
A Novel Atomic Annotator for Quality Assurance of Biomedical
Ontologies
Rashmi Burse, Michela Bertolotto and Gavin Mcardle
University College Dublin, Belfield, Dublin 4, Ireland
Keywords:
Biomedical Named Entity Recognition, Lexical Auditing, Semantic Analysis, Quality Assurance, Biomedical
Ontologies, SNOMED.
Abstract:
Existing lexical auditing techniques for Quality Assurance (QA) of biomedical ontologies exclusively consider
lexical patterns of concept names and do not take semantic domains associated with the tokens constituting
those patterns into consideration. For many similar lexical patterns the corresponding semantic domains may
not be similar. Therefore, not considering the semantic aspect of similar lexical patterns can lead to poor QA
of biomedical ontologies. Semantic domain association can be accomplished by using a Biomedical Named
Entity Recognition (Bio-NER) system. However, the existing Bio-NER systems are developed with the goal
of extracting information from natural language text, like discharge summaries, and as a result do not annotate
individual tokens of a clinical concept. Annotating individual tokens of a clinical concept with their semantic
domains is important from a QA perspective, since these annotations can be leveraged to gain insight into
the type of attributes that should be associated with the concept. In this paper we present an annotator that
atomically annotates the tokens of a clinical concept by crafting atomic dictionaries from the sub-hierarchies
of Systematized Nomenclature of Medicine (SNOMED). Semantic analysis of lexically similar concepts by
atomically annotating semantic domains to the tokens will ensure improved QA of biomedical ontologies.
1 INTRODUCTION
Incomplete and inconsistent representations of
biomedical ontologies reduce their expressiveness
and represent real-world facts inaccurately. Clinical
concepts represented in biomedical ontologies are re-
ferred to by various Electronic Health Record (EHR)
systems, discharge summaries and several Health
Information Systems (HIS). Therefore, it is crucial
to ensure that the data represented in biomedical on-
tologies is accurate and impeccable. Over the years,
several auditing techniques have been developed
to identify inconsistent/incorrect representations,
missing relationships, and incomplete definitions of
clinical concepts. Based on their approach to iden-
tify inconsistencies, they can be roughly classified
into lexical, structural, and ontological techniques.
Structural techniques focus on the graphical structure
of biomedical ontologies to identify missing edges
(relationships), ontological methods evaluate the
soundness of an ontology based on ontological
principals, and lexical techniques exploit the names
of biomedical concepts to refine their definitions.
Each of these have their own strengths and can be
used to identify a particular set of inconsistencies.
However, a robust technique that can be employed to
seamlessly identify all inconsistencies is still an area
of ongoing research. Given the large variety of incon-
sistencies that can be identified by lexical auditing
techniques (Rector et al., 2011), this paper focuses on
the lexical auditing techniques and aims to improve
their quality by analyzing semantic aspects along
with the lexical aspects. Lexical aspects include
consideration of common words and the sequence in
which they appear in the Fully Specified Name (FSN)
whereas semantic aspects also consider the meaning
of the word by associating a semantic domain to it.
The paper presents a method to associate semantic
domains to the individual lexical tokens of a concept
name, which will ensure better identification of
inconsistencies in an ontology and therefore suggest
more appropriate attribute relationships to correct
those inconsistencies, as compared to purely lexical
auditing techniques. The remainder of the paper
is organized as follows: Section 2 describes the
state of the art and several gaps identified with the
existing lexical auditing techniques and Bio-NER
systems. Section 3 discusses the proposed method
Burse, R., Bertolotto, M. and Mcardle, G.
A Novel Atomic Annotator for Quality Assurance of Biomedical Ontologies.
DOI: 10.5220/0010782500003123
In Proceedings of the 15th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2022) - Volume 5: HEALTHINF, pages 35-44
ISBN: 978-989-758-552-4; ISSN: 2184-4305
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
35
to atomically annotate the individual tokens of a
concept name with their semantic domains. Section
4 presents the obtained results along with a detailed
discussion. Finally, Section 5 concludes the paper
and discusses some directions for future work.
2 RELATED WORK
2.1 Lexical Auditing Techniques
The existing lexical auditing techniques employ a va-
riety of approaches to exploit several lexical features
to identify inconsistencies in clinical concepts. For
example, (Bodenreider et al., 2002) identified miss-
ing concepts in SNOMED by targeting concepts con-
taining binary antonymous adjectives such as (acute ,
chronic ), (unilateral , bilateral ), (primary, secondary)
etc. The method suggested new concepts by creating
combinations of adjectives and nouns. (Bodenreider
et al., 2001) identified missing hyponomic relation-
ships by intuitively assuming that concepts conform-
ing to a “modifier+noun” form should be hyponyms
of the “noun” form concepts: e.g., acute appendici-
tis should be a child of appendicitis. (Pacheco et al.,
2009) developed a method by eliminating the com-
mon sub-words appearing in both parent and child
concept’s Fully Specified Name (FSN) to suggest at-
tribute relationships. (Agrawal and Elhanan, 2014)
created similarity sets containing concepts whose
FSNs were lexically similar and identified 5 types of
inconsistencies by comparing the concepts within a
similarity set. (Bodenreider, 2016) re-created logical
definitions from the lexical features of a concept name
and inferred hierarchical relationships among these
newly defined concepts. The newly obtained hierar-
chy was then compared with the original SNOMED
hierarchy to detect differences. (Schulz et al., 2017)
detected ambiguities in hierarchy tags, attribute rela-
tionships, and IS-A relationships based on the lexical
features of SNOMED concepts. (Rector and Iannone,
2012) focused on finding concepts from the findings
and diseases sub-hierarchies of SNOMED that should
be classified as chronic or acute according to CORE
problem list but currently are not and studied the
effect of this misclassification on post-coordination
queries. (Ceusters et al., 2007) scrutinized concepts
containing negation words like absence, negation, and
not and misclassification caused due to these words.
The author introduced a new ”lacks” relationship to
correctly classify such negative concepts. (Agrawal
et al., 2013) presented the results of a study that statis-
tically concluded that the complexity and thereby the
chances of identifying errors increases with the length
(number of words) of a concept name and the number
of parents of a concept. (Agrawal, 2018; Agrawal and
Qazi, 2020) proposed an auditing method based on
the hypothesis that if two concepts are lexically sim-
ilar then their structural and logical modeling should
also be similar. (Cui et al., 2017) proposed a hybrid
method combining the structural and lexical aspects
of a CT system and identified four lexical patterns in
non-lattice subgraphs that suggested potential missing
hierarchical relationships and potential missing con-
cepts. (Damme et al., 2018) suggested OWL axioms
to be added in a concept definition by analyzing con-
cept FSNs having a similar lexical structure.
2.1.1 Discussion
Based on this literature survey, we observed that the
auditing techniques focusing on the lexical features of
concept names fail to integrate the semantic meaning
of the tokens constituting the concept name, leading
to many false positives in the identification of incon-
sistencies and subsequently suggestion of inappropri-
ate relationships to rectify those inconsistencies. For
example, the axioms suggested by (Damme et al.,
2018), based purely on lexical analysis, suggest the
attribute “finding site” to be present in all disorders
containing “of aorta”. However, this axiom only holds
true if the identified lexical pattern contains a body
structure after ”of”. If the token following ”of” were
to belong to another hierarchy, the method would
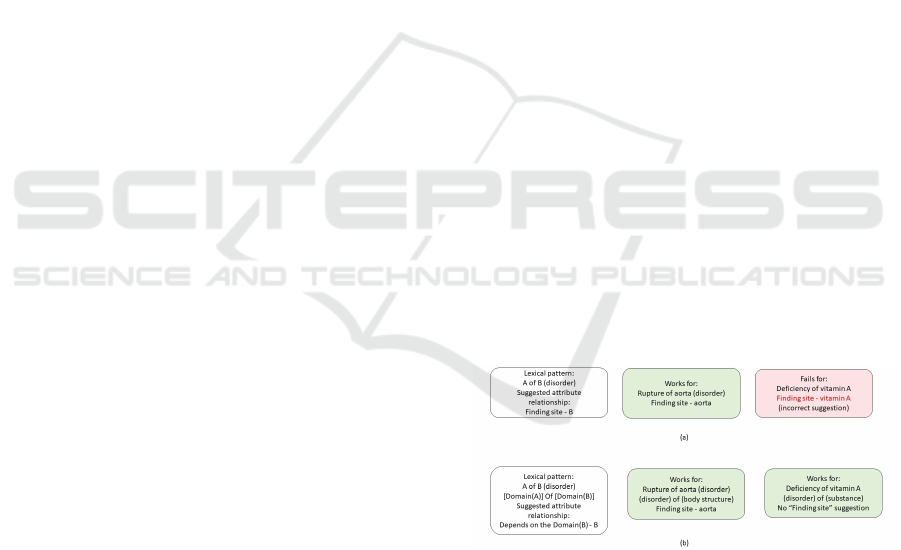
not work. Figure 1 illustrates the shortcomings of
a purely lexical approach with an example and how
the proposed work would improve on the existing ap-
proach, by including a semantic perspective.
Figure 1: (a) Shortcomings of a purely lexical approach (b)
Atomic annotation solution to address the shortcoming.
In Figure 1 (a), both “Rupture of aorta (disorder)”
and “Deficiency of vitamin A (disorder)” have sim-
ilar lexical patterns, “A of B (disorder)”. However,
the attribute suggestion “finding site” while suitable
for “Rupture of aorta (disorder)” turns out to be an
inappropriate attribute suggestion for “Deficiency of
vitamin A (disorder)”, since vitamin A is a substance
and not a body structure. For many similar lexical
patterns the corresponding semantic domains associ-
HEALTHINF 2022 - 15th International Conference on Health Informatics
36

ated with the tokens in the lexical pattern may not be
similar, which is the primary cause of false positives
in the identification of inconsistencies in such meth-
ods. In cases where both the part of speech (POS) and
lexical pattern are similar, the semantic domain is the
deciding factor for suggesting an appropriate attribute
relationship for the concept. We hypothesize that the
rate of incorrect attribute suggestions can be reduced
by basing suggestions on lexical similarities and also
taking the semantic domains associated with the to-
kens in the lexical pattern into consideration. In order
to improve the performance of purely lexical audit-
ing techniques, we propose to add a semantic layer in
the analysis (Figure 1 (b)). We propose to associate
a semantic tag (semantic domain) to each individual
token in an FSN and then suggest attributes to rectify
the identified missing relationships based on both lex-
ical and semantic aspects associated with individual
tokens. The next section discusses the state of the art
in named entity recognition for annotating biomedical
entities.
2.2 Biomedical Named Entity
Recognition
Named entity recognition is a subtask of Information
Extraction (IE) that includes locating and classifying
named entities from unstructured text into certain pre-
defined categories. With huge amounts of biomedical
text being generated, domain specific Named Entity
Recognizers (NERs) were developed that could iden-
tify named entities, like disorders, treatment, diagno-
sis, etc. The growth of Bio-NERs led to a demand
for biomedical lexicons that could aid the process
of identifying clinical entities in free, unstructured
text. As a result the major biomedical ontologies like
UMLS (Bodenreider, 2004), SNOMED (IHTSDO,
2021), Gene Ontology (Consortium, 2003) etc. were
used as dictionaries to identify biomedical entities in
free text. Along with these dictionaries a number of
manually annotated corpora including NCBI disease
corpus (Dogan et al., 2014), GENIA corpus (Ohta
et al., 2002), MCN corpus (Luo et al., 2019), cor-
pus of manually annotated clinical notes (Pakhomov
et al., 2006), (Ogren et al., 2008), BioScope corpus
(Vincze et al., 2008), Distributional Semantics Re-
sources corpus (Pyysalo et al., 2013), CLEF corpus
(Roberts et al., 2007) were developed to train NLP
algorithms. Genia (Ohta et al., 2002) is to date one
of the most widely used corpora and specialises in
gene and protein identification. With dictionaries and
corpora in order, various Bio-NER methods were em-
ployed to extract biomedical concepts from clinical
narratives like discharge summaries. Commonly used
approaches for Bio-NER (Allahyari et al., 2017) can
be classified into:
• Dictionary-based approaches (Friedman et al.,
2004; Long, 2005), that extract biomedical enti-
ties by searching for a match in the biomedical
dictionaries.
• Rule-based approaches (Hina et al., 2010; Ina
et al., 2013), that aid the dictionary-based ap-
proaches by defining specific rules for matching
biomedical concept patterns.
• Statistical approaches (Kulick et al., 2004) that
employ various statistical models for extracting
named entities.
• Machine Learning (ML) based approaches that
employ artificial neural network models like SVM
(Ju et al., 2011), KNN classifiers (Keretna et al.,
2015), Conditional Random Field (CRF) (Skepp-
stedt et al., 2014; Lee et al., 2018), biLSTM-
CRF (Lample et al., 2016), LSTM-CRF (Habibi
et al., 2017), Recurrent Neural Networks (RNNs)
(Unanue et al., 2017), unsupervised models
(Zhang and Elhadad, 2013; P
´
erez et al., 2017),
deep learning models (Liu et al., 2019), and the
latest of all BERT (Lee et al., 2020). Although
ML based techniques automated the process of
Bio-NER to a large extent, a major requirement
was the provision of huge volumes of manually
annotated corpora to train the ML models. Man-
ual annotation of huge volumes of biomedical
data was both laborious and time intensive. This
led to the development of methods that attempted
to eliminate the need for creation of manually an-
notated datasets (Chen et al., 2015; Ghiasvand and
Kate, 2018; Tulkens et al., 2019; Usami et al.,
2011).
• Finally, Hybrid models that combine the strengths
of aforementioned approaches for improved Bio-
NER (Sasaki et al., 2008; Wang et al., 2019).
To make Bio-NERs reachable to non-expert users,
easy-to-use pipelines (Dernoncourt et al., 2017),
online web services (Jonquet et al., 2009a), and
tools like CONANN (Reeve and Han, 2007), Ci-
mind (Cabot et al., 2019), ABNER(Settles, 2005),
CLAMP(Soysal et al., 2018), CliNER (Boag et al.,
2018), MetaMap (Aronson and Lang, 2010) and
CTakes (Savova et al., 2010) were also developed.
(Rais et al., 2014) has presented a comparative study
of seven Machine Learning methods and (Sniegula
et al., 2019) has systematically reviewed all NER
methods employed in the biomedical domain and
highlighted specific areas that need to be attended in
order to improve the performance of Bio-NERs.
A Novel Atomic Annotator for Quality Assurance of Biomedical Ontologies
37

2.2.1 Discussion
The existing Bio-NERs were developed with the goal
of extracting biomedical entities from free unstruc-
tured text like discharge summaries, medical ab-
stracts, and other clinical narratives. As a result, these
Bio-NERs use existing biomedical ontologies as a
reference source / dictionary in order to locate and
annotate biomedical concepts appearing in free text.
These methods try to club as many sequential words
together until they can match a concept name in one
of the biomedical ontologies. However, when anno-
tating clinical concepts from the perspective of Qual-
ity Assurance (QA) of biomedical ontologies, con-
cept names tagged with their respective semantic cat-
egories are already available in the biomedical ontol-
ogy. On the contrary, the annotation process from a
QA perspective requires tokenizing a concept name
and annotating semantic domains to individual tokens
in the name in order to gain insight into the type of at-
tributes that can be associated with the concept. This
makes the end goal and therefore the annotation ap-
proach applied for QA different from the existing Bio-
NER systems. After extensively reviewing the liter-
ature and assessing the functionality of some of the
existing Bio-NERs (Aronson and Lang, 2010; Jon-
quet et al., 2009b; Mahi, 2019; Kocaman and Talby,
2021), we came to the conclusion that none of the ex-
isting tools could be satisfactorily used to serve the re-
quirement of atomic annotation. For example, when
the input “injury of knee” was fed to National Cen-
ter for Biomedical Ontology (NCBO) Bio-ontology
Annotator (Jonquet et al., 2009b), the annotator ei-
ther tagged “Injury of knee” as a “clinical finding”
or “injury” was individually tagged as a “traumatic
abnormality” and “knee” was left unannotated. In-
teractive MetaMap tool (Aronson and Lang, 2010)
tagged “Injury of knee” as “knee injuries” / “injury
or poisoning”. While an implementation of Scispacy
(Mahi, 2019) using the en ner bionlp13cg md model
in python only tagged “knee” as an organ and left “in-
jury of” unannotated. Based on a cursory examina-
tion of the demo provided by SparkNLP (Kocaman
and Talby, 2021), a licensed software tool, we noted
that the entire disorder “malignant neoplasm of thy-
roid gland” was tagged as a “Problem”. Some of the
limitations that do not allow us to use existing Bio-
NER tools for the purpose of atomic annotation are
listed below.
• Non-uniformity: The tags used by each of the
tools were different and there was no uniformity
among them. For example, NLTK tagged knee as
an “organ” instead of “body structure” which is a
more general term and used widely in SNOMED.
SparkNLP (Kocaman and Talby, 2021) followed a
more generic approach and tagged the entire dis-
order as a “problem” instead of a more specific
“traumatic abnormality” as tagged by bio-portal’s
annotator (Jonquet et al., 2009b).
• Dataset Variation: Firstly, we cannot use the ex-
isting trained ML models for atomic annotation
as the datasets employed in their training are dif-
ferent. For example, if an ML model is trained on
a dataset that has manually annotated “injury of
knee” as a disorder, it will not tag the individual
elements atomically. Manually annotating new
datasets to train a model for atomic annotation
is a very laborious and time intensive task. Au-
tomated creation of annotated datasets discussed
earlier is also not of much use since they use ex-
isting biomedical ontologies as dictionaries and
this would again tag the entire disorder instead of
tagging its individual elements. Secondly, given
the highly constrained lexical structure of clini-
cal concept FSNs, the computing requirement for
context detection is very limited and employing
the time and memory intensive ML models for
this purpose seems unnecessary. The task of an-
alyzing such highly constrained lexical clinical
concept FSNs can be easily accomplished by us-
ing a simple dictionary and rule based approach.
• Partial annotation: While some bio-NERs par-
tially annotated the FSN, this is not sufficient. We
require uniform atomic annotation of all tokens in
the FSN, in order to create semantic patterns for
analysis in the future. For example, NLTK anno-
tated knee as an organ in “injury of knee” but left
“injury of” unannotated. We need to annotate both
injury as a disorder and knee as a body structure
in order to classify a concept in a semantic pattern
“disorder of body structure”.
After reviewing the existing lexical auditing tech-
niques we proposed combining semantic aspects
along with lexical aspects to improve the QA of
biomedical ontologies. Based on the literature sur-
vey of the state-of-the-art Bio-NERs we found that
none of the existing Bio-NERs could be satisfactorily
used to serve the purpose of atomic annotation in or-
der to add a semantic aspect into lexical auditing tech-
niques. In this paper, we present a method to develop
an atomic annotator that uniformly annotates individ-
ual tokens in concept FSNs using the SNOMED hier-
archy / semantic tags. The next section discusses the
method in detail.
HEALTHINF 2022 - 15th International Conference on Health Informatics
38

3 METHOD
As discussed in Section 2.2, based on an analysis of
our requirements we adopted a hybrid approach that
combined the strengths of dictionary-based and rule-
based approaches to develop the atomic annotator.
3.1 Dataset
RF2 files of the SNOMED International Edition re-
leased in January 2021 were used to test and validate
our approach. Based on a preliminary manual inspec-
tion of the Disorder subhierarchy of SNOMED, we
found that a lexical pattern “A of B” had at least 3
different semantic patterns associated with it. There-
fore, Disorder subhierarchy was chosen to evaluate
the performance of our atomic annotator. We created
a subset of the Disorder subhierarchy to only include
disorders containing the stop word ”of”. The dataset
was further simplified by limiting the number of to-
kens in the FSN to a maximum of 3 words, exclud-
ing the semantic tag “(disorder)”. This reduced the
complexity of the dataset without hindering the per-
formance evaluation process of the atomic annotator.
Finally, the dataset consisted of 2777 three-word dis-
order concepts containing the stop-word “of”.
3.2 Atomic Dictionary Creation
In order to atomically annotate each token in the lex-
ical pattern “A of B”, 3 atomic dictionaries were cre-
ated. Based on the semantic patterns observed, these
included:
• Atomic disorder dictionary (tag “DIS”)
• Atomic body structure dictionary (tag “BOD”)
• Atomic substance dictionary (tag “SUB”)
Each of these dictionaries was created by process-
ing the respective SNOMED subhierarchies. The to-
ken “of” was annotated using the tag GEN (for gen-
eral English word) followed by the token itself, i.e.,
“GEN-of”. Tokens that could not be annotated using
any of the aforementioned tags, were annotated as un-
known (tag “UNK”). The detailed process employed
in the creation of each of the atomic dictionaries is
described next.
3.2.1 Atomic Disorder Dictionary
All single-word disorders extracted from the Disor-
der subhierarchy were stripped of their semantic tag
“(disorder)” and added to the atomic disorder dictio-
nary. Since the atomic disorder dictionary only con-
tained disorders, the semantic tag “(disorder)” was re-
moved to avoid redundancy (this step was repeated for
all atomic dictionaries). To ensure high annotation
performance for disorders, we logically assumed that
if “A of B” is a disorder then “A” must be a disorder.
For example, consider if “Carcinoma” was not present
as a single-word concept in the Disorder subhierarchy
of SNOMED, then it would not have been added to
the atomic disorder dictionary. As a result, the atomic
annotator would have tagged “Carcinoma” as “UNK”
in the concept “Carcinoma of breast”, in spite of a
disorder being clearly present in the FSN. Based on
this assumption, we added all tokens appearing before
“of” to the dictionary. A few exceptions were elimi-
nated from the dictionary after consulting a medical
expert. A medical expert manually examined all to-
kens appearing before ”of” and checked if they could
be atomically added to the disorder dictionary based
on his medical knowledge. For example, in the con-
cept “Band of Ladd”, “Band” was not included in the
atomic disorder dictionary but in the concept ”Edema
of pharynx”, ”Edema” was included in the atomic dis-
order dictionary. For future references, we define this
as refinement 1.
• Refinement 1: All tokens appearing before “of”
were included in the atomic disorder dictionary,
except a few that were removed based on the opin-
ion of a medical expert.
3.2.2 Atomic Body Structure Dictionary
The approach followed for atomic disorder dictionary
creation would not work well for a body structure
dictionary because SNOMED follows a Structure En-
tire Part (SEP) model to represent body structures, in
which a body structure is preceded by words like en-
tire, part of, etc. For example, ”knee” is present as
“entire knee joint” or “entire left knee” in the Body
Structure sub-hierarchy of SNOMED. As a result, the
body structure “knee” would not have been added to
the atomic body structure dictionary, if only single-
word concepts from the Body Structure sub-hierarchy
of SNOMED were included. Therefore, in case of a
concept like “Injury of knee”, the atomic annotator
would have annotated “knee” as “UNK”, in spite of
a body structure being present in the FSN. After tak-
ing SNOMED’s SEP model into consideration, we re-
fined the atomic body structure dictionary. For future
references, we define this as refinement 2.
• Refinement 2: The atomic body structure dictio-
nary was recreated by extracting two-word con-
cepts (excluding the (body structure) tag) from the
Body Structure subhierarchy of SNOMED.
A Novel Atomic Annotator for Quality Assurance of Biomedical Ontologies
39

3.2.3 Atomic Substance Dictionary
An atomic substance dictionary was created by ex-
tracting single-word concepts from the Substance
subhierarchy of SNOMED. To ensure high annota-
tion performance for substances we added a few more
entries that included (a) substances missing from the
Substance subhierarchy of SNOMED, which were
identified after an analysis of the common ”UNK”
tags and (b) substances that were usually referred to
in their plural forms in the concept FSNs. In most
of the cases, substances listed as proper nouns ap-
peared in their singular form whereas a general ref-
erence to substances was always represented in the
plural form. For example, “Deficiency of vitamin A”
vs “Deficiency of vitamins”. We did not find it accu-
rate to process the disorder FSNs to eliminate plurals
as we are not medical experts and instead refined the
atomic substance dictionary to include such entries.
Let us define this as refinement 3.
• Refinement 3: The substance hierarchy was re-
fined to include plural forms of certain substances
and a few substances that were missing in the Sub-
stance subhierarchy of SNOMED.
3.3 Atomic Annotation
After creating and refining the atomic dictionaries,
the dataset of 2777 concepts was passed as input for
atomic annotation of tokens. In cases where a token
could be annotated using multiple tags, rules were de-
fined to ensure that the tokens were annotated accu-
rately. The rules were defined after a manual inspec-
tion and taking medical expert opinion into consid-
eration. The highest priority was given to disorder
followed by body structure and lastly substance. In-
deed, based on the position of the token, if the token
appeared before “of”, it was always tagged as a dis-
order. For example, in “Dehiscence of fascia (disor-
der)”, Dehiscence could be tagged as both a disorder
and a body structure but higher priority was given to
disorder based on the position. In the case of “Necro-
sis of flap (disorder)”, flap could be tagged as both
a substance and a body structure but higher priority
was given to body structure, as suggested by the med-
ical expert. While searching for a token in the body
structure dictionary a partial match logic was applied
since the body structure present in the concept FSN
was always a substring of the body structure modelled
using the SEP model in the atomic body structure dic-
tionary. Minute considerations like adding trailing
spaces to tokens before applying partial match logic
were made to ensure accurate atomic annotation. For
example, not adding trailing spaces to a token would
have resulted in inaccurate results like “liver” being
tagged as a “DIS” instead of a “BOD” due to the pres-
ence of “Hyperbiliverdinemia” in the atomic disorder
dictionary. So trailing spaces were appended to both
the entries in the atomic dictionary and the tokens be-
fore matching to avoid such mishaps. For disorder
and substance tagging, a complete match was consid-
ered while searching through atomic dictionaries. A
concept was considered to be correctly annotated if
• All individual tokens of the FSN were annotated.
• The semantic pattern formed after atomically an-
notating the FSN belonged to one of these pat-
terns : “DIS GEN-of BOD”, “DIS GEN-of DIS”,
or “DIS GEN-of SUB”.
• The medical expert found the annotated concept
to be semantically sound after manually inspect-
ing the tags
The next section discusses the results obtained by the
atomic annotator.
4 RESULTS & DISCUSSION
The atomic annotator correctly annotated 2653 out of
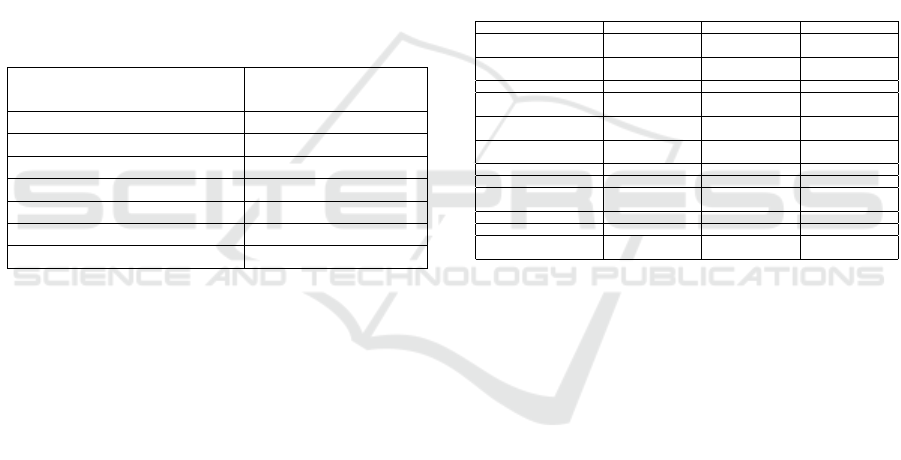
the 2777 concepts (95.53%) passed to it. Table 1 de-
scribes the gradual improvement in annotation results
after applying each of the refinements defined in sec-
tion 3.2. In table 1, column 1 describes the num-
Table 1: Improvement in annotation results after applying
each refinement.
Refinements
applied
# correct an-
notations
% correct an-
notations
None 342 12.32
1 361 12.99
1,2 2627 94.59
1,2,3 2653 95.53
ber of dictionary refinements that were applied be-
fore annotating the dataset. Column 2 displays the
number of concepts that were correctly annotated by
the atomic annotator, as per our definition. Column
3 displays the respective percentages calculated out
of a total 2777 concepts fed to the atomic annota-
tor. Initially when all atomic dictionaries were created
by adding single-word concepts from the respective
SNOMED sub-hierarchies, only 12.32 % of the con-
cepts were correctly annotated. After applying refine-
ment 1 to the atomic disorder dictionary, this percent-
age increased from 12.32 % to 12.99 %. After taking
into consideration the SEP model of SNOMED and
refining the atomic body structure dictionary 94.59 %
HEALTHINF 2022 - 15th International Conference on Health Informatics
40
of the concepts were correctly annotated. The rea-
son for this drastic improvement is the fact that the
majority of the disorder concepts in our input data set
belonged to the semantic pattern ”DIS GEN-of BOD”
and after refinement 2 the majority of them were an-
notated correctly. Finally, after applying refinement 3
to the atomic substance dictionary, the results further
improved to 95.53 %. An additional output was the
identification of missing concepts in the SNOMED
Substance sub-hierarchy, which were added to the
atomic substance dictionary as a part of refinement
3. This list of identified missing substances will be
submitted to SNOMED authors for review. The 124
concepts that could not be annotated correctly by the
atomic annotator include rare tokens that could not be
found in any of the aforementioned atomic dictionar-
ies. Table 2 displays some of the patterns that were
not correctly annotated by the atomic annotator.
Table 2: Examples of concepts incorrectly annotated by the
atomic annotator.
Concept FSN (excluding
(disorder) tag)
Annotations by
atomic annotator
Caries of infancy DIS GEN-of UNK
Disorder of fluency DIS GEN-of UNK
Gangrene of newborn DIS GEN-of UNK
Vegetation of heart UNK GEN-of BOD
Barotrauma of ascent DIS GEN-of UNK
Barotrauma of descent DIS GEN-of UNK
Fibroepithelioma of Pinkus DIS GEN-of UNK
The majority of the cases where concepts were
incorrectly annotated belonged to the semantic pat-
tern “DIS GEN-of UNK”. In a few of the cases,
the token appearing after “of” was a qualifier value
(e.g. infancy), a social concept (e.g. newborn), an
observable entity (e.g. fluency). In “Barotrauma of
ascent” and “Barotrauma of descent”, “ascent” was
listed as an Event but “descent” was listed as a Sit-
uation, Finding and an Event in SNOMED. Analyz-
ing “UNK” tags will provide interesting insights into
the lexical modelling of SNOMED FSNs and high-
light additional erroneous and inconsistent regions of
SNOMED. In rare cases like “Vegetation of heart”,
the token before “of” was not annotated as a disorder.
This happened because of the manual elimination of
a few concepts appearing before “of” from the atomic
disorder dictionary, based on the opinion of a medi-
cal expert. In a few cases, for example, “Fibroepithe-
lioma of Pinkus”, The token after “of”, i.e., “Pinkus”
represents the name of the person who discovered the
disorder. It would be more ideal to model such disor-
ders using “Pinkus’s Fibroepithelioma” instead.
To conduct a comparative evaluation of our atomic
annotator with the state-of-the-art, a set of three
concepts belonging to each semantic pattern, which
were picked randomly, was passed to two of the
most widely used Bio-NERs , i.e., Bioportal (Jon-
quet et al., 2009b) and MetaMap (Aronson and Lang,
2010). Furthermore, the aforementioned concepts,
represented in table 2, that could not be annotated
by our atomic annotator were also passed to bioportal
(Jonquet et al., 2009b) and metaMap (Aronson and
Lang, 2010), to check if they could annotate them
individually and extract semantic patterns. Table 3
presents a comparative evaluation of the annotation
results obtained by our atomic annotator vs Bioportal
(Jonquet et al., 2009b) and MetaMap (Aronson and
Lang, 2010).
Table 3: Comparative evaluation of the atomic annotator
with Bioportal and MetaMap.
Concept FSN Atomic annotator Bioportal annotator MetaMap annotator
Calcification of lung DIS GEN-of BOD calcification of lung
structure
Disease or Syndrome
Tuberculosis of bronchus DIS GEN-of BOD Tuberculosis of
bronchus
Disease or Syndrome
Lipoma of hip DIS GEN-of BOD hip Neoplastic Process
Overdose of metformin DIS GEN-of SUB Overdose of met-
formin
Injury or Poisoning
Abuse of laxatives DIS GEN-of SUB Abuse of laxatives Mental or Behavioral
Dysfunction
Extravasation of urine DIS GEN-of SUB Extravasation of
urine
Pathologic Function
Sequela of trachoma DIS GEN-of DIS Sequela of trachoma Pathologic Function
Rupture of neoplasm DIS GEN-of DIS Rupture of neoplasm Neoplastic Process
Hyperkeratosis of pinta DIS GEN-of DIS Hyperkeratosis of
pinta
Disease or Syndrome
Caries of infancy DIS GEN-of UNK Caries of infancy Disease or Syndrome
Disorder of fluency DIS GEN-of UNK Disorder of fluency Disease or Syndrome
Fibroepithelioma of Pinkus DIS GEN-of UNK Fibroepithelioma of
Pinkus
Neoplastic Process
Based on a comparative evaluation, a notable fea-
ture of bioportal (Jonquet et al., 2009b) was that it
was able to tokenize the disorder phrase. However,
despite tokenizing, the atomic annotations rendered
were of no use since they were only mapped to the
same term in a reference biomedical ontology, rather
than being mapped to its semantic domain. Figure
2 displays a partial view of the bioportal annotator
results illustrating a few annotations for each of the
tokens of the disorder ”Tuberculosis of bronchus”.
Comparatively, metaMap (Aronson and Lang, 2010),
did annotate the entire phrase with its semantic do-
main but could not tokenize the phrase and annotate
its individual elements. Figure 3 displays the annota-
tion results of metaMap for the disorder ”Tuberculosis
of bronchus”. It is clearly evident from the compara-
tive evaluation results that our atomic annotator out-
performs both bioportal and metaMap annotators as
far as atomic annotation is concerned. The tags anno-
tated by bioportal and metaMap, although useful in IE
from discharge summaries, cannot be used to extract
semantic patterns for QA of biomedical ontologies.
A Novel Atomic Annotator for Quality Assurance of Biomedical Ontologies
41
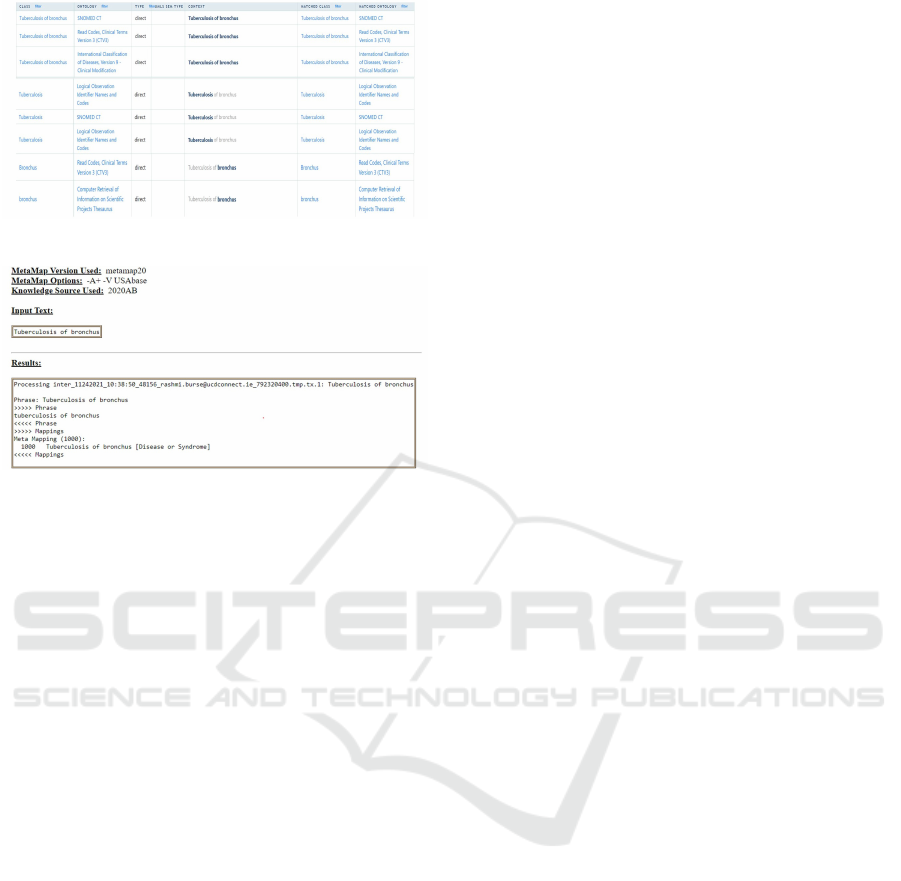
Figure 2: Bioportal annotator results.
Figure 3: MetaMap annotator results.
5 CONCLUSION
In this work, we highlighted the importance of taking
into account a semantic aspect along with the lexical
aspect in order to reduce the number of false positives
in the identification of inconsistencies in biomedical
concepts. In order to introduce a semantic perspec-
tive to the exclusive lexical auditing techniques, cur-
rently employed in the QA of biomedical ontologies,
we presented an atomic annotator that annotates in-
dividual tokens of a concept FSN with their semantic
domains to provide insight into the nature of attributes
that should be suggested for an inconsistent or incom-
pletely defined concept. The atomic annotator has
shown a promising potential by correctly annotating
95.53% of the concepts from the input dataset. The
atomic annotator presented in this paper is a part of
ongoing work. In the future we plan on validating the
semantic patterns identified by our atomic annotator
and using the atomically annotated output to extract
specific attribute suggestions for inconsistent biomed-
ical concepts based on the semantic pattern to which
the concept belongs.
ACKNOWLEDGEMENTS
We would like to thank Dr. K.S. Burse (M.S.
(ENT)) for providing his expert medical opinion
which tremendously helped us in refining the atomic
dictionaries.
REFERENCES
Agrawal, A. (2018). Evaluating lexical similarity and
modeling discrepancies in the procedure hierarchy of
snomed ct. BMC Medical Informatics and Decision
Making, 18.
Agrawal, A. and Elhanan, G. (2014). Contrasting lexical
similarity and formal definitions in snomed ct: Con-
sistency and implications. Journal of biomedical in-
formatics, 47:192–8.
Agrawal, A., Perl, Y., Chen, Y., Elhanan, G., and Liu, M.
(2013). Identifying inconsistencies in snomed ct prob-
lem lists using structural indicators. AMIA ... Annual
Symposium proceedings. AMIA Symposium, 2013:17–
26.
Agrawal, A. and Qazi, K. (2020). Detecting modeling in-
consistencies in snomed ct using a machine learning
technique. Methods.
Allahyari, M., Pouriyeh, S., Assefi, M., Safaei, S., Trippe,
E. D., Guti
´
errez, J. B., and Kochut, K. (2017). A brief
survey of text mining: Classification, clustering and
extraction techniques. ArXiv, abs/1707.02919.
Aronson, A. and Lang, F.-M. (2010). An overview of
metamap: historical perspective and recent advances.
Journal of the American Medical Informatics Associ-
ation : JAMIA, 17 3:229–36.
Boag, W., Sergeeva, E., Kulshreshtha, S., Szolovits, P.,
Rumshisky, A., and Naumann, T. (2018). Cliner 2.0:
Accessible and accurate clinical concept extraction.
ArXiv, abs/1803.02245.
Bodenreider, O. (2004). The unified medical language sys-
tem (umls): integrating biomedical terminology. Nu-
cleic acids research, 32 Database issue:D267–70.
Bodenreider, O. (2016). Identifying missing hierarchi-
cal relations in snomed ct from logical definitions
based on the lexical features of concept names. In
ICBO/BioCreative.
Bodenreider, O., Burgun, A., and Rindflesch, T.
(2001). Lexically-suggested hyponymic relations
among medical terms and their representation in the
umls.
Bodenreider, O., Burgun-Parenthoine, A., and Rindflesch,
T. (2002). Assessing the consistency of a biomedical
terminology through lexical knowledge. International
journal of medical informatics, 67 1-3:85–95.
Cabot, C., Darmoni, S., and Soualmia, L. (2019). Cimind:
A phonetic-based tool for multilingual named entity
recognition in biomedical texts. Journal of biomedical
informatics, 94:103176.
Ceusters, W., Elkin, P., and Smith, B. (2007). Negative
findings in electronic health records and biomedical
ontologies: A realist approach. International journal
of medical informatics, 76 Suppl 3:S326–33.
Chen, Y., Lasko, T., Mei, Q., Denny, J., and Xu, H. (2015).
A study of active learning methods for named entity
recognition in clinical text. Journal of biomedical in-
formatics, 58:11–18.
Consortium, G. O. (2003). The gene ontology ( go )
database and informatics resource gene ontology con-
sortium.
HEALTHINF 2022 - 15th International Conference on Health Informatics
42

Cui, L., Zhu, W., Tao, S., Case, J., Bodenreider, O., and
Zhang, G.-Q. (2017). Mining non-lattice subgraphs
for detecting missing hierarchical relations and con-
cepts in snomed ct. Journal of the American Medical
Informatics Association : JAMIA, 24:788 – 798.
Damme, P., Quesada-Mart
´
ınez, M., Cornet, R., and
Fern
´
andez-breis, J. (2018). From lexical regulari-
ties to axiomatic patterns for the quality assurance of
biomedical terminologies and ontologies. Journal of
biomedical informatics, 84:59–74.
Dernoncourt, F., Lee, J. Y., and Szolovits, P. (2017).
Neuroner: an easy-to-use program for named-entity
recognition based on neural networks. In EMNLP.
Dogan, R., Leaman, R., and Lu, Z. (2014). Ncbi disease
corpus: A resource for disease name recognition and
concept normalization. Journal of biomedical infor-
matics, 47:1–10.
Friedman, C., Shagina, L., Lussier, Y., and Hripcsak, G.
(2004). Research paper: Automated encoding of clin-
ical documents based on natural language processing.
Journal of the American Medical Informatics Associ-
ation : JAMIA, 11 5:392–402.
Ghiasvand, O. and Kate, R. J. (2018). Learning for clinical
named entity recognition without manual annotations.
Informatics in Medicine Unlocked, 13:122–127.
Habibi, M., Weber, L., Neves, M., Wiegandt, D., and Leser,
U. (2017). Deep learning with word embeddings im-
proves biomedical named entity recognition. Bioin-
formatics, 33:i37 – i48.
Hina, S., Atwell, E., and Johnson, O. (2010). Secure in-
formation extraction from clinical documents using
snomed ct gazetteer and natural language processing.
2010 International Conference for Internet Technol-
ogy and Secured Transactions, pages 1–5.
IHTSDO (2021). SNOMED International. last accessed
24/08/2021.
Ina, S., Twell, E. R. A., and Ohnson, O. W. J. (2013).
Snomedtagger : A semantic tagger for medical nar-
ratives.
Jonquet, C., Shah, N., and Musen, M. (2009a). The open
biomedical annotator. Summit on Translational Bioin-
formatics, 2009:56 – 60.
Jonquet, C., Shah, N., Youn, C., Musen, M., Callendar, C.,
and Storey, M. (2009b). Ncbo annotator : Semantic
annotation of biomedical data.
Ju, Z., Wang, J., and Zhu, F. (2011). Named entity recogni-
tion from biomedical text using svm. 2011 5th Inter-
national Conference on Bioinformatics and Biomedi-
cal Engineering, pages 1–4.
Keretna, S., Lim, C., Creighton, D., and Shaban, K. (2015).
Enhancing medical named entity recognition with an
extended segment representation technique. Com-
puter methods and programs in biomedicine, 119
2:88–100.
Kocaman, V. and Talby, D. (2021). Spark nlp: Natu-
ral language understanding at scale. Softw. Impacts,
8:100058.
Kulick, S., Bies, A., Liberman, M., Mandel, M. A., McDon-
ald, R. T., Palmer, M., Schein, A., Ungar, L., Winters,
S., and White, P. S. (2004). Integrated annotation for
biomedical information extraction. In HLT-NAACL
2004.
Lample, G., Ballesteros, M., Subramanian, S., Kawakami,
K., and Dyer, C. (2016). Neural architectures for
named entity recognition. In NAACL.
Lee, J., Yoon, W., Kim, S., Kim, D., Kim, S., So, C. H.,
and Kang, J. (2020). Biobert: a pre-trained biomedi-
cal language representation model for biomedical text
mining. Bioinformatics, 36:1234 – 1240.
Lee, W., Kim, K., Lee, E. Y., and Choi, J. (2018). Condi-
tional random fields for clinical named entity recogni-
tion: A comparative study using korean clinical texts.
Computers in biology and medicine, 101:7–14.
Liu, X., Zhou, Y., and Wang, Z. (2019). Recognition and ex-
traction of named entities in online medical diagnosis
data based on a deep neural network. J. Vis. Commun.
Image Represent., 60:1–15.
Long, W. (2005). Extracting diagnoses from discharge sum-
maries. AMIA ... Annual Symposium proceedings.
AMIA Symposium, pages 470–4.
Luo, Y.-F., Sun, W., and Rumshisky, A. (2019). Mcn: A
comprehensive corpus for medical concept normaliza-
tion. Journal of biomedical informatics, 92:103132.
Mahi, M. (2019). scispaCy for Bio-medical Named Entity
Recognition. last accessed 30/08/2021.
Ogren, P. V., Savova, G., and Chute, C. (2008). Construct-
ing evaluation corpora for automated clinical named
entity recognition. In LREC.
Ohta, T., Tateisi, Y., and Kim, J.-D. (2002). The genia cor-
pus: an annotated research abstract corpus in molecu-
lar biology domain.
Pacheco, E., Stenzhorn, H., Nohama, P., Paetzold, J., and
Schulz, S. (2009). Detecting underspecification in
snomed ct concept definitions through natural lan-
guage processing. AMIA ... Annual Symposium pro-
ceedings. AMIA Symposium, 2009:492–6.
Pakhomov, S. V. S., Coden, A., and Chute, C. (2006). De-
veloping a corpus of clinical notes manually annotated
for part-of-speech. International journal of medical
informatics, 75 6:418–29.
P
´
erez, A., Weegar, R., Casillas, A., Gojenola, K., Oronoz,
M., and Dalianis, H. (2017). Semi-supervised medical
entity recognition: A study on spanish and swedish
clinical corpora. Journal of biomedical informatics,
71:16–30.
Pyysalo, S., Ginter, F., Moen, H., Salakoski, T., and Ana-
niadou, S. (2013). Distributional semantics resources
for biomedical text processing.
Rais, M., Lachkar, A., Lachkar, A., and Ouatik, S. A.
(2014). A comparative study of biomedical named en-
tity recognition methods based machine learning ap-
proach. 2014 Third IEEE International Colloquium
in Information Science and Technology (CIST), pages
329–334.
Rector, A. and Iannone, L. (2012). Lexically suggest, logi-
cally define: Quality assurance of the use of qualifiers
and expected results of post-coordination in snomed
ct. Journal of biomedical informatics, 45 2:199–209.
Rector, A. L., Iannone, L., and Stevens, R. (2011). Quality
assurance of the content of a large dl-based terminol-
A Novel Atomic Annotator for Quality Assurance of Biomedical Ontologies
43

ogy using mixed lexical and semantic criteria: experi-
ence with snomed ct. In K-CAP ’11.
Reeve, L. H. and Han, H. (2007). Conann: An online
biomedical concept annotator. In DILS.
Roberts, A., Gaizauskas, R., Hepple, M., Davis, N.,
Demetriou, G., Guo, Y., Kola, J., Roberts, I., Setzer,
A., Tapuria, A., and Wheeldin, B. (2007). The clef
corpus: Semantic annotation of clinical text. AMIA
... Annual Symposium proceedings. AMIA Symposium,
pages 625–9.
Sasaki, Y., Tsuruoka, Y., McNaught, J., and Ananiadou, S.
(2008). How to make the most of ne dictionaries in
statistical ner. BMC Bioinformatics, 9:S5 – S5.
Savova, G., Masanz, J. J., Ogren, P. V., Zheng, J., Sohn, S.,
Schuler, K., and Chute, C. (2010). Mayo clinical text
analysis and knowledge extraction system (ctakes):
architecture, component evaluation and applications.
Journal of the American Medical Informatics Associ-
ation : JAMIA, 17 5:507–13.
Schulz, S., Mart
´
ınez-Costa, C., and Mi
˜
narro-Gim
´
enez, J. A.
(2017). Lexical ambiguity in snomed ct. In JOWO.
Settles, B. (2005). Abner: an open source tool for automat-
ically tagging genes, proteins and other entity names
in text. Bioinformatics, 21:3191–3192.
Skeppstedt, M., Kvist, M., Nilsson, G., and Dalianis, H.
(2014). Automatic recognition of disorders, find-
ings, pharmaceuticals and body structures from clin-
ical text: An annotation and machine learning study.
Journal of biomedical informatics, 49:148–58.
Sniegula, A., Poniszewska-Mara
´
nda, A., and Chomatek, L.
(2019). Study of named entity recognition methods in
biomedical field. In EUSPN/ICTH.
Soysal, E., Wang, J., Jiang, M., Wu, Y., Pakhomov, S. V. S.,
Liu, H., and Xu, H. (2018). Clamp – a toolkit for effi-
ciently building customized clinical natural language
processing pipelines. Journal of the American Medi-
cal Informatics Association : JAMIA, 25:331 – 336.
Tulkens, S., Suster, S., and Daelemans, W. (2019). Unsu-
pervised concept extraction from clinical text through
semantic composition. Journal of biomedical infor-
matics, 91:103120.
Unanue, I. J., Borzeshi, E. Z., and Piccardi, M. (2017).
Recurrent neural networks with specialized word em-
beddings for health-domain named-entity recognition.
Journal of biomedical informatics, 76:102–109.
Usami, Y., Cho, H.-C., Okazaki, N., and Tsujii, J. (2011).
Automatic acquisition of huge training data for bio-
medical named entity recognition. In BioNLP@ACL.
Vincze, V., Szarvas, G., Farkas, R., M
´
ora, G., and Csirik, J.
(2008). The bioscope corpus: biomedical texts anno-
tated for uncertainty, negation and their scopes. BMC
Bioinformatics, 9:S9 – S9.
Wang, Q., Xia, Y., Zhou, Y., Ruan, T., Gao, D., and He,
P. (2019). Incorporating dictionaries into deep neural
networks for the chinese clinical named entity recog-
nition. Journal of biomedical informatics, 92:103133.
Zhang, S. and Elhadad, N. (2013). Unsupervised biomedi-
cal named entity recognition: Experiments with clini-
cal and biological texts. Journal of biomedical infor-
matics, 46 6:1088–98.
HEALTHINF 2022 - 15th International Conference on Health Informatics
44
