
Using Merged Cancer Registry Data for Survival Analysis in Patients
Treated with Integrative Oncology: Conceptual Framework and First
Results of a Feasibility Study
Thomas Ostermann
1
, Sebastian Appelbaum
1,2
, Stephan Baumgartner
3
, Lukas Rist
4
and Daniel Krüerke
3,4
1
Methods and Statistics in Psychology, Faculty of Health, Witten/Herdecke University, Witten, Germany
2
Trimberg Research Academy, University of Bamberg, Bamberg, Germany
3
Society for Cancer Research, Hiscia Institute, Arlesheim, Switzerland
4
Clinic Arlesheim, Research Department, Arlesheim, Switzerland
daniel.krueerke@klinik-arlesheim.ch
Keywords: Survival Analysis, Clinical Registry, Cancer, Integrative Oncology.
Abstract: Survival analysis is the basis for research into all types of treatments aimed at prolonging the overall survival
of a cancer entity. Before we use data from a cancer registry at the Clinic Arlesheim (CRCA) for more
sophisticated survival analysis in relation to integrative oncology treatments, we wanted to learn more about
the possible differences between the clientele in this database and the public. In a first step we compared
survival rates for breast cancer and pancreatic cancer analyzed from CRCA-data with the cor-responding
survival rate (all stages) available at the Robert-Koch-Institute. Furthermore, we differentiated the survival
rates from CRCA-patients with respect to the fraction of the survival time in the care of the clinic Arlesheim.
While the survival rates of CRCA-patients with breast cancer or with pancreatic cancer show similar survival
rates compared to corresponding data from the Robert-Koch-Institute, the sensitivity analysis suggests that
the longer the fraction of the survival time in the care of the clinic Arlesheim the higher the expected survival
rates. In conclusion, the analysis and comparison of the survival rates of a clinical population of a cancer
registry, such as CRCA, may lead to a better identification of responders and non-responders and thus in the
long run may help to optimise integrative and patient cantered treatment strategies.
1 INTRODUCTION
Cancer in many cases still is a disease with fatal
outcome. According to GLOBOCAN database, 18.1
million new cancer cases and 9.6 million cancer
deaths worldwide were counted in 2018 with a 20%
risk of getting a cancer before age of 75 and a 10%
risk of dying from it (Ferlay et al., 2019). Thus, there
is an absolute necessity to be able to provide
statistical data on cancer incidence and treatments.
This is mainly done by cancer surveillance initiatives.
Cancer surveillance according to the National
Cancer Institute is the “ongoing, timely, and
systematic collection and analysis of information on
new cancer cases, extent of disease, screening tests,
treatment, survival, and cancer deaths (Stillman et al.,
2012).
Consequently, the reliability and functionality of
cancer surveillance relies on the ability to transfer
cancer data from hospitals, physicians and
laboratories into an environment where data can be
exchanged and made available (Pollack et al., 2020).
In a pragmatic view this is already the definition
of a cancer registry, which according to Bianconi et
al. (2012) is defined as “a systematic collection of a
clearly defined set of health and demographic data for
patients with specific health characteristics, held in a
central database for a predefined purpose”.
The history of cancer registries however started
very early before modern computer technology was
able to assist in fulfilling its purpose.
Ostermann, T., Appelbaum, S., Baumgartner, S., Rist, L. and Krüerke, D.
Using Merged Cancer Registry Data for Survival Analysis in Patients Treated with Integrative Oncology: Conceptual Framework and First Results of a Feasibility Study.
DOI: 10.5220/0010826400003123
In Proceedings of the 15th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2022) - Volume 5: HEALTHINF, pages 463-468
ISBN: 978-989-758-552-4; ISSN: 2184-4305
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
463

1.1 History of Cancer Registries
With a deeper understanding of the pathogenesis of
cancer in the 19th century first ideas were developed
to gather reliable statistics in terms of cancer related
mortality or morbidity rates. In 1900, a first
nationwide survey on cancer was started by the
German Committee for Cancer Research (Wagner,
1991). According to (Meyer, 1911) “the committee's
first work should be directed towards the strength of
the enemy to be fought […]. Preparing for his
suppression means first of all to get a statistic of
cancer”. Therefore, questionnaires were sent to every
physician to record mortality rates due to cancer.
Another 30 years later according to Alam (2011),
a first population-based cancer registry was
established in Germany allowing to follow up the
treatment process including the duration of survival
of cancer patients, which was one of the starting
points of cancer epidemiology.
Today, the Federal Cancer Register Data Act from
2009, obligated the federal states to transmit data
from the federal states to the Center for Cancer
Registry Data at the Robert Koch-Institute, where
data is stored and used for epidemiological and
scientific purposes (Arndt et al., 2019) such as the
calculation of survival rates (SRs)
As already stated above, SRs provide information
about the percentage of people with the same cancer
and stage of cancer who survived a certain period
after diagnosis (usually five years) after a given
therapy. This information can be used to predict
therapy success on a probabilistic basis.
In particular, cancer registry data can be used to
identify patients with prolonged survival, which is
one of the main objectives in clinical oncology.
Keeping in mind that integrative treatment of cancer
may have a survival benefit for cancer patients (Bae
et al., 2019; Ostermann et al., 2020), registry data
from integrative inpatient treatment is a highly
valuable source not to be underestimated in health
services research.
1.2 The Cancer Registry of the Clinic
Arlesheim (CRCA)
The CRCA dates back to the late 1950
th
and the early
1960
th
(Leroi, A., 1959). The main idea of the
“medical records archive” as it was called at that time,
was to collect and process as many clinical
experiences as possible. In regular steps of one or two
years after inpatient treatment physicians were
contacted, to find out how the patients were doing.
In house, the medical records collected in the
archive were available to the physicians for clinical
preparation as well as for research in a hanging card
register (Figure 1).
Figure 1: Structure of the former hanging card register of
the Clinic Arlesheim including organ areas
(“Organgebiete”) as well as informations on recurrent
cancer (“Rezidive) and type of therapy.
And indeed at that time first case reports on the
treatment of cancer were published (Leroi-von Mai,
1962; Leroi, A., & Wrede, E.;1967) but also a large
cohort study in 1.042 breast cancer patients was
published (Leroi, A. 1958).
Today, the CRCA includes the documentation in
the international oncology database QuaDoSta
(Jeschke et al., 2007) located in Berlin Havelhöhe
(quality, documentation and statistics) since 2010, the
own hospital information system (HIS) since 2016
and a Follow-Up database since 1961. They
contribute with different size to the documentation of
the clinical course of various cancer entities in the
CRCA. Figure 2 describes how different sources of
documentation interact and contributing to finally
create the CRCA structure with all data sources.
Figure 2: Schematic representation of the CRCA structure
with all data sources contributing to the documentation.
HEALTHINF 2022 - 15th International Conference on Health Informatics
464
Before using data from the CRCA for more
sophisticated survival analysis regarding integrative
oncology treatments, the present study aims at
investigating the data about possible differences
between the clientele in the database and the public.
Thus, in a first step we compared SRs for patients
with breast cancer and pancreatic cancer respectively
analyzed from CRCA-data with the corresponding
SRs (all stages) available at the Center for Cancer
Registry Data at the Robert Koch-Institute.
Furthermore, we differentiated the SR from
CRCA-patients according to the fraction of the
survival time in the care of the clinic Arlesheim.
2 MATERIAL AND METHODS
2.1 Data Sources and Data
Management
We used the anonymized data for date of first
diagnosis, age at diagnosis, date of admission to the
Clinic Arlesheim and date of dead from the CRCA to
analyse survival times and rates for breast cancer and
pancreatic cancer patients.
Advanced data analysis is based on a conceptual
approach that contributes to a better understanding
which parameters and treatment modalities have a
positive impact on survival of major cancer entities.
The CRCA provides clinical information of more
than 14,000 cancer patients treated with integrative
medical concepts between 2003 and 2017 at either the
Lukas Clinic or the Ita Wegman Hospital and the
Clinic Arlesheim (the latter was founded in 2014 by
the fusion of the other two institutes). The CRCA
contains information on tumour location, date of
cancer diagnosis, TNM, consultations, diagnostics,
duration and frequency of conventional treatments as
well as integrative therapies, date and cause of death,
medical treatment regimens including doses and
application forms and many other detailed clinical
documentation on the course of cancer diseases.
Inclusion criteria for this extended documentation of
disease progression were:
- Diagnosis of malignancy at any of the following
sites:
• Pancreas (ICD10 C25),
• Colon and Rectum (C18 - C20),
• Lung (C34),
• Breast (C50),
• Prostate (C61).
- Valid informed consent
- At least 3 medical consultations within the first year
after registration in the CRCA (outpatients)
- At least 4 days of hospitalization within the first year
of registration in the CRCA (inpatients)
Exclusion Criteria:
- Medical consultation only within the first year of
registration in the CRCA.
- No malignant tumour
We selected freely available data from the Robert
Koch Institute (RKI) to compare our results with a
large national cancer registry (RKI 2017).
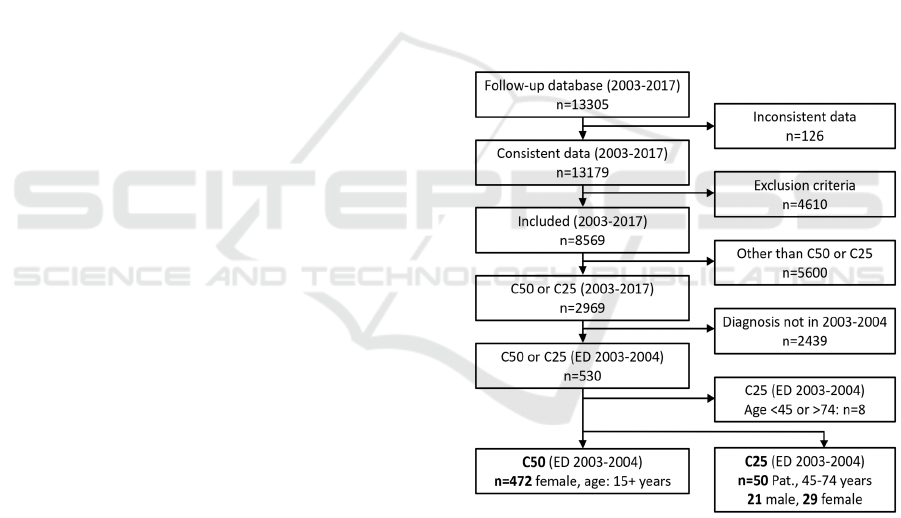
For a 10-year survival comparison, we had to take
data from the CRCA Follow-Up database with initial
diagnosis for C25 and C50 in 2003-2004. From this
database, we were able to use 472 breast cancer
patients (C50) and 50 pancreas cancer patients (C25)
for the following survival analysis, as shown in the
flowchart (Figure 3).
Figure 3: Patient flow chart for CRCA Follow-Up database
extraction of C50 and C25 patients, processed in the further
survival analysis.
2.2 Statistical Analysis
In a preliminary analysis, the data were prepared and
adjusted to be comparable to the survival data of the
RKI.
Survival time was defined as the time from the
date of first cancer diagnosis derived from the records
of the Clinic Arlesheim until the last follow-up date
or documentation of death.
Using Merged Cancer Registry Data for Survival Analysis in Patients Treated with Integrative Oncology: Conceptual Framework and First
Results of a Feasibility Study
465
The survival curves were estimated by the
Kaplan-Meier method (Schober & Vetter; 2018).
3 RESULTS
The C50 and C25 incidences and age distributions for
the CRCA Follow-Up database extraction and for the
RKI database query are summarized in Table 1.
The average age of the corresponding patients at
the clinic Arlesheim are about 5 to 10 years younger
than that of the patients registered in the RKI
database. This could be related to the fact, that
younger people are more open to integrative medicine
treatments than older generations.
Table 1: Sex-specific incidences and age distributions for
the patients from the CRCA Follow-Up database and for the
patients from the RKI database query (first diagnosed 2003-
2004, f=females, m=males).
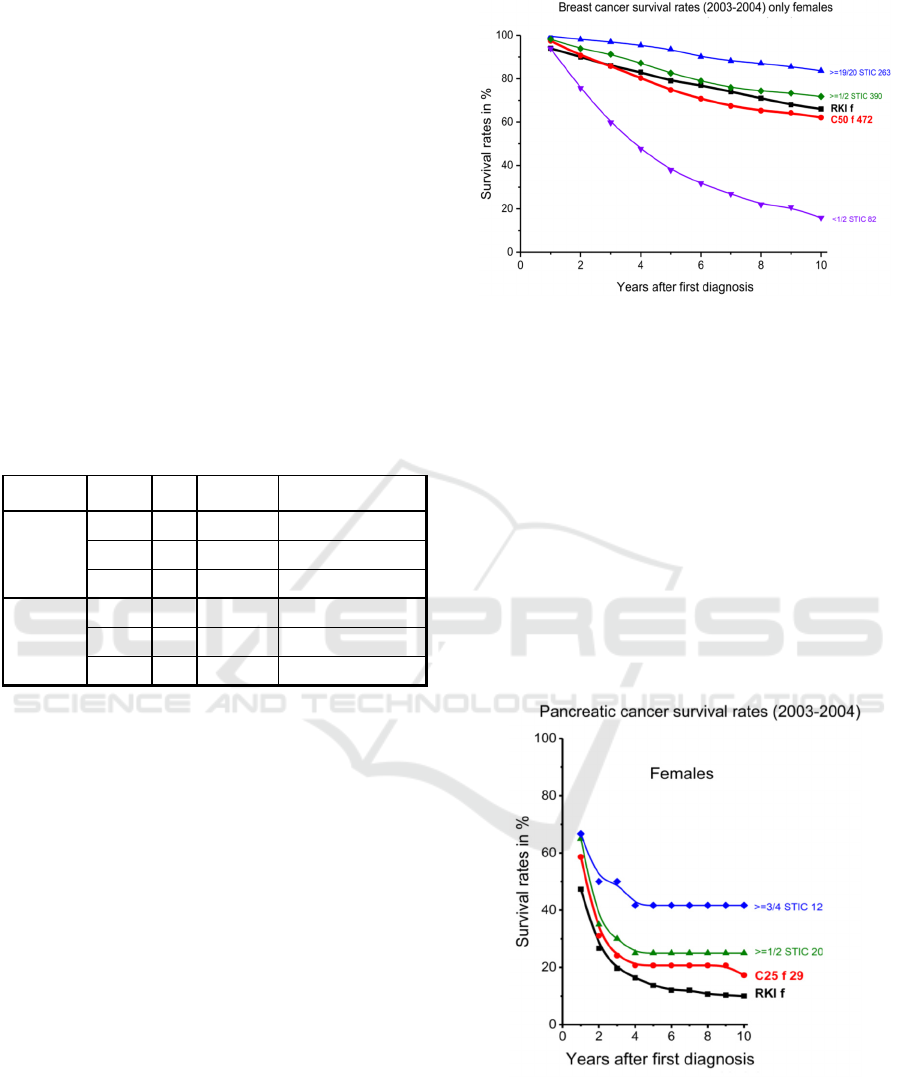
SR of CRCA patients with breast cancer (age over
15y) or with pancreatic cancer (age 45-74y)
diagnosed 2003-2004, show similar survival curves
compared to corresponding data from the RKI
(compare Figure 4 and Figure 5a and 5b). The relative
3-year SR for breast cancer are 83% and 82% RKI,
for pancreatic cancer 20% and 21% RKI (women),
14%, and 15% RKI (men).
The sensitivity analysis suggests that the longer
the fraction of the survival time in the care of the
clinic Arlesheim (x/y STIC n), the higher the
expected SR (e.g. the 3-year SR for the >=1/2 STIC
group with 390 patients is about 7% higher than for
the overall group of 472 patients).
The obvious dependency of SR on the fraction of
the survival time in the care of the clinic Arlesheim
can have many origins as we possess only limited
knowledge of detailed treatments outside the clinic.
Patients who used the integrative care at the clinic
Arlesheim only during a short period of their survival
time, may have undergone inadequate treatment
Figure 4: Kaplan-Meier estimates of SR for breast cancer
patients of the Clinic Arlesheim (C50) compared to data
from the Robert Koch Institute (RKI), diagnosed between
2003 to 2004 (age over 15y). [x/y STIC n = fraction of
Survival Time In Care of Clinic Arlesheim and number of
patients in this group (f=females, m=males)].
elsewhere or despite extensive conventional
treatments they showed poor prognosis and used
integrative treatments only in progressed states. It’s
noteworthy that the x/y STICn-factor seems to be
capable to distinguish between different groups with
different survival time. Therefore, statistical concepts
such as random forest analysis are currently being
adapted to these results in order to gain a deeper
understanding of the parameters and treatment
modalities that influence SR.
Figure 5a: Kaplan Meier estimates of SR for female
pancreatic cancer patients of the Clinic Arlesheim (C25)
compared to data from the Robert Koch Institute (RKI),
diagnosed between 2003 to 2004 (age 45-74y). [x/y STIC
n: see caption Fig. 2].
Registry ICD10 Sex N Age ± SD / y
C50 f 472 53.9 ± 12.5
CRCA
C25 f 29 61.8 ± 9.7
C25 m 21 62.4 ± 7.5
C50 f 122922 63.2 ± 13.8
RKI
C25 f 14119 72.4 ± 11.0
C25 m 13572 67.8 ± 10.7
HEALTHINF 2022 - 15th International Conference on Health Informatics
466
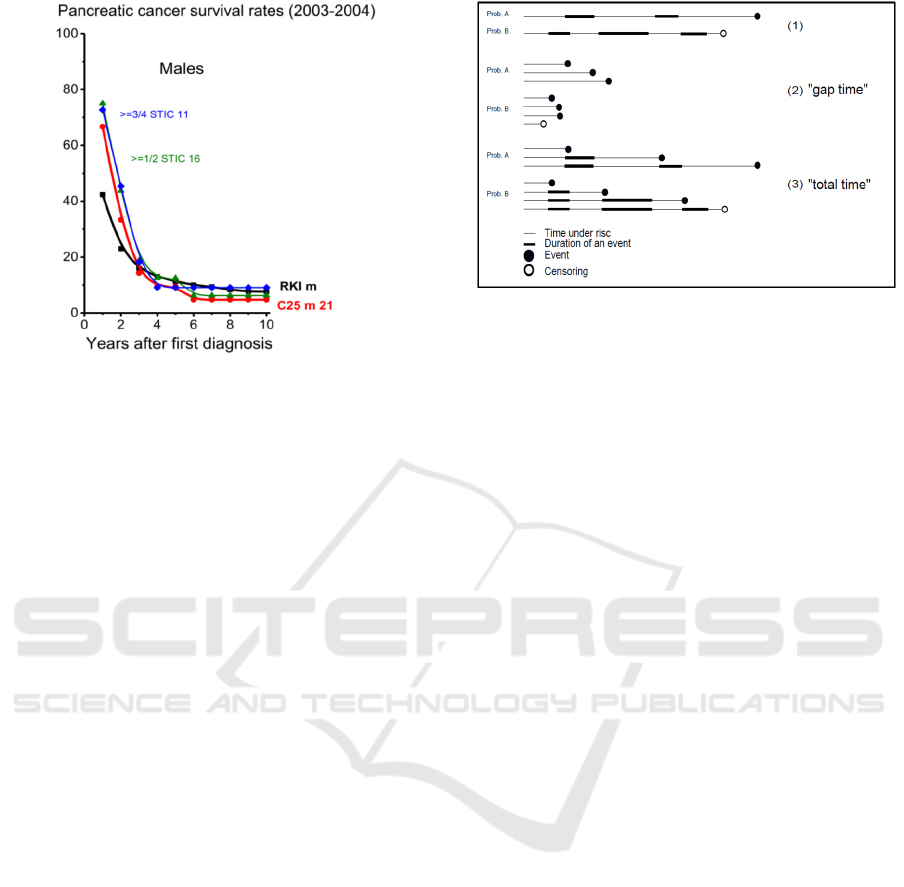
Figure 5b: Kaplan Meier estimates of SR for male
pancreatic cancer patients of the Clinic Arlesheim (C25)
compared to data from the Robert Koch Institute (RKI),
diagnosed between 2003 to 2004 (age 45-74y). [x/y STIC
n: see caption Fig. 3].
4 CONCLUSIONS
The analysis and comparison of the SR of a clinical
population of a cancer registry, such as CRCA, may
lead to a better identification of responders and non-
responders to integrative treatments (Winkler et al.,
2018). For this purpose, a high data quality of the
patient's treatment documentation is indispensable for
comprehensive statistics from the cancer registry to
contribute to cancer prevention in integrative
oncology.
From a methodological point of view, complex
statistical approaches such as the concept of frailty to
introduce random effects, association and unobserved
heterogeneity into models for survival data according
to (Martins et al.; 2019) is a current challenge which
extends the Cox model of proportional hazards model
by introducing individual factors such as therapeutic
gap times to survival analysis and will be applied to
this data (Hirsch et al., 2016; Yazdani et al., 2019).
Figure 5 illustrates typical sequences of therapy
and non-therapy sections in the courses of a disease,
which can be analysed concerning e.g. SR with
respect to “gap time” or “total time” for instance.
As a consequence this might not only lead to an
identification of responders in cancer patients but also
to a detection of optimal treatment strategies for
patient subgroups undergoing an integrative
oncologic treatment (Haller et al., 2021).
Figure 6: Frailty model to model therapeutic “gap times”.
ACKNOWLEDGEMENT
The authors would like to thank Prof. Dr. med.
Andreas Wienke (University Halle/Saale) for the
provision of the illustration in Figure 5. We also
would like to explicitly appreciate the friendly
support and constant helpfulness with all questions
concerning the QDS system by the FIH team (Antje
Merkle, Danilo Pranga and Friedemann Schad).
REFERENCES
Alam, A.S. (2011): Cancer Registry and Its Different
Aspects. Journal of Enam Medical College; 1(2): 76-80.
Arndt, V., Holleczek, B., Kajüter, H., Luttmann, S.,
Nennecke, A., Zeissig, S. R., Kraywinkel, K. &
Katalinic, A. (2020). Data from population-based
cancer registration for secondary data analysis:
methodological challenges and perspectives. Das
Gesundheitswesen, 82(S 01), S62-S71.
Bae K, Kim E, Kong JS, et al. (2019): Integrative cancer
treatment may have a survival benefit in patients with
lung cancer: A retrospective cohort study from an
integrative cancer center in Korea. Medicine; 98(26):
e16048.
Bianconi, F., Brunori, V., Valigi, P., La Rosa, F., & Stracci,
F. (2012). Information technology as tools for cancer
registry and regional cancer network integration. IEEE
Transactions on Systems, Man, and Cybernetics-Part
A: Systems and Humans, 42(6), 1410-1424.
Ferlay, J., Colombet, M., Soerjomataram, I., Mathers, C.,
Parkin, D. M., Piñeros, M., Znaor, A. & Bray, F. (2019).
Estimating the global cancer incidence and mortality in
2018: GLOBOCAN sources and methods. International
journal of cancer, 144(8), 1941-1953.
Haller, H., Voiß, P., Cramer, H., Paul, A., Reinisch, M.,
Appelbaum, S., Dobos, G., Sauer, G., Kümmel, S. &
Thomas Ostermann & Ostermann, T. (2021). The
INTREST registry: protocol of a multicenter
Using Merged Cancer Registry Data for Survival Analysis in Patients Treated with Integrative Oncology: Conceptual Framework and First
Results of a Feasibility Study
467

prospective cohort study of predictors of women’s
response to integrative breast cancer treatment. BMC
Cancer, 21(1), 1-9.
Hirsch K, Wienke A, Kuss O (2016): Log-normal frailty
models fitted as Poisson generalized linear mixed
models. Computer methods and programs in
biomedicine; 137: 167-175.
Jeschke, E., Schad, F., Pissarek, J., Matthes, B., Albrecht,
U., & Matthes, H. (2007). QuaDoSta—a freely
configurable system which facilitates multi-centric data
collection for healthcare and medical research. MS
Medizinische Informatik, Biometrie und
Epidemiologie, 2007-3.
Leroi, A. (1958). Post-operative Iscador therapy for breast
carcinoma. British Homeopathic Journal, 47(03), 191-
200.
Leroi, A. (1959). Jahresbericht des Vereins für
Krebsforschung" Arlesheim.
Leroi, A. & Wrede, E. (1967). Progress in iscador therapy
of malignant tumours. British Homeopathic Journal,
56(01), 2-19.
Leroi-von May, R. (1962). Progress in Iscador therapy of
malignant tumours. British Homeopathic Journal,
51(03), 176-185.
Martins, A., Aerts, M., Hens, N., Wienke, A., & Abrams, S.
(2019). Correlated gamma frailty models for bivariate
survival time data. Statistical methods in medical
research, 28(10-11), 3437-3450.
Meyer G (1911): Bericht über die zehnjährige Wirksamkeit
des Deutschen Zentralkomitees für Krebsforschung.
Zeitschrift für Krebsforschung; 10: 8–33.
Ostermann T, Appelbaum S, Poier D et al. (2020): A
Systematic Review and Meta-Analysis on the Survival
of Cancer Patients Treated with a Fermented Viscum
album L. Extract (Iscador): An Update of Findings.
Complementary Medicine Research 27(4), 1-12.
Pollack, L. A., Jones, S. F., Blumenthal, W., Alimi, T. O.,
Jones, D. E., Rogers, J. D., Benard, V.B. & Richardson,
L. C. (2020). Population Health Informatics Can
Advance Interoperability: National Program of Cancer
Registries Electronic Pathology Reporting Project. JCO
Clinical Cancer Informatics, 4, 985-992.
RKI, Krebsregisterdaten im Robert Koch-Institut,
https://www.krebsdaten.de/Krebs/EN/Database
(database query 27.11.2017)
Schober, P., & Vetter, T. R. (2018). Survival analysis and
interpretation of time-to-event data: the tortoise and the
hare. Anesthesia and analgesia, 127(3), 792-98.
Stillman, F. A., Kaufman, M. R., Kibria, N., Eser, S.,
Spires, M., & Pustu, Y. (2012). Cancer registries in four
provinces in Turkey: a case study. Globalization and
health, 8(1), 1-8.
Wagner G (1991): History of cancer registration. In: Jensen
OM, Parkin DM, MacLennan R et al, (eds). Cancer
registration: principles and methods. IARC scientific
publication 95. Lyon: International Agency for
Research on Cancer, 3-6.
Winkler M, Reissmann R, Sauer G et al. (2018): Einfluss
eines integrativonkologischen Therapieprogramms auf
die Therapieresponse von Brustkrebspatientinnen: eine
prospektive Kohortenstudie (INTEREST). Senologie-
Zeitschrift für Mammadiagnostik und –therapie;
15(02), 152.
Yazdani A, Yaseri M, Haghighat S et al. (2019):
Investigation of Prognostic Factors of Survival in
Breast Cancer Using a Frailty Model: A Multicenter
Study. Breast cancer: basic and clinical research; 13:
1178223419879112.
HEALTHINF 2022 - 15th International Conference on Health Informatics
468
