
The Effects of High Fat High Carbohydrate (HFHC) Diet on Body
Weight in Overweight Sprague Dawley Rats
Novia Zuriatun Solehah
1a
, Adi Prayitno
2,3 b
and Eti Poncorini Pamungkasari
2,4 c
1
Postgraduated Student of Human Nutrition Science, Universitas Sebelas Maret, Surakarta, Indonesia
2
Postgraduated Program of Nutrition Science, Universitas Sebelas Maret, Surakarta, Indonesia
3
Departement of Pathology Anatomic, Faculty of Medicine,Universitas Sebelas Maret, Surakarta, Indonesia
4
Departement of Public Health, Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia
Keywords: High Fat High Carbohydrate Diet, Body Weight, Overweight.
Abstract: Excessive body weight is caused by an inappropriate diet. Foods that are high in fat and carbohydrates may
lead to overweight. When overweight condition is followed by an increase in the body fat level, it also
increases the production of Reactive Oxygen Species (ROS) in the blood circulation and adipose cells. This
study aims to determine the effect of High Fat High Carbohydrate (HFHC) diet on body weight of overweight
Sprague dawley rats. This study used an experimental design with a pre-post-test control group design. Male
Sprague dawley rats (n=10) were randomized into two groups. The normal group was given standard rats and
the overweight group was given diet HFHC for 14 days. Their body weights were measured before and after
treatment. After 14 days of treatment, the body weight mean was (210 ±1,5) in the treatment group increased
significantly, compared with body weight mean (193±1,3) and before treatment (183,2±1,6) in the control
group. In conclusion, those induced to be overweight used HFHC diet for 14 days and increased body weight.
This finding can be used to induce overweight rats for a short time.
1 INTRODUCTION
Global prevalence of overweight rose trifold (1.9
billion) of world population and around 13.6% in
Indonesia by 2018 (Khadaee and Saidi, 2016; Rebello
et.al., 2020; Riskesdas, 2018). Overweight and
obesity is the fifth leading cause of mortality globally
(Sudargo et.al., 2018). Overweight is a starting point
for obesity which then will lead to various metabolic
syndromes including diabetes mellitus, hypertension,
stroke, coronary heart disease, dyslipidaemia and
cancer (Muhammad et.al., 2019). Results of a study
stated that the increase of Body Mass Index (BMI)
within five years between the age of 25-74 years
significantly increased the risk of hypertension up to
30% compared to people without an increase of BMI
(Hall et.al., 2019). In addition, a rise in body weight
may trigger insulin resistance of peripheral tissue
through disfunction or adipose tissue lipotoxicity,
inflammation, dysfunction of mitochondria,
a
https://orcid.org/0000-0003-0827-2695
b
https://orcid.org/0000-0001-5548-4848
c
https://orcid.org/0000-0002-4197-3226
hyperinsulinemia and endoplasmic reticulum stress
mechanisms (ER) (Longo et.al., 2019).
The accumulation of excess fat causes the
release of high amount of free fatty acid to various
organs thus generating metabolic syndrome. The
prevalence of metabolic syndrome grows as the
prevalence of obesity rises. One of the factors that
sets overweight off is excessive fat and carbohydrate
intake in addition to a sedentary lifestyle. In recent
days, the diet pattern of Indonesians shifts as it
receives heavy influence from western diet pattern
where people consume more high fat and high
carbohydrate food (Fernandez et.al., 2018). Food
with high content of carbohydrate, protein, and fat
may contribute to the increase of oxidative stress and
cause inflammation by forming white adipose tissue
which secretes proinflammation factor (Tan et.al.,
2018; Tan and Norhaizan, 2019). Increased oxidative
stress is the main cause of numerous metabolic
diseases.
106
Solehah, N., Prayitno, A. and Pamungkasari, E.
The Effects of High Fat High Carbohydrate (HFHC) Diet on Body Weight in Overweight Sprague Dawley Rats.
DOI: 10.5220/0010760400003235
In Proceedings of the 3rd International Conference on Social Determinants of Health (ICSDH 2021), pages 106-110
ISBN: 978-989-758-542-5
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

Imbalanced energy intake and outtake give rise
to excessive fat accumulation not only in adipose
tissue but also in ectopic tissue like the liver. If
prolonged, it will cause excessive fat in liver thus lead
to impaired liver function, fatty liver and liver failure
(Rahman et.al., 2017). The sign of accumulated fat in
ectopic tissue is the increased percentage of visceral
fat in intra-abdominal linked to abdominal obesity
(Tchernof et.al., 2013). Also, excessive fat and
carbohydrate intake may change oxygen metabolism
which then triggers oxidation reaction. Excessive
intake may also induce enlargement of adipose tissue
through adipocyte hypertrophy and hyperplasia. The
imbalance between ROS and antioxidants causes an
increase in oxidative stress, causing systemic
inflammation.
Observing overweight model mice with diet
induction can give illustration of the effect of poor
eating habits among human. Several studies in animal
used high fat high carbohydrate feed in inducing
overweight to animals. This diet is believed to be the
best method in following the pathogenesis of the
development of metabolic syndrome, one of which is
obesity (Panchal et.al., 2011). Although, several
studies conducting the induction of overweight
animal models require quite a long time. Therefore,
this study aimed to examine the effect of the high fat
high carbohydrate (HFHC) diet on overweight rats for
14 days.
2 MANUSCRIPT PREPARATION
2.1 Animal Experimental Protocol
This study has received approval from the Health
Research Ethics Commission, Faculty of Medicine,
Sebelas Maret University Surakarta (KEPK UNS)
No.23/UN27.06.6.1/KEP/EC/2021.
2.2 Samples
The samples in this study were Rattus norvegicus
strain male Sprague dawley rats aged 8-12 week with
the average of weight of 150-200 gram. The subjects
are 10 rats divided into normal or control groups and
HFHC diet group. The subjects were obtained from
the Nutrition Laboratory of the Food and Nutrition
Studies Centre PAU, Gadjah Mada University,
Yogyakarta.
2.3 Study Design
This is an experimental study with pre posttest with
control group design. 10 rats are divided into two
groups, control group and HFHC diet group. Control
group was given standard Comfeed which was AD II
feed. All the rats were acclimatized for seven days in
individual cages and were given standard Comfeed
AD II with ad libitum drinking water. Every 100 gram
of standard AD II feed contains 12% water, 7% ash,
15% crude protein, 3-7% crude fat, 0.9-11% calcium,
0.6-0.9% phosphor, antibiotic, and coccidiostat
maximum 20 mg/h. The raw materials used include
yellow corn, SBM, MBM, CGM Palm olein, essential
amino acid, essential mineral, premix, and vitamin.
The average feed intake was 5 g/100gBB/h. The rat
rearing cage was a plastic cage sized 25 cm x 15 cm
x 7 cm. Each cage is used by only one rat. The
subjects were reared in a specific room with
controlled temperature (27-29ºC) inside a hygienic
polypropylene cage. The room is set with a 12 hours
light and 12 hours dark cycle (lights were turned on
at 07.00 pm, and 70-90% humidity. The rats were
considered stable if the feed consumption and drink
was sufficient. The HFHC group was given HFHC
feed. The composition of the HFHC feed includes 5%
cheese, 10% egg yolk, 15% cow fat, oil 15%, rice
45% and standard feed 20% (Ardiansyah et.al.,
2018). The HFHC diet was given for 14 days. All the
feed was given ad libitum. In a study conducted by
Adriansyah et al, the induction of obese rats was
carried out for 8 weeks. And in this study, HFHC feed
was added with 0.02 g of cholic acid wihch functions
as a fat binder in the body and produce overweight
rats in a shorter time.
2.4 Anthropometry Measurement
The body weight measurement was conducted using
a digital scale. It was a digital scale with 0.1 accuracy.
The measurement was done three times, after 7 days
acclimatization period, after 7 days diet induced
overweight, and after 14 days diet induced
overweight right before termination.
2.5 Statistical Analysis
The statistical analysis was conducted using SPSS
version 16.0. The data was presented with a mean
value and standard deviation. To analyse the mean
difference between groups a paired t test and oneway
anova was performed. The result is considered
significant if the p value is <0.05.
The Effects of High Fat High Carbohydrate (HFHC) Diet on Body Weight in Overweight Sprague Dawley Rats
107
3 RESULT AND DISCUSSION
The development of overweight model rats was
carried out by feeding HFHC. The difference in the
composition of HFHC with the standard can be seen
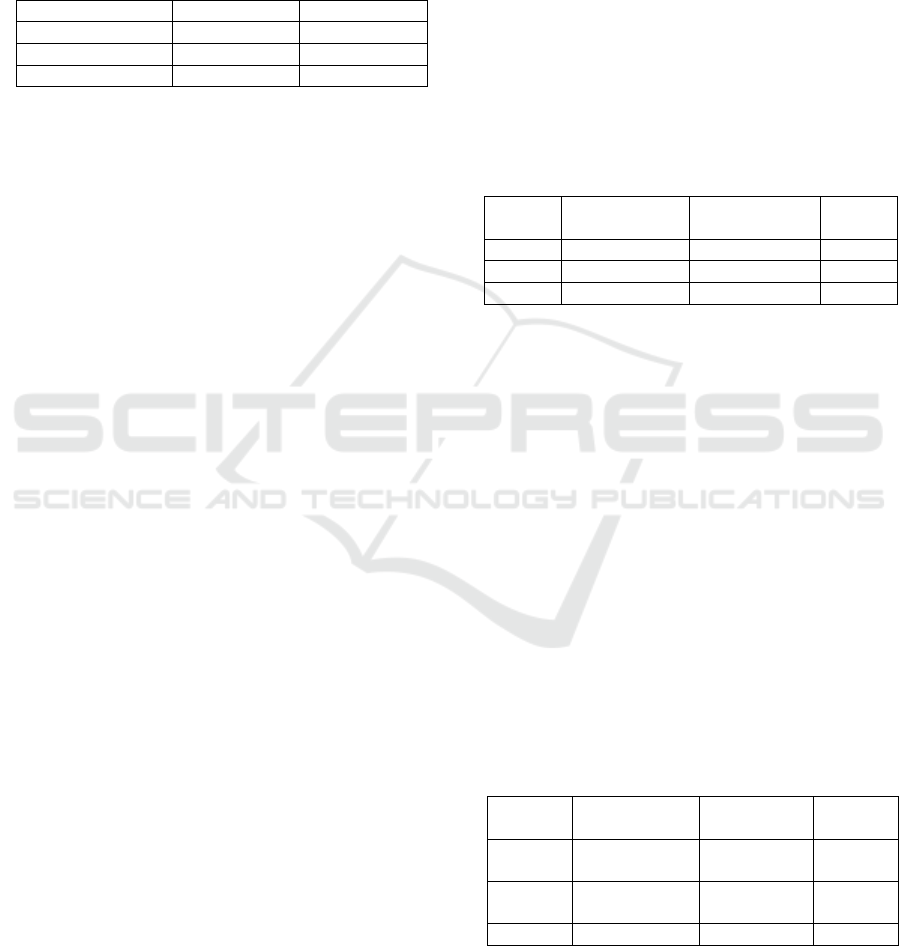
in Table 1.
Table 1: Composition of Standart and HFHC
In
g
redients Standar
d
HFHC
Carbohydrate 53-57 % 67.69%
Protein 16% 22%
Fat 3-7% 74%
Table 1 shows that the composition of HFHC feed
contains higher carbohydrates and fats compared to
standard feed. The body weight increased due to the
administration of feed with 67.69% carbohydrate and
74% fat from the total calorie. Results displayed that
consumption of fat >30% of total calories can cause
obesity (Hariri and Tibault, 2010). In this study, the
composition of the HFHC feed includes cheese, egg
yolk, cow fat, oil and rice. In addition, the mice
induced with HFHC feed tend to consume a lot of
water. Water is essential in digestive system
especially during the enzymatic hydrolysis and lipid
absorption. Lipase enzyme plays a role to hydrolyse
triglycerides into diglycerides, monoglycerides fatty
acid and glycerol (Udomkasemsab and Prangthip,
2019). The animal response to the diet given to them
can be used to evaluate the rate of success during the
overweight induction. Thus, the composition of
HFHC diet compared to control group’s standard diet
must have a similar basic nutritional content except
for the types of the macronutrients, namely
carbohydrate and fat. For this research, the
composition of macronutrients in HFHC diet include
cheese, cow fat, egg yolk, and rice.
On the other hand, the composition of
macronutrients in control group’s standard feed
included yellow corn as a carbohydrate. The content
of carbohydrate and fat in the HFHC diet is
significantly higher compared to the standard feed
which only contains 53-57% carbohydrate and 3-7%
fat. Results of the systematic review conducted by
Tibault and Hariri (2010) demonstrated that the best
method to induce obesity into mice is by using high
semi-pure fat which contains 40% animal fat with a
low amount of amino acid omega 3 and pure
vegetable oil and high in amino acid omega 6 and
omega 9. The composition of selected raw ingredients
will influence the amount consumed. This will also
affect the increase of the body weight. The
consumption of food high in fat, sugar, and salt
contributes to the occurrence of overweight and
obesity by changing the expression of gene and
sending dopamine signal in the brain. Several
researches mentioned that high fat diet is a source of
fat which in it includes saturated fatty acid (de Moura
e Dias et.al., 2021). Results indicated that there is a
positive association between body adiposity and HFD
including saturated fatty acid (Norris et.al., 2017).
Weighing was carried out to evaluate whether
there was a difference in the weight of rats fed HFHC
for 14 days with rats fed only standard feed. Rats were
weighed 3 times before HFHC induction, after HFHC
induction on the 7 days, and after HFHC induction on
the 14 days. The average of body weight before and
after induction are presented in Table 2.
Table 2: Average on Body Weight Before and After
Induced HFHC
Group
Pre test
(Mean ± SD)
Post test
(Mean ± SD)
p
Normal 183,2 ± 3,70 193,0 ± 2,92 >0.005
a
HFHC 184,2 ± 3,77 210,6 ± 3,36 >0.005
a
p
>0.005
b
>0.005
b
In table 2 shows that the administration of the
HFHC diet for 14 days significantly increased body
weight (p>0.005), the mean body weight rose from
184,2 ± 3,77 to 210,6 ± 3,36. Within control group
which was given standard feed, the mean body weight
was lower than the HFHC group. This supports the
findings of a study conducted by Wong (2018) which
concluded that HFHC diet for 6 weeks significantly
increased rats body weight to obesity. Other study on
Sprague dawley rats displayed that the administration
of high fat diet of 61% for 8 weeks did develop
obesity and increase visceral fat accumulation
(Udomkasemsab and Prangthip, 2019).
Rats overweight determined based on the Lee
index. The rats stated if overweight index value Lee
> 300. Heavy rats soul uses scales and the height is
measured using ruler. The average of Lee index can
be seen in Tabel 3.
Table 3: Indicator of Overweight
Grou
p
Post test
(Mean ± SD)
Lee Indekx p
Normal
193,0 ± 2,92 285,97
±1,77
>0.005
HFHC
210,6 ± 3,36 324,76
±4,62
>0.005
p
>0.005
*
>0.005
*
Consumption of food high in fat can cause
excessive fat accumulation in our body which then
resulted in the increase of free fatty acid in adipose
ICSDH 2021 - International Conference on Social Determinants of Health
108

tissue and release a high level of triglycoid (Marques
et.al., 2016). Also, excessive fat accumulation will
also be stored in ectopic tissue like liver, muscle, and
heart which will be indicated by hypertrophy and
hypoxia thus lead to inflammation and insulin
resistance (Longo et.al., 2019).
The incidence of overweight and obesity occur
due to the intake of fat that is way higher than the
outtake thus the excess will be stored in the form of
fat in our body, especially adipose tissue. The
increase in adipose tissue mass causes changes in
adipokine production where adipose tissue plays a
role in energy storage which is an important
endocrine. Furthermore, the enlargement of adipose
tissue and the increase of adipocyte progressive may
disturb the blood flow (McArdle et.al., 2013; Exley
MA et.al., 2014). High fat diets have been proven to
increase body weight, fat deposition, increase of
oxidate stress biomarkers, increase of fasting plasma
glucose and insulin. The occurrence of inflammation
around the blood vessel is triggered by the
accumulation of fat in cellular molecules by releasing
inflammation adipocytes like tumour necrosis factor
(TNF) and interleukin-6 (IL-6).
In overweight, the production of adipocytes is
disrupted, including leptin, resistin, adiponectin,
Monocyte Chemoattractant Protein-1 (MCP-1),
Tumor Necrosis Factor-α (TNF-α), and interleukin-6
(IL-6) (Mesquida et.al., 2020). Animal research
conducted by Feillet-Coundray et.al. (2019) showed
that the administration of high fat/high fructose cause
overweight, glucose intolerance, and increase of IL-
6. Analysis results review done by Tan et.al. (2018)
mentioned that high fat diet for 12 months increased
plasma triglycerides and total cholesterol which led to
increase of oxidative stress biomarker in blood. High
fat diet not only worsen lipid profile but also increase
the accumulation of ROS and trigger damage to
mitochondria. The mechanism of inflammation in
adipose tissue is caused by the activation of
proinflammation line in this case being nuclear
factor-kappaB- (NF-κB-). The occurrence of this
inflammation can be detected by the increase
inflammation biomarker and cytokines. In addition,
the occurrence of inflammation is also linked to the
increase of oxidative stress (de Melo et.al., 2017).
Overweight and obese people go through an increase
in the production of raective oxygen species (ROS)
due to imbalance between prooxidant and antioxidant
(Bondia-Pons et.al., 2012; Yosika et.al., 2020). This
condition contributes to the incidence of metabolic
disordersnamely insulin resistance, type 2 diabetes
mellitus, hyperlipidaemia, and atherosclerosis.
4 CONCLUSIONS
Feeding HFHC diet for 14 days can increase body
weight in overweight induced mice. However, further
studies are needed to further analyse the effect of
HFHC to overall health. Furthermore, this study can
be used as a reference to rear overweight model mice
in shorter period of time.
ACKNOWLEDGEMENTS
We would like to thank all staffs in Laboratory of
Food and Nutrition Study Centre of PAU Gadjah
Mada University Yogyakarta for the support in
rearing the rats during this study.
REFERENCES
Ardiansyah SA, Hidayat DS, and Simbolon NS. 2018. Uji
Aktivitas Antiobesitas dari Ekstrak Etanol Daun
Malaka (Phyllanthus emblica L.) Terhadap Tikus Putih
Jantan Galur Wistar. Indonesian Journal of
PharmaceuticalScience and Technology.
Bondia-Pons I, Ryan L, and Martinez JA. 2012. Oxidative
Stress and Inflammation Interactions in Human
Obesity. Journal Physiol Biochem.
de Mello AH, Costa AB, Engel JDG, and Rezin GT. 2017.
Mitochondrial Dysfuntion in Obesity. Life Sciences.
de Moura e Dias M, dos Reis SA, da Conceicao LL, de
Oliveira Sediyama CMN, Pereira SS, de Oliveira LL,
do Carmo Gouveia Peluzio M, Martinez JA, and
Milagro FI. 2021. Diet-Induced Obesity in animal
Models: Points to Consider and Influence on Metabolic
Markers. Diabetology & Metabolic Syndrome.
Exley MA, Hand L, O’Shea D, and Lynch L. 2014.
Interplay Between the Immune System and Adipose
Tissue in Obesity. Journal of Endrocrinology.
Feillet-Coudray C, Fouret G, Vigor C, Bonafos B, Jover B,
Blachino-Zabielska A, Rieusset J, Casas F, Gaillet S,
Landrier JF, Durand T, and Coudray C. 2019. Long-
Term Measurres of Dyslipidemia, Inflamation, and
Oxidative Stress in Rats Fed a High-Fat/High-Fructose
Diet. Lipids.
Fernandez AM, Rimon MG, Vera G, Astier J, Landrier JF,
and Miguel M. 2018. High Fat/High Glucose Diet
Induces Metabolic Syndrome in an Experimental Rat
Model. Nutrients.
Hall JE, do Carmo JM, da Silva AA, Wang Z, and Hall ME.
2019. Obesity, Kidney Dysfunction Hypertension:
Mechanistic Links. Nature Reviews Nephrology.
Khadaee GH and Saeidi M. 2016. Increases of obesity and
overweight in children: an Alarm of parents and
policymakers. International journal of Pediatrics.
Longo M, Zetterale F, Naderi J, Parrillo L, Formisano P,
Raciti GA, Beguinot F, and Miele C. 2019. Adipose
The Effects of High Fat High Carbohydrate (HFHC) Diet on Body Weight in Overweight Sprague Dawley Rats
109

Tissue Dysfunction as Determinant of Obesity-
Associated Metabolic Complications. International
Journal of Molecular Sciences.
Marques C, Meireles M, Norberto S, Leite J, Freitas J,
Pestana D, Faria A, and Calhau C. 2016. High Fat Diet
Induced Obesity Rat Model: a Comparison between
Wistar And Sprague Dawley Rat. Adipocyte.
McArdle MA, Finucane OM, Connaughton RM,
McMaorrow AM, amd Roche HM. 2013. Mechanism
of Obesity Induced Inflammation and Insulin
Resistance: Insight into the Emerging Role of
Nutritional Strategies. Frontiers in Endocrinology.
Mesquida MM, Liabres MQ, Capo X, Bouzas C, Mateos D,
Pons A, Tur JA, and Sureda A. Metabolic Syndrome is
Associated with Oxidative Stress and
Proinflammantory State. Antioxidants,
Muhammad HFL. 2020. Prevention of Weight Gain During
Self-Isolation in COVID-19 Pandemic Era: A Narrative
Review. Journal of Community Empowerment for
Health.
Norris GH, Porter CM, Jiang C, Millar CL, and Blesso CN.
2017. Dietary Sphingomyelin Attenuates Hepatic
Steatosis and Adipose Tissue Inflammation in High Fat
Diet-Induced Obese Mice. The Journal of Nutritional
Biochemistry.
Rahman MM, Alam MN, Ulla A, Sumi FA, Subhan N,
Khan T, Sikder B, Hossain H, Reza HM, and Alam MA.
2017. Cardamon Powder Supplementation Prevents
Obesity, Improves Glucose Intolerance, Inflamation
and Oxidative Stress in Liver Of High Carbohydrate
High Fat Diet Induced Obese Rats. Lipids In Health and
Disease.
Rebello CJ, Kirwan JP, and Greenway FL. 2020. Obesity,
The Most Common Comorbidity in SARS-CoV-2: is
Leptin the Link? Journal of Obesity.
Sudargo T, Muhammad HFL, Rosiyani F, and Kusmayanti
NA. 2014. Pola Makan dan Obesitas. Yogyakarta.
Gadjah Mada University Press.
Tan BL and Norhaizan ME. 2019. Effect of High-Fat Diet
on Oxidative Stress, Cellular Inflammatory Response
and Cognitive Function. Nutrients.
Tan BL, Norhaizan ME, and Pui-Pui Liew W. 2018.
Nutrients and Oxidative Stress: Friend or Foe?
Oxidative Medicine and Cellular Longevity.
Tchernof A and Despres JP. 2013. Pathophysiology of
Human Viseceral Obesity: An Update. Physio Rev.
Thibault L and Hariri N. 2010. High Fat Diet Induced
Obesity in Animal Models. Nutrition Research
Reviewes.
Udomkasemsab A, and Prangthip P. 2019. High Fat Diet
Induced Dyslipidemia and Cardiac Pathological
Alterations in Wistar Rats Compared to Sprague
Dawley Rats. Clin Investig Arterioscler.
Panchal SK, Poudyal H, Iyer A, Nazer R, Alam MA, Diwan
V, Kauter K, Sernia C, Campbell F, Ward L, Gobe G,
Fenning A, and Brwon L. 2011. High-Carbohydrate,
High-Fat-Induced Metabolic Syncdrome and
Cardiovascular Remodeling in Rats. J Cardiovasc
Pharmacol.
Wong SK, Chin KY, Suhaimi FH, Ahmad F, Jamil NA, and
Nirwana SI. 2018. Osteoporosis is Associated with
Metabolic Syndrome Induced by High-Carbohydrate
High-Fat in A Rat Model. Biomedicine &
Pharmavotherapy.
Yosika GF, Sukoco P, Pranoto A, and Purwoto SP. 2020.
Penurunan Malodialdehyde Serum Setelah Latihan
Interval dan Continous Di Pagi Hari Pada Perumpuan
Obesitas. Jurnal SPORTIF.
ICSDH 2021 - International Conference on Social Determinants of Health
110
