
Community and Distribution of Living Coccolithophores in the
Yellow Sea and East China Sea
Jian Zhang
1
, Yi Yang
1,*
, Zhaoyang Liu
1
, Youquan Zhang
2
and Xueding Li
2
1
National Marine Data and Information Service,Tianjin, 300171, China
2
Fu Jian Marine Forecasts, Fujian, 350003, China
Keywords: Living coccolithophores, Community and distribution, Yellow Sea and East China Sea, Summer and Winter
Abstract: Based on the investigation and study on the community and distribution of Living coccolithophores (LC) in
the Yellow Sea and East China Sea in summer and winter 2016. In summer, 21 species of LC were found in
the survey area, and their dominant species were E. huxleyi, G. oceanica, U. tenuis and F. profunda. The cell
abundance ranged from 0.023 to 1.762 × 104 cells/L, with an average of 0.284 × 104 cells/L. In winter, 20
species of LC were found in the survey area, and their dominant species were E. huxleyi, G. oceanica, F.
profunda and U. tenuis. The abundance of LC ranged from 0.012 to 3.535×104 cells/L, with an average of
0.384×104 cells/L. This thesis investigated and analyzed the LC community and distribution in two seasons
(summer and winter) of the Yellow Sea and East China Sea, which enriched the LC studies in the coastal
seas of China and also provided the basic data for understanding carbon cycle and carbon flux in China Sea
waters.
1 INTRODUCTION
Living coccolithophores (LC) refer to those who live
in the sea today, with calcium carbonate shells at
certain stages of life history, and play an important
role in the marine ecosystem (Billard & Inouye,
2004). LC take a great part in the marine carbon cycle
process, and as one of the most important producers
in the marine ecosystem. With its unique carbonate
counter pump and organic carbon pump, LC take a
great part in the ocean’s carbon cycle (Sun, 2007). So
far, the community and distribution (especially
vertical distribution) of LC is still not clear in China
Sea. Based on the investigations of the communities
and distribution of LC in the Yellow Sea and East
China Sea in summer (20th July to 1st September)
and winter (23th December to 5th February) in 2016,
we made a report about the LC species composition,
cell abundance, dominant species, horizontal
distribution, vertical distribution, diversity index and
evenness index from upper body water (0 ~ 200m).
Lacking of internationally harmonized method for
quantitative sampling and sample analysis, This
study applied the polarizing microscope method
(Bollmann et al., 2002) which is widely recognized
internationally and can truly reflect quantitative
information, and its description and analysis was
made in order to provide reliable information on the
basis of the study of LC community about
distribution, and for China's coastal waters follow-up
studies of carbon fluxes, coccolithophores
calcification and its response to global climate
change and other support.
2 SURVEY AREAS AND
RESEARCH METHODS
2.1 The Survey Areas
This study was based on China's coastal waters
investigation. Respectively, in summer (July 20 to
September 1) and winter (December 23 to February
5th) of China Sea waters (27.00 ° ~ 36.50 ° N, 121.50
° ~ 127.00 ° E), including water chemistry, chemical
and biological, with a comprehensive field
investigation nested two quarters of the month. In
summer and winter, 17 and 19 survey sampling
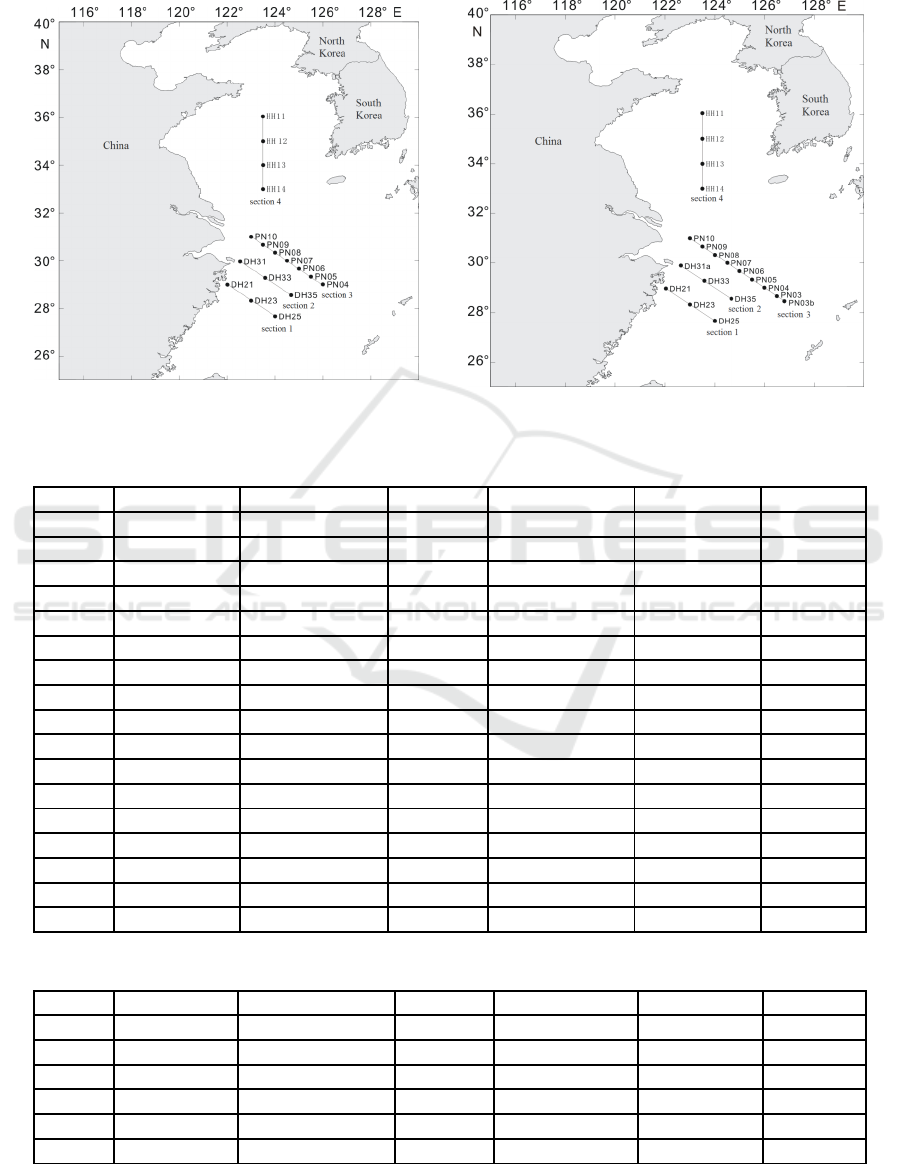
stations were set up in the survey areas (Figure 1,
Figure 2). Meanwhile, there were four sections
(section 1, section 2, section 3, section 4) located in
the survey area. The information of sampling stations
was listed in Table 1 and Table 2. LC samples were
collected from the surface (~ 2 m) below the natural
Zhang, J., Yang, Y., Liu, Z., Zhang, Y. and Li, X.
Community and Distribution of Living Coccolithophores in the Yellow Sea and East China Sea.
In Proceedings of the 7th International Conference on Water Resource and Environment (WRE 2021), pages 151-163
ISBN: 978-989-758-560-9; ISSN: 1755-1315
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
151
water by using CTD, and then poured into the sea to
get a 1L polyethylene bottle, immediately adding the
right amount of weakly alkaline solution of formalin,
making the concentration of formaldehyde in the
sample of 1% to 2% (Sun, 2007). Temperature and
salinity were determined by the ship's CTD.
Figure 1: 2016 summer survey stations bitmap. Figure 2: 2016 winter survey stations bitmap.
Table 1: Summer survey stations of 2016.
Stations Regions Date Time Longitude Latitude Depth
DH21 ECS 2016-8-20 8:29 122.0113
28.9926
15
DH23 ECS 2016-8-20 15:41 123.0002
28.3306
74
DH25 ECS 2016-8-20 23:40 124.0016
27.6627
97
DH35 ECS 2016-8-22 3:55 124.6662
28.5573
89
DH33 ECS 2016-8-22 11:07 123.5851
29.2843
69
DH31 ECS 2016-8-22 20:00 122.5414
29.9651
23
PN10 ECS 2016-8-24 17:57 123.0006
31.0004
49
PN09 ECS 2016-8-24 21:38 123.4950
30.6715
59
PN08 ECS 2016-8-25 2:19 124.0011
30.3325
51
PN07 ECS 2016-8-25 6:10 124.4998
29.9981
66
PN06 ECS 2016-8-25 10:41 124.9993
29.6716
83
PN05 ECS 2016-8-25 15:04 125.4994
29.3309
94
PN04 ECS 2016-8-25 20:25 125.9969
29.0026
117
HH14 YS 2016-8-29 14:06 123.4990
32.9972
37.4
HH13 YS 2016-8-29 23:30 123.5035
34.0009
70
HH12 YS 2016-8-30 9:00 123.5012
34.9981
77.8
HH11 YS 2016-8-31 12:23 123.4938
36.0400
75
Table 2: Winter survey stations of 2016.
Stations Regions Date Time Longitude Latitude Depth
PN03b ECS 2016-12-26 16:30 126.8008 28.4618 209
PN03 ECS 2016-12-26 19:59 126.5000 28.6667 134
PN04 ECS 2016-12-27 0:58 126.0000 29.0000 118
PN05 ECS 2016-12-27 5:11 125.5067 29.3256 94
PN06 ECS 2016-12-27 9:55 125.0000 29.6710 83
PN07 ECS 2016-12-27 13:28 124.5000 30.0000 68
WRE 2021 - The International Conference on Water Resource and Environment
152
PN08 ECS 2016-12-28 8:30 124.0028 30.3266 50
PN09 ECS 2016-12-28 11:50 123.5003 30.6683 58
PN10 ECS 2016-12-28 16:29 123.0000 31.0000 49
DH31a ECS 2016-12-29 5:06 122.6394 29.8914 36
DH33 ECS 2016-12-29 12:16 123.5792 29.2799 69
DH35 ECS 2016-12-29 19:42 124.6691 28.5618 92
DH25 ECS 2016-12-31 7:51 124.0000 27.6689 98
DH23 ECS 2016-12-31 19:52 123.0022 28.3286 78
DH21 ECS 2017-01-01 4:40 122.0384 28.9669 13.7
HH14 YS 2017-02-04 15:00 123.4989 33.0030 38
HH13 YS 2017-02-04 20:00 123.5014 33.9980 70
HH12 YS 2017-02-05 1:33 123.4990 35.0004 77
HH11 YS 2017-02-05 6:45 123.4990 36.0442 77
2.2 Research Methods
2.2.1 Sample Preparation
The samples were carried back to the laboratory,
taking 400ml to filter through polycarbonate
membrane (diameter 25mm, pore size 0.45μm), the
filter pressure is less than 100mmHg. Immediately
wash the filter membrane with weak alkaline distilled
water to remove excess salt after filtered. Then,
placed the filter in a plastic Petri dish, and set aside in
oven at 50 ℃ for drying treatment. Finally, removed
the filter after drying, clipping appropriate size with
scissors, and placed on glass slides, dropping
appropriate amount of Canada neutral resin, and then
mounted the coverslips. After the production is
finished, the samples were then put into the oven (50
℃) for 2~3 days.
The quantity of LC was carried out under the
polarizing microscope (MoticBA300pol) 1000×.
According to the characteristics of birefringence, free
pellets and stone balls were identified and counted.
According to the statistical requirements, the visual
field should be randomly selected and 300 stone or
100 stone balls should be detected as far as possible.
The dominant species are identified based on
morphological characteristics of coccolithophores
(Heimdal, 1993) by using scanning electron
microscopy (Jordan & Kleijne, 1994), and
determined the ation of the average maximum and
average grain length ball diameter.
2.2.2 The Main Formulas
The formula for calculating cell abundance of LC is
modified by Bollmann’s formula (Bollmannet al.,
2002):
sb
N
Sa
1000
A
(1)
A is the cell abundance of LC (cells / L); N is the
number of horizons observed on each slide; a is the
number of LC in the N field of vision; b is the
volume of the filtered sample (ml); S is the effective
filtration area of membrane; s is a single vision for
the polarizing microscope under 1000× area.
According to the number of stone grains per unit cell,
each species of stone balls will be transformed into
cell numbers.
Diversity index of Species (H’) is calculated by
Shannon - Wiener index:
S
i
ii
PPH
1
2
log'
(2)
Evenness index of Species (J) is calculated by
Pielou:
S
H
J
2
log
'
(3)
Dominance index (Y), which is calculated as:
i
i
f
N
n
Y
(4)
N for total number of LC cells, S is the total
number of species in the sample, ni is the total
number of individuals of the i species, Pi = ni / N is
the first species in the sample i cell abundance
probability, fi for the frequencies present in each
sample.
Using surfer9.3 and CorelDRAW to map out the
Community and Distribution of Living Coccolithophores in the Yellow Sea and East China Sea
153

data, we can get the distribution map of LC.
2.3 Results and Discussion
2.3.1 Species Composition and Dominant
Species
In summer, 21 kinds of living coccolithophores were
found in the survey area, most of them are
heterococcolithophores, only a handful of
holococcolithophores (Winter & Siesser, 1994). The
dominant species were Emiliania huxleyi,
Gephyrocapsa oceanica, Umbellosphaera tenuis,
Florisphaera profunda, Helicopontosphaera carteri
and Umbilicosphaera sibogae. Emiliania huxleyi and
Gephyrocapsa oceanica were the dominant species,
and the relative abundance of the cells were 36.77%
and 32.90%. The frequency of occurrence were 1.00;
The relative abundance of Umbellosphaera tenuis
was 14.87%, the frequency of occurrence was 0.69.
Species composition of LC is shown in Table 3.
Table 3: Species composition of living coccolithophores in summer of 2016.
Species Abundance Frequency Dominant
Emiliania huxleyi 36.77 % 1.00 0.36774
Gephyrocapsa oceanica 32.90 % 1.00 0.32896
Umbellosphaera tenuis 14.87 % 0.69 0.10223
Florisphaera profunda 3.66 % 0.38 0.01374
Calcidiscus leptoporus 3.02 % 0.34 0.00913
Umbilicosphaera sibogae 2.09 % 0.30 0.00719
Calciosolenia murrayi 1.20 % 0.22 0.00263
Syracosphaera pulchra 1.14 % 0.17 0.00171
Algirosphaera robusta 1.03 % 0.16 0.00166
Discosphaera tubifera 0.93 % 0.15 0.00145
Oolithotus antillarum 0.42 % 0.13 0.00052
Helicosphaera carteri 0.41 % 0.11 0.00049
Rhabdosphaera clavigera 0.38 % 0.10 0.00039
Umbilicosphaera foliosa 0.30 % 0.09 0.00026
Syracosphaera rotula 0.28 % 0.07 0.00019
Calicasphaera disconstricta 0.25 % 0.06 0.00018
Umbellosphaera irregularis 0.14 % 0.06 0.00008
Florisphaera profunda var. elongata 0.09 % 0.05 0.00003
Gephytocapsa ericsonii 0.06 % 0.03 0.00003
Pontosphaera discopora 0.05 % 0.01 ——
Michaelsarsia adriaticus 0.01 % 0.01 ——
In winter, 20 kinds of living coccolithophores
were found in the survey area. Most of them are
heterococcolithophores, a small number of
holococcolithophores. The dominant species is E.
huxleyi, G. oceanica, F. profunda, U. tenuis, S.
pulchra and U. sibogae. The relative abundance of
cell density in E. huxleyi and G. oceanica has an
absolute advantage in the survey area, respectively,
accounted for 42.97% and 32.06%, both the
frequency of 1.00 and 0.98, respectively; F.
profunda’s cell abundance was 5.60%, the frequency
of occurrence is 0.64. U. tenuis’s relative abundance
and frequency of cells respectively 3.54%
percentage, abundance 0.51. Species composition of
living coccolithophores is shown in Table 4.
Table 4: Species composition of living coccolithophores in winter of 2016.
Species Abundance Frequenc
y
Dominant
Emiliania huxleyi 42.97 % 1.00 0.42971
Ge
p
h
y
roca
p
sa oceanica 32.06 % 0.98 0.32064
Floris
p
haera
p
ro
f
unda 5.60 % 0.64 0.05597
Umbellos
p
haera tenuis 3.54 % 0.51 0.03545
Syracosphaera pulchra 0.61 % 0.26 0.00612
Umbilicosphaera sibogae 0.44 % 0.31 0.00444
Helicos
p
haera carteri 0.31 % 0.22 0.00306
WRE 2021 - The International Conference on Water Resource and Environment
154

A
l
g
iros
p
haera robusta 0.15 % 0.17 0.00146
Discosphaera tubifera 0.10 % 0.15 0.00101
Calcidiscus leptoporus 0.08 % 0.20 0.00077
Ge
p
h
y
toca
p
sa ericsonii 0.04 % 0.15 0.00044
Calciosolenia murra
y
i 0.04 % 0.12 0.00043
Florisphaera profunda var.
elongata
0.03 % 0.10 0.00029
Pontos
p
haera bi
g
elowi 0.02 % 0.09 0.00021
Umbellos
p
haera irre
g
ularis 0.01 % 0.07 0.00007
Oolithotus antillarum 0.01 % 0.08 0.00006
Pontosphaera discopora 0.00 % 0.03 0.00001
Syracosphaera rotula 0.00 % 0.02
——
Pontos
p
haera disco
p
ora 0.00 % 0.03
——
M
ichaelsarsia adriaticus 0.00 % 0.01
——
2.3.2 Horizontal Distribution
In summer 2016, the cell abundance of LC in the
survey area was between 0.23 ~ 17.62 × 10
3
cells / L,
with an average of 2.84 × 10
3
cells / L. Cell
abundance of Emiliania huxleyi was between 0.79 ~
7.4 × 10
3
cells / L, with an average of 1.04 × 10
3
cells / L. Cell abundance of Gephyrocapsa oceanica
was between 0.29 ~ 7.6 × 10
3
cells / L, with an
average of 0.93 × 10
3
cells / L. Umbellosphaera
tenuis was between 0 ~ 2.22 × 10
3
cells / L, with an
average of 0.42 × 10
3
cells / L. (Figure 3)
In summer, the distribution of LC in the study
area is uneven, and a high value suddenly appears at
a certain site in the investigation area. This is due to
the fact that the distribution of LC in summer is not
only affected by light and nutrients, but also
influenced by the interaction of the warm Kuroshio
in the South and the cold water mass of the Yellow
Sea in the north. At the same time, as the largest
diluted water runoff in the coastal areas of China, the
influence of Yangtze River on the distribution of LC
can not be ignored
(Honjo, 1976).
Figure 3: The distribution of living coccolithophores' cell abundance at surface water in summer of 2016 (cells / L, a:
coccolithophores; b: Emiliania huxleyi; c: Gephyrocapsa oceanica; d: Umbellosphaera tenuis)
Community and Distribution of Living Coccolithophores in the Yellow Sea and East China Sea
155
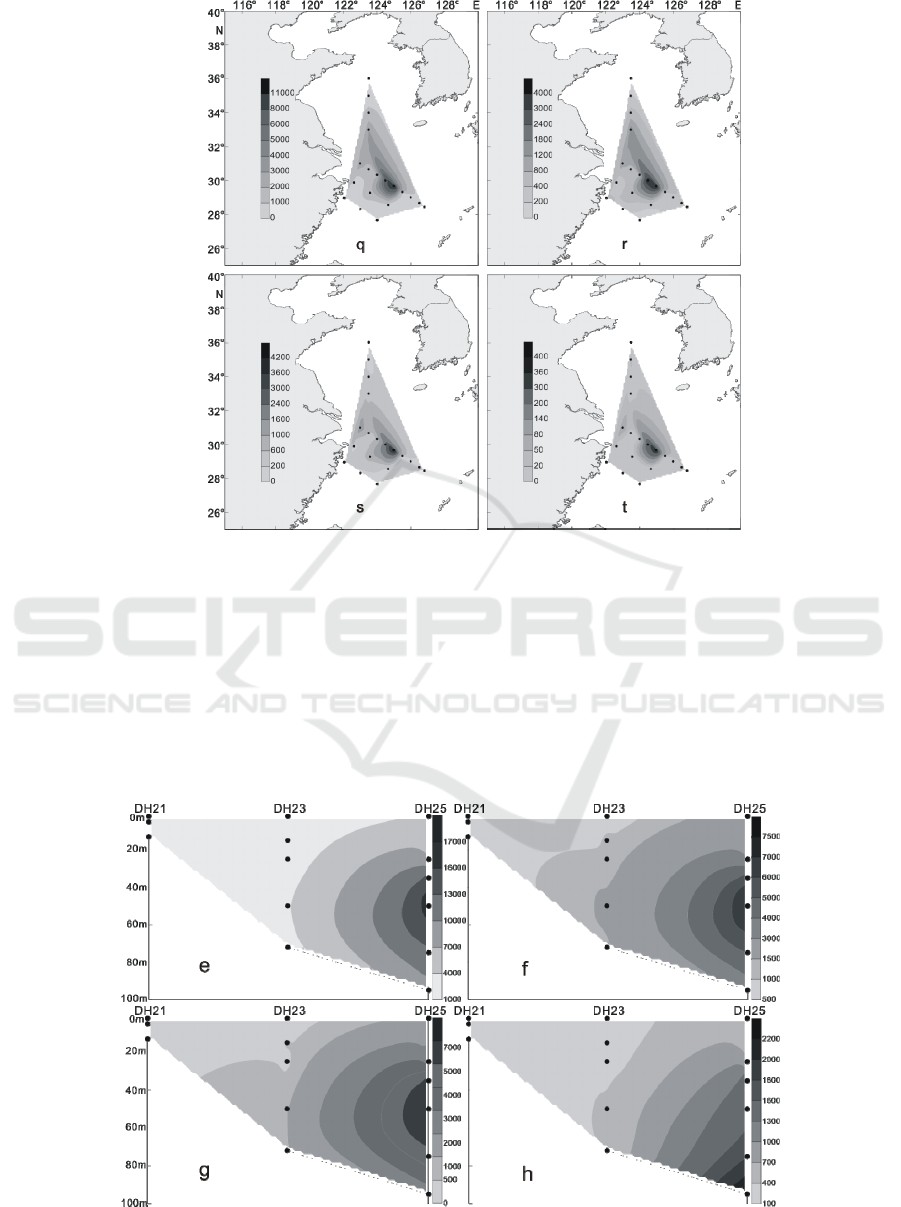
Figure 4: The distribution of living coccolithophores' cell abundance at surface water in winter of 2016 (cells / L, q:
coccolithophores; r: Emiliania huxleyi; s: Gephyrocapsa oceanics; t: Florisphaera profunda)
2.3.3 Vertical Distribution
In summer, LC were mostly located in 35 m, 50 m
and 75 m water layer, the maximum cell abundance
appears in DH25 station of section 1 of 50 m layer
of water, reaching 17.62 × 10
3
cells / L, and in the
stations of 35 m and 75 m layer, layer respectively
reached 14.83 × 10
3
cells / L and 12.46 × 10
3
cells /
L of higher value. Meanwhile, in the section 3 of
PN09 station, there’s a high value of 10.28 × 10
3
cells / L. (Figures 5-8) Vertical distribution of the
survey area presents a patchy and “bull's eyes”
distribution mode (Zou et al., 2001), a sudden
abundance of high value appears in a water layer in
some stations.
Figure 5: The vertical distribution of living coccolithophores' cell abundance in section 1 in summer of 2016 (cells / L, e:
coccolithophores; f: Emiliania huxleyi; g: Gephyrocapsa oceanica; h: Umbellosphaera tenuis) .
WRE 2021 - The International Conference on Water Resource and Environment
156
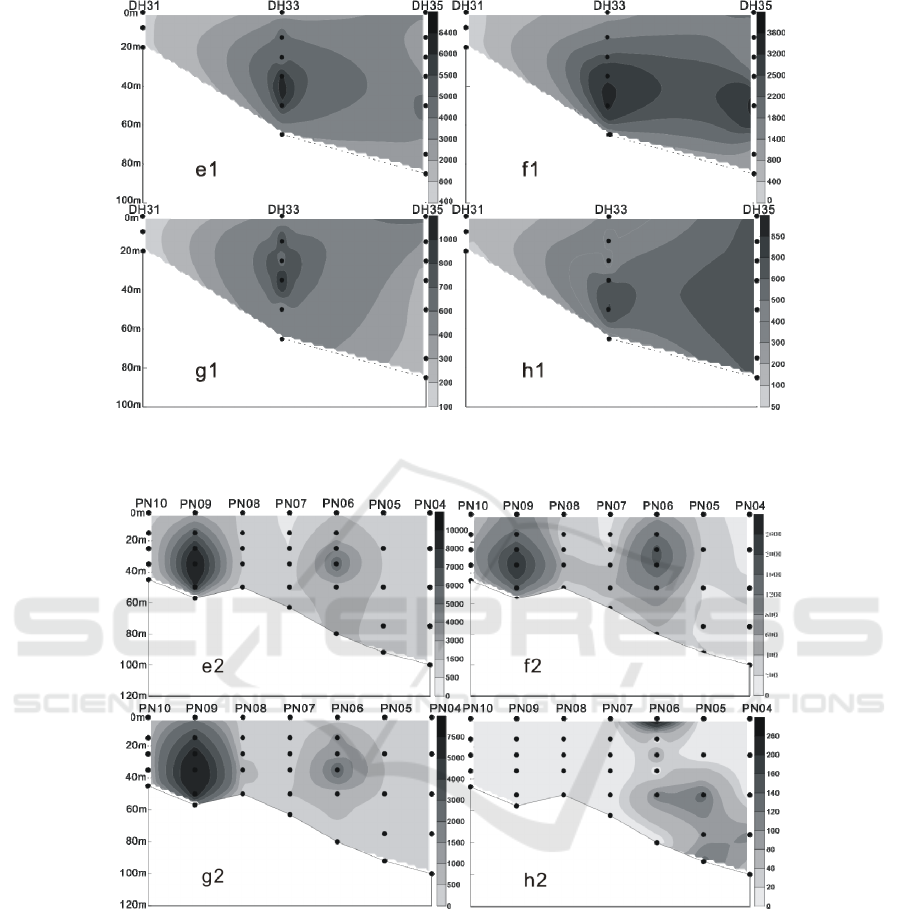
Figure 6: The vertical distribution of living coccolithophores' cell abundance in section 2 in summer of 2016 (cells / L, e1:
coccolithophores; f1: Emiliania huxleyi; g1: Gephyrocapsa oceanica; h1: Umbellosphaera. tenuis).
Figure 7: The vertical distribution of living coccolithophores' cell abundance in section 3 in summer of 2016 (cells / L, e2:
coccolithophores; f2: Emiliania huxleyi; g2: Gephyrocapsa oceanica; h2: Umbellosphaera tenuis).
In the winter of 2016, the LC in the Yellow Sea
and East China Sea were distributed in the 3 water
layers of 25 m, 50 m and 75 m. The maximum
abundance of LC appeared in the 50 m layer of the
PN06 station of section 3, and reached 35.35 × 10
3
cells / L.At the same time, the 25 m and 75 m layer
of the sation also reached 26.45 × 10
3
cells / L and
20.67 × 10
3
cells / L. In addition to the PN06
station, the high values of 8.66 × 10
3
cells / L and
8.47 × 10
3
cells / L were achieved at the 55 m layer
of PN09 and the 65 m layer of PN07. Different from
summer, the higher value of the abundance of LC
appeared in the section 4 of the survey area, which
appeared respectively at the 50 m layer of HH13
station and the 35 m layer of the HH14 station, and
the value reached 6.97 × 10
3
cells / L and 5.56 × 10
3
cells / L. (Figures 9-12) The vertical distribution of
LC in winter is similar to that in summer, also
showing the characteristics of uneven distribution,
high value abundance will suddenly appear at a
certain water level in some stations.
Community and Distribution of Living Coccolithophores in the Yellow Sea and East China Sea
157
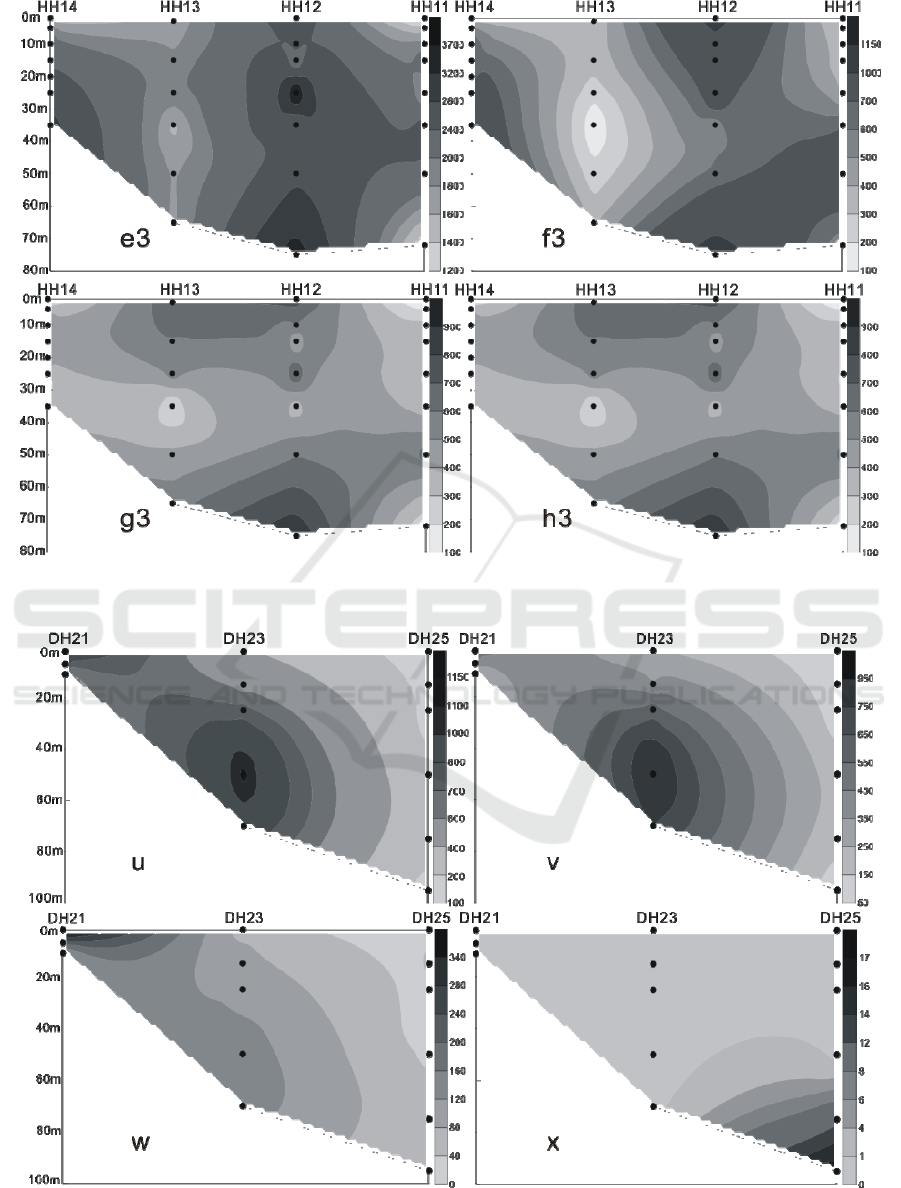
Figure 8: The vertical distribution of living coccolithophores' cell abundance in section 4 in summer of 2016 (cells / L, e3:
coccolithophores; f3: Emiliania huxleyi; g3: Gephyrocapsa oceanica; h3: Umbellosphaera tenuis).
Figure 9: The vertical distribution of living coccolithophores' cell abundance in section 1 in winter of 2016 (cells / L, u:
coccolithophores; v: Emiliania huxleyi; w: Gephyrocapsa oceanica; x: Florisphaera profunda).
WRE 2021 - The International Conference on Water Resource and Environment
158
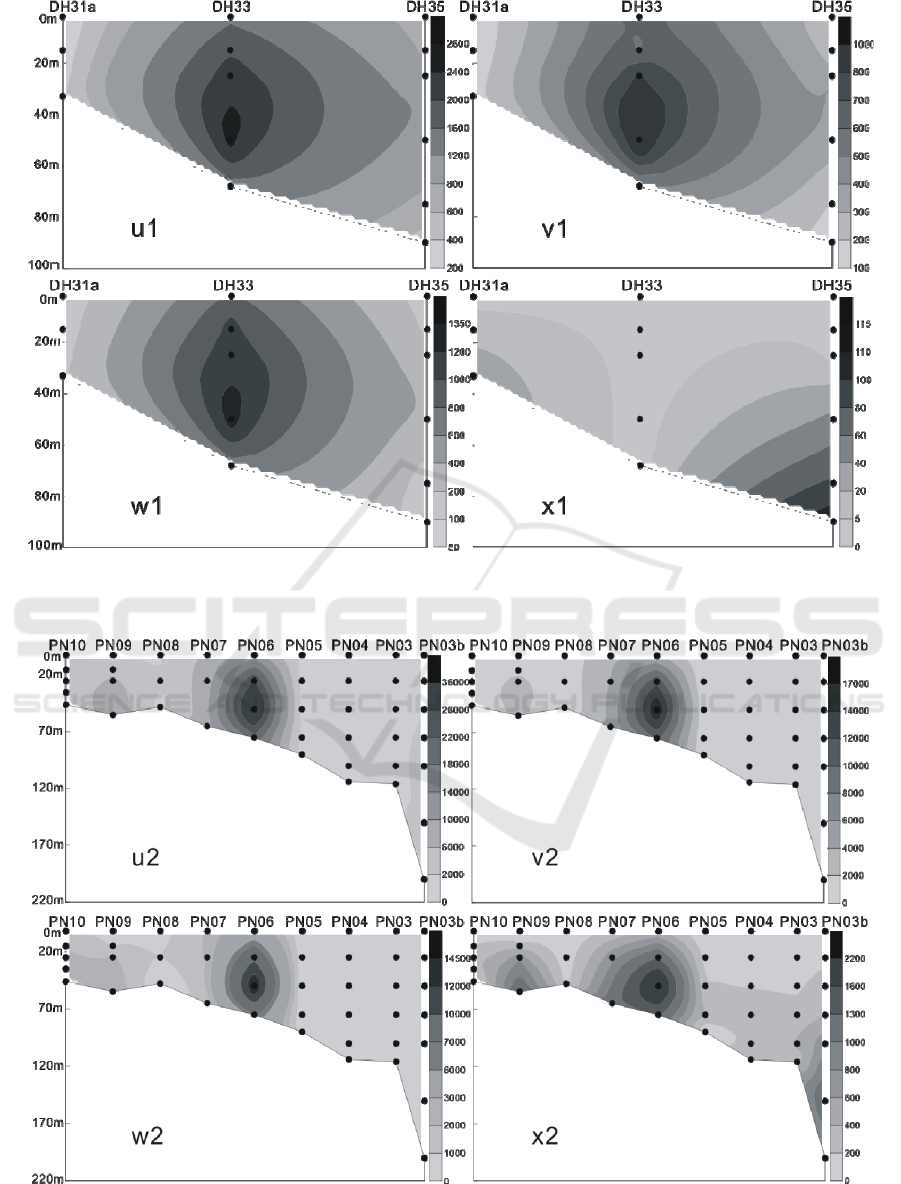
Figure 10: The vertical distribution of living coccolithophores' cell abundance in section 2 in winter of 2016 (cells / L, u1:
coccolithophores; v1: Emiliania huxleyi; w1: Gephyrocapsa oceanica; x1: Florisphaera profunda).
Figure 11: The vertical distribution of living coccolithophores' cell abundance in section 3 in winter of 2016 (cells / L, u2:
coccolithophores; v2: Emiliania huxleyi; w2: Gephyrocapsa oceanica; x2: Florisphaera profunda).
Community and Distribution of Living Coccolithophores in the Yellow Sea and East China Sea
159
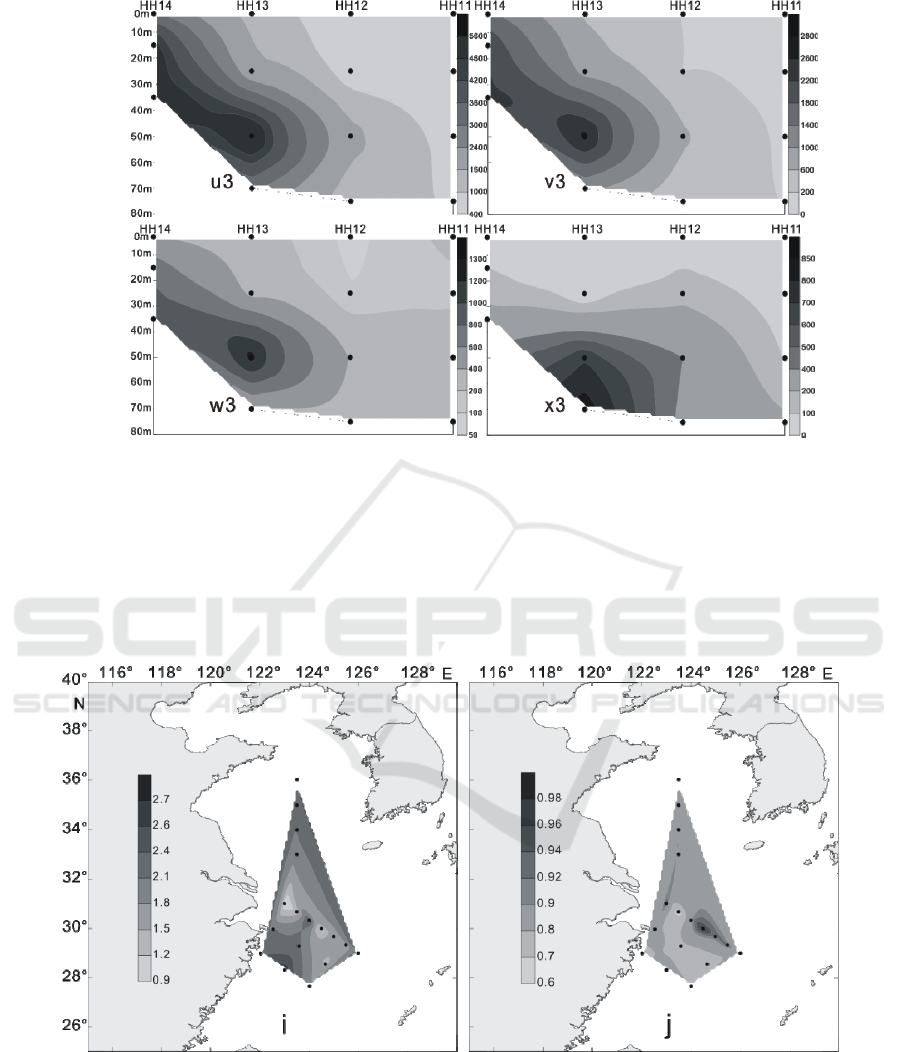
Figure 12: The vertical distribution of living coccolithophores' cell abundance in section 4 in winter of 2016 (cells / L, u3:
coccolithophores; v3: Emiliania huxleyi; w3: Gephyrocapsa oceanica; x3: Florisphaera profunda).
2.3.4 Diversity Index and Evenness
In summer, the community diversity index (Figure
13i) of LC was between 0.72 to 2.35, with an
average of 1.84. The diversity index is higher in the
north and south of the investigation area and the
adjacent sea area, and there is a high value in the
PN08 station in the central area. The evenness index
(Figure 13j) was between 0.49 to 0.99, with an
average of 0.82, which has a higher value in the
central and eastern waters of the study area.
Figure 13: Distribution of Shannon-Wiener diversity index and Pielou evenness index at surface water of survey area in
summer of 2016 (i: Shannon-Wiener diversity index; j: Pielou evenness index).
In the winter of 2016, the species diversity index
(Figure 14y) in the Yellow Sea and East China Sea
was between 1.11~ 2.62, with an average of 1.85.
The highest value of species diversity index appears
in HH12 station and its adjacent waters. The
diversity index of the whole survey area is higher in
the northern, Eastern and southern areas and
adjacent sea areas, and the stations with higher
values are HH12, PN06 and DH35.The distribution
of Pielou evenness index and the distribution of
WRE 2021 - The International Conference on Water Resource and Environment
160
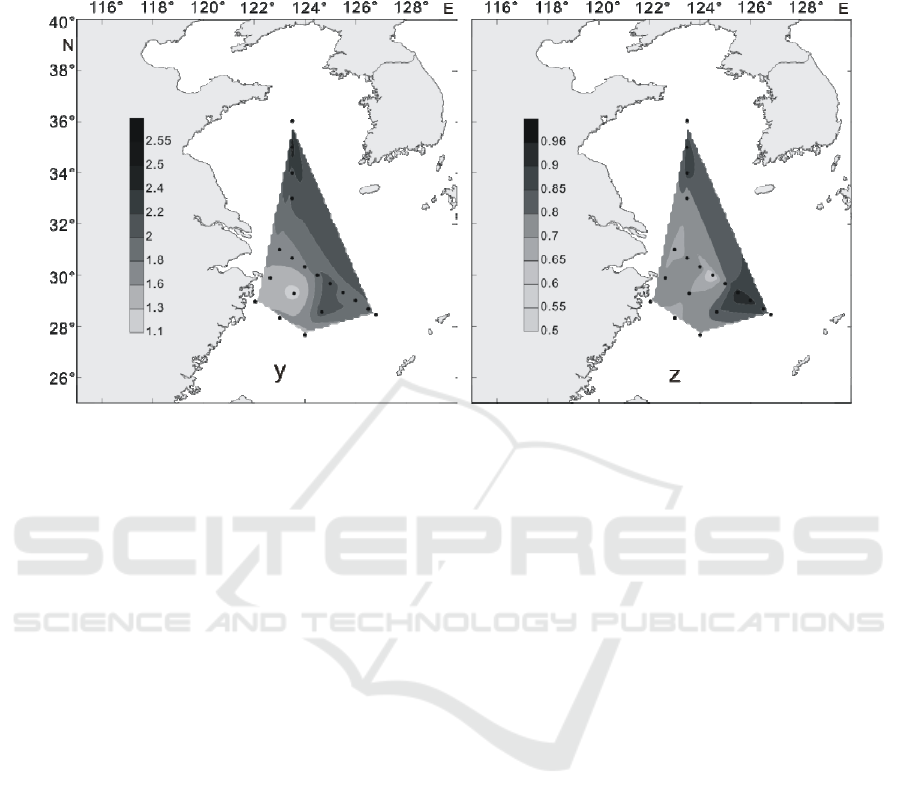
diversity index in the investigation area showed a
more consistent feature, with a value of 0.57~0.95,
with an average value of 0.78 (Figure 14z), which
had a higher value in the middle and adjacent waters
of the investigation area.
Figure 14. Distribution of Shannon–Wiener diversity index and Pielou evenness index at surface water of the survey area in
the winter of 2016(y: Shannon-Wiener diversity index; z: Pielou evenness index).
2.3.5 Distribution of Surface Temperature and
Salinity of the Survey Area
In the summer of 2016, the distribution of surface
temperature and salinity in the Yellow Sea and East
China Sea was shown in figure 15. The surface
temperature of the investigated area is 25.52 ℃ ~
29.99 ℃, with an average value of 28.34 ℃. The
highest value of temperature appeared in the eastern
and southern parts of the investigation area, and the
highest value appeared at DH33 station, with a value
of 29.99 ℃. At the PN10 station closest to the
Yangtze River Estuary, the temperature reaches a
minimum of 25.52 ℃. As can be seen from figure
15o, the trend of surface temperature distribution in
the Yellow Sea and East China Sea area in summer
is from north to south, from nearshore to distant sea,
and the temperature is getting higher and higher.
The salinity of the surface layer in summer survey
area is 22.39~33.69, with an average of 29.96. The
high salinity area appeared in the eastern part of the
survey area, and the highest value appeared at PN04
station, reaching 33.69. At the southern DH35
station, salinity reached a minimum value of 22.39.
As can be seen from figure 15p, the surface salinity
of Yellow Sea and East China Sea in summer is
greatly influenced by the Yangtze River fresh water.
The surface salinity of the near shore is low, and it is
increasing along the direction of fresh water to the
outer sea.
In the winter of 2016, the distribution of surface
temperature and salinity in the Yellow Sea and East
China Sea was shown in figure 16. The surface
temperature of the investigated area is
7.63~22.87 ℃, with an average value of 15.73 ℃.
The highest value of temperature appeared in the
southeastern part of the investigation area, and the
highest value appeared at PN03b station, with a
value of 22.87 ℃. Meanwhile, at the North HH11
station, the temperature reached a minimum of
7.63 ℃. As can be seen from figure 16E, the surface
temperature distribution trend in winter is similar to
that in summer, which is from north to south, from
near shore to far sea, and the temperature is getting
higher and higher.
The salinity of the surface layer in winter survey
area is 27.06~34.58, with an average of 33.24. The
high salinity area appeared in the eastern part of the
survey area, and the highest value appeared at PN03
station, reaching 34.58. At the DH21 station near the
Gulf of Hangzhou, salinity reached a minimum
value of 27.06. As can be seen from figure 16F, the
surface salinity in winter is still influenced by inland
runoff, and the surface salinity is low in the near
shore, increasing along the direction of fresh water
to the outer sea.
Community and Distribution of Living Coccolithophores in the Yellow Sea and East China Sea
161
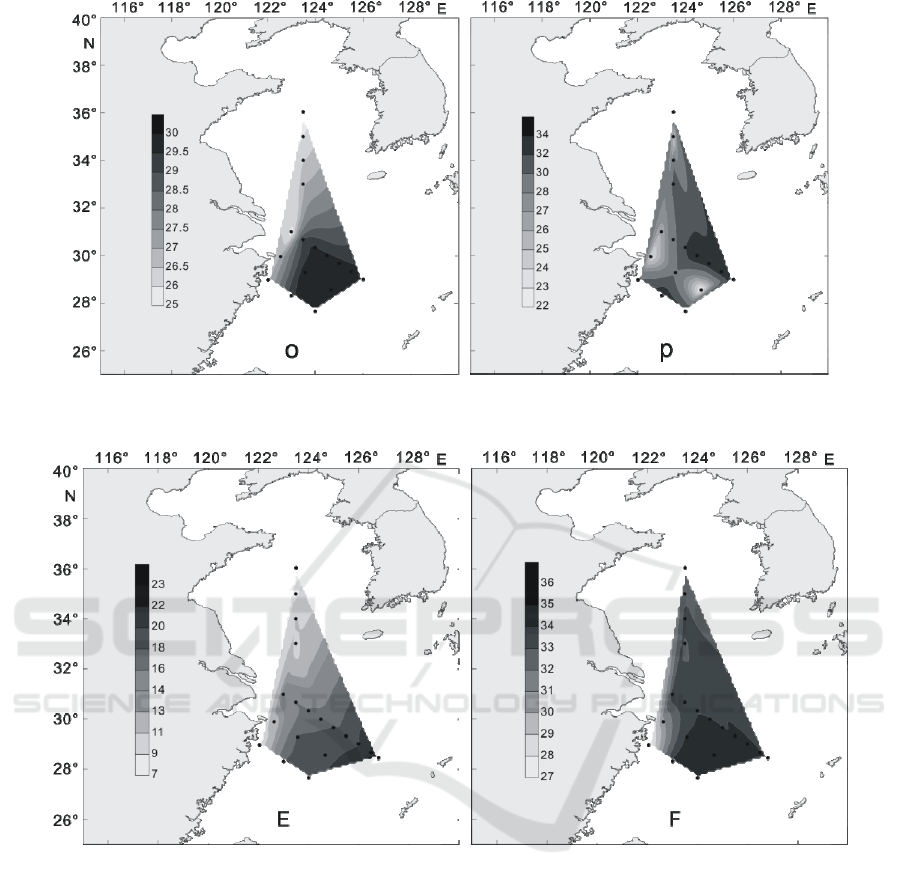
Figure 15. The distribution of temperature and salinity in surface layer of the survey area in summer of 2016 (o: the
distribution of temperature in surface layer (℃); p: the distribution of salinity in surface layer)
Figure 16: The distribution of temperature and salinity in surface layer of the survey area in winter of 2016 (E: the
distribution of temperature in surface layer (℃); F: the distribution of salinity in surface layer).
3 SUMMARY
This paper mainly studied the community and
distribution of LC in the summer of 2016 (July 20th
to September 1st) and winter (December 23rd to
February 5th) of the Yellow Sea and East China Sea
in China.
21 kinds of living coccolithophores were found in
the survey area in summer, most of them are
heterococcolithophores, only a few of
holococcolithophores. The dominant species were
Emiliania huxleyi, Gephyrocapsa oceanica,
Umbellosphaera tenuis, Florisphaera profunda,
Helicopontosphaera carteri and Umbilicosphaera
sibogae. The cell abundance of LC in the survey
area was between 0.23 ~ 17.62 × 10
3
cells / L, with
an average of 2.84 × 10
3
cells / L.The high value
areas of these dominant species usually appear in the
southern part of the investigation area and the
surrounding waters. This is due to the fact that the
distribution of LC is not only affected by light and
nutrients, but also influenced by the interaction of
the warm Kuroshio in the South and the cold water
mass of the Yellow Sea in the north
(Yang & We i,
2003). At the same time, the influence of the
Yangtze River is very significant.
WRE 2021 - The International Conference on Water Resource and Environment
162

20 kinds of living coccolithophores were found in
the survey area in winter, and Most of them are
heterococcolithophores. The dominant species were
E. huxleyi, G. oceanica, F. profunda, U. tenuis, S.
pulchra and U. sibogae. The cell abundance of LC
was between 0.12 ~ 35.35 × 10
3
cells / L, with an
average of 3.84 × 10
3
cells / L. Compared with
summer, the abundance of LC increased greatly in
winter, but the species of the dominant species
changed little. The high value area of the abundance
of LC appeared in the middle and eastern waters,
and its abundance coverage is much wider than that
in summer.
The reasons for the difference between summer
and winter were mainly due to the effect of strong
monsoon in the sea area in winter, and the effect of
upwelling in the shelf of Yellow Sea and East China
Sea was remarkable. The upwelling transported
nutrients from bottom of the sea water to the upper
water body in a certain area
(Yu et al., 2006)., and
the warm Kuroshio water also changed the living
environment of LC to a great extent. Together, they
give the necessary nutrients and environment for the
growth of the algae, resulting in higher cell
abundance.
ACKNOWLEDGMENTS
We are grateful to the research vessel Xiang Yang
Hong 01, for providing the seawater samples. The
research was funded by National Key R&D Program
of China (2016YFC1401906).
REFERENCES
Billard, C., & Inouye, I. (2004). What is new in
coccolithophore biology? In: Coccolithophore: from
molecular processes to global impact. Thierstein H R,
Young J R, eds. Berlin: Springer-Verlag Berlin, 35-37.
Bollmann, J., Cortés, M. Y., & Haidar, A. T. (2002).
Techniques for quantitative analyses of calcareous
marine phytoplankton. Marine Micropaleontology, 44,
163-185.
Heimdal, B. R. (1993). Modern coccolithophorid. In:
Tomas C R, eds. Identifying Marine Phytoplankton.
United Kingdom: Academic Press, 147-247.
Honjo, S. (1976). Coccoliths production, transportation,
sedimentation. Marine Micropaleontology, 1, 65-79.
Jordan, R. W. & Kleijne, A. (1994). A classification
system for coccolithophores. In: Winter A, Siesser W
G, eds. Coccolithophores. United Kingdom:
Cambridge University Press, 83-105.
Sun, J. (2007). Organic carbon pump and carbonate
counter pump of living coccolithophorid. Advances in
earth science, 22(12), 1231-1239.
Winter, A., & Siesser, G. (1994). Atlas of
coccolithophores. In: Winter A and Siesser W G, eds
Coccolithophores. Cambridge: Cambridge University
Press, 107-159.
Yang, T. N., & Wei, K. Y. (2003). How many coccoliths
are there in a coccosphere of the extant
coccolithophorids? A compilation. Journal of
Nannoplankton Research, 25, 7-15.
Yu, F., Zhang, Z., Diao, X. Y., Guo, J. X., & Tang, Y. X.
(2006). Analysis of evolution of the Huanghai Sea
Cold Water Mass and its relationship with adjacent
water. Acta Oceanologica Sinica, 28(5), 26-34.
Zou, E. M., Guo, B. H., Tang, Y. X., Li, Z. X., & Li, X. Z.
(2001). A number of hydrological characteristics and
circulation analysis in Southern Yellow Sea and
Northern East China Sea in summer. Oceanologia et
Limnologia Sinica, 32(3), 340-348.
Community and Distribution of Living Coccolithophores in the Yellow Sea and East China Sea
163
