entire heart anatomy. Similarly, the final version of
the ARLE will include improvements to animated
physiological processes so that the heart circulation
may be represented with greater accuracy and realism.
Finally, an evaluation will be conducted of the
educational impact of the prototype ARLE on cardiac
anatomy knowledge acquisition and learning
experience. Once development and a study design are
complete, the project will progress to empirical user
studies involving selected medical student cohorts in
order to observe interaction behaviour and learning
efficacy while using the ARLE. There are also plans
to develop AI-based evaluation methods. This
includes plans to model the patient-specific heart
structure with AI algorithms using CT images, to
detect possible diseases using the patients’
Electrocardiogram (ECG) data, and to animate both
the heartbeat rhythm and diseases on the 3D model to
support decision making and educational purposes.
ACKNOWLEDGEMENTS
This project was supported by grant from the
Afyonkarahisar Health Sciences University Scientific
Research Projects Coordinatorship (Project No: 19.
TEMATIK.001).
REFERENCES
Ba, R. K. T. A., Cai, Y., and Guan, Y. (2018, December).
Augmented reality simulation of cardiac circulation
using APPLearn (heart). In 2018 IEEE International
Conference on Artificial Intelligence and Virtual
Reality (AIVR), pp. 241-243.
Bakar, W. A. W. A., Solehan, M. A., Man, M., and Sabri, I.
A. A. (2021). GAAR: Gross Anatomy using
Augmented Reality Mobile Application. International
Journal of Advanced Computer Science and
Applications, 12(5), 162-168.
Billinghurst, M. & Henrysson, A., 2009. Mobile
architectural augmented reality. In X. Wang & M. A.
Schanbel (Eds.), Mixed reality in architecture, design
and construction (pp. 93-104). Springer.
Birbara, N. S., Sammut, C., and Pather, N. (2020). Virtual
reality in anatomy: a pilot study evaluating different
delivery modalities. Anatomical Sciences Education,
13(4), 445-457.
GRAPP 2022 - 17th International Conference on Computer Graphics Theory and Applications
210

Bogomolova, K., Hierck, B. P., Looijen, A. E., Pilon, J. N.,
Putter, H., Wainman, B., and van der Hage, J. A.
(2021). Stereoscopic three‐dimensional visualisation
technology in anatomy learning: A meta‐ analysis.
Medical Education, 55(3), 317-327.
Bork, F., Stratmann, L., Enssle, S., Eck, U., Navab, N.,
Waschke, J., and Kugelmann, D. (2019). The benefits
of an augmented reality magic mirror system for
integrated radiology teaching in gross anatomy.
Anatomical Sciences Education, 12(6), 585-598.
Cakmak, Y. O., Daniel, B. K., Hammer, N., Yilmaz, O.,
Irmak, E. C., & Khwaounjoo, P. (2020). The human
muscular arm avatar as an interactive visualization tool in
learning anatomy: Medical students’ perspectives. IEEE
Transactions on Learning Technologies, 13(3), 593-603.
Celik, C., Guven, G., and Cakir, N. K. (2020). Integration
of mobile augmented reality (MAR) applications into
biology laboratory: Anatomic structure of the heart.
Research in Learning Technology, 28.
Eichinger, M., Heussel, C. P., Kauczor, H. U., Tiddens, H.,
and Puderbach, M. (2010). Computed tomography and
magnetic resonance imaging in cystic fibrosis lung
disease. Journal of Magnetic Resonance Imaging,
32(6), 1370-1378.
Gloy, K., Weyhe, P., Nerenz, E., Kaluschke, M., Uslar, V.,
Zachmann, G., and Weyhe, D. (2021). Immersive
Anatomy Atlas: Learning Factual Medical Knowledge
in a Virtual Reality Environment. Anatomical Sciences
Education.
Gonzalez, A. A., Lizana, P. A., Pino, S., Miller, B. G., and
Merino, C. (2020). Augmented reality-based learning
for the comprehension of cardiac physiology in
undergraduate biomedical students. Advances in
Physiology Education, 44(3), 314-322.
Jamali, S. S., Shiratuddin, M. F., Wong, K. W., and Oskam,
C. L. (2015). Utilising mobile-augmented reality for
learning human anatomy. Procedia - Social and
Behavioral Sciences, 197 (February), 659–668.
Kalana, M. H. A., Junaini, S. N., and Fauzi, A. H. (2020).
Mobile augmented reality for biology learning: Review
and design recommendations. Journal of Critical
Reviews, 7(12), 579-585.
Kiourexidou, M., Natsis, K., Bamidis, P., Antonopoulos, N.,
Papathanasiou, E., Sgantzos, M., and Veglis, A. (2015).
Augmented reality for the study of human heart anatomy.
International Journal of Electronics Communication and
Computer Engineering, 6(6), 658-663.
Küçük, S., Kapakin, S., and Göktaş, Y. (2016). Learning
anatomy via mobile augmented reality: Effects on
achievement and cognitive load. Anatomical Sciences
Education, 9(5), 411–421.
Kurniawan, M. H., Suharjito, D., & Witjaksono, G. (2018).
Human anatomy learning systems using augmented
reality on mobile application. Procedia Computer
Science, 135, 80-88.
Kurul, R., Ögün, M. N., Neriman Narin, A., Avci, Ş., and
Yazgan, B. (2020). An alternative method for anatomy
training: Immersive virtual reality. Anatomical
Sciences Education, 13(5), 648-656.
Layona, R., Yulianto, B., and Tunardi, Y. (2018). Web
based augmented reality for human body anatomy
learning. Procedia Computer Science, 135, 457-464.
Mandalika, V. B. H., Chernoglazov, A. I., Billinghurst, M.,
Bartneck, C., Hurrell, M. A., Ruiter, N. de, Butler, A.
P. H., and Butler, P. H. (2017). A hybrid 2D/3D user
interface for radiological diagnosis. Journal of Digital
Imaging, 31(1), 56–73.
Maresky, H. S., Oikonomou, A., Ali, I., Ditkofsky, N.,
Pakkal, M., and Ballyk, B. (2019). Virtual reality and
cardiac anatomy: Exploring immersive three ‐
dimensional cardiac imaging, a pilot study in
undergraduate medical anatomy education. Clinical
Anatomy, 32(2), 238-243.
Moro, C., Phelps, C., Redmond, P., and Stromberga, Z.
(2021). HoloLens and mobile augmented reality in
medical and health science education: A randomised
controlled trial. British Journal of Educational
Technology, 52(2), 680-694.
Nuanmeesri, S. (2018). The Augmented Reality for
Teaching Thai Students about the Human Heart.
International Journal of Emerging Technologies in
Learning, 13(6), 203-213.
Piumsomboon, T., Altimira, D., Kim, H., Clark, A., Lee,
G., & Billinghurst, M. (2014, September). Grasp-Shell
vs gesture-speech: A comparison of direct and indirect
natural interaction techniques in augmented reality. In
2014 IEEE International Symposium on Mixed and
Augmented Reality (ISMAR) (pp. 73-82). IEEE.
Romli, R. and Wazir, F. N. H. B. M. (2021, July). AR Heart:
A Development of Healthcare Informative Application
using Augmented Reality. Journal of Physics:
Conference Series, 1962(1), 012065.
Rosni, N. S., Kadir, Z. A., Mohamed Noor, M. N. M.,
Abdul Rahman, Z. H., and Bakar, N. A. (2020).
Development of mobile markerless augmented reality
for cardiovascular system in anatomy and physiology
courses in physiotherapy education. In 14
th
International Conference on Ubiquitous Information
Management and Communication (IMCOM), 2020, pp.
1-5, doi: 10.1109/IMCOM48794.2020.9001692.
Safadel, P. and White, D. (2019). Facilitating molecular
biology teaching by using Augmented Reality (AR) and
Protein Data Bank (PDB). TechTrends, 63(2), 188-193.
Samosky, J. T., Nelson, D. A., Wang, B., Bregman, R.,
Hosmer, A., Mikulis, B., and Weaver, R. (2012,
February). BodyExplorerAR: enhancing a mannequin
medical simulator with sensing and projective
augmented reality for exploring dynamic anatomy and
physiology. In Proceedings of the Sixth International
Conference on Tangible, Embedded and Embodied
Interaction, pp. 263-270.
Venkatesan, M., Mohan, H., Ryan, J. R., Schürch, C. M.,
Nolan, G. P., Frakes, D. H., and Coskun, A. F. (2021).
Virtual and augmented reality for biomedical
applications. Cell Reports Medicine, 2(7), 100348.
Wainman, B., Pukas, G., Wolak, L., Mohanraj, S., Lamb, J.,
and Norman, G. R. (2020). The critical role of stereopsis
in virtual and mixed reality learning environments.
Anatomical Sciences Education, 13(3), 401-412.
A Natural Interaction Paradigm to Facilitate Cardiac Anatomy Education using Augmented Reality and a Surgical Metaphor
211
