
GIMO-PD: Towards a Health Technology Proposal for Improving
the Personalized Treatment of Parkinson’s Disease Patients
E. Enamorado-Díaz and J. A. García-García
University of Seville, Escuela Técnica Superior de Ingeniería Informática, Avda. Reina Mercedes s/n, 41012 Seville, Spain
Keywords: Clinical Practice Guideline, Computer-Interpretable Guidelines, Model-Driven Engineering Paradigm,
Parkinson’s Disease.
Abstract: Parkinson's disease (PD) is the second most common neurodegenerative disease and its pharmacological
treatment usually has unwanted side effects (motor fluctuations, dyskinesias and other motor alterations).
These effects vary from patient to patient, resulting in the use of «trial and error» manual methods by
healthcare professionals to optimize treatment. The GIMO-PD project (Mobile health solution based on
Genetic profile, Image analysis and the permanent Monitoring of symptoms for the personalized management
of Parkinson’s Disease patients) aims to present a technological solution for improving clinical decision-
making on the allocation of appropriate personalized treatments according to the characteristics of each PD
patient. This clinical decision support system integrates and combines patient biomarkers (such as genetic and
neurological markers), motor markers (based on the computerised monitoring of activity and movement) and
the digitization of clinical practice guidelines to optimise the diagnosis and treatment processes of patients
with PD and to improve their quality of life.
1 INTRODUCTION
Parkinson's disease (PD) is the second most common
neurodegenerative disease (Dorsey et al., 2007). One
of the limitations of its treatment is the appearance of
unwanted effects like motor fluctuations, dyskinesias
and other motor disorders. Furthermore, the way in
which patients respond to treatment is not always the
same, resulting in a great disparity of responses and a
high degree of variability in clinical progression
(Jankovic, 2005).
In medical practice, clinicians often still use a
«trial and error» approach to optimizing their patients'
treatments (e.g., increasing or reducing doses,
deciding whether to change one drug or combine it
with another). This approach typically involves high
socio-economic and clinical expenses (Olesen et al.,
2012), a problem compounded by the increase in the
prevalence of PD as the population ages. There is
therefore an urgent need to develop new paradigms in
the PD patient care model.
Another constraint of current clinical practice is
the limited monitoring of patients with PD. Clinical
examinations and follow-ups are limited to short
visits excessively spaced in time. The adoption of
new activity and movement monitoring
methodologies, and the use of new computing,
storage, and data analysis techniques would allow
continuous monitoring and make it possible to detect
symptoms of great value for optimising PD
treatments.
There are also other limitations that currently
make such systems difficult to implement, such as the
lack of digitization of Clinical Practice Guidelines for
treating Parkinson's disease and for integrating those
guidelines with other sources of clinical information
(Espay et al., 2016).
Over the last ten years, basic clinical research has
contributed a considerable amount of Clinical
Knowledge based on highly effective biological
markers capable of accurately predicting the
evolution of PD and patients’ response to treatment
(Poewe et al., 2017). However, these markers are
applied manually by healthcare professionals,
causing variability in clinical practice.
This paper presents the objectives of the GIMO-
PD project (Mobile health solution based on Genetic
profile, Image analysis and permanent Monitoring of
symptoms for the personalized management of
Parkinson’s Disease patients). GIMO-PD will
propose a technological solution for improving
clinical decision-making on the allocation of
Enamorado-Díaz, E. and García-García, J.
GIMO-PD: Towards a Health Technology Proposal for Improving the Personalized Treatment of Parkinson’s Disease Patients.
DOI: 10.5220/0010651900003058
In Proceedings of the 17th International Conference on Web Information Systems and Technologies (WEBIST 2021), pages 267-274
ISBN: 978-989-758-536-4; ISSN: 2184-3252
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
267

appropriate, personalized treatments adapted to the
characteristics of each PD patient. This clinical
decision support system will integrate and combine:
1. Patient biomarkers. A personalized medicine
model is applied to PD, proposing the
integration of information from different
biological biomarkers, both genetic (drug-
gene interaction and genetic risk factors) and
neuroimaging (SPECT with the [123I] FP-CIT
technique).
2. Motor markers based on the computerised
monitoring of activity and movement. The
GIMO-PD platform will include technologies
based on wearable devices for detecting
symptoms and motor disorders.
3. Digitization of Clinical Practice Guidelines
(CPGs)
1
associated with the treatment of PD.
For this purpose, our objective is to apply the
Model-Driven Engineering (MDE) paradigm
(Schmidt, 2006) to systematize and automate
the management, definition, and execution of
clinical guidelines.
The integration of these technologies for
monitoring, diagnosing, and treating patients with
Parkinson's disease will make it possible to optimise
their diagnostic and treatment processes and thus
improve their quality of life.
In this regard, GIMO-PD presents a technological
proposal for integrating clinical information obtained
from multiple sources (such as genetic analysis,
molecular markers, neuroimaging, motor monitoring
and clinical practice recommendations).
GIMO-PD also aims to further existing
knowledge about the etiology of PD and introduce
standard mechanisms (supported by Information and
Communications Technologies) to diagnose and treat
patients with this disease. These mechanisms can help
define digitized clinical practice processes to establish
personalized medical treatments for each patient.
Another objective of GIMO-PD is to reduce the costs
incurred through the ineffective use of drugs and
hospital visits, expenditure which has a great sanitary
and socio-economic impact. From a technological
point of view, the project’s application of the MDE
paradigm to the healthcare context is an important
innovation in terms of its potential results (reduction of
errors and costs, increase in quality, etc.).
This paper is organized as follows. Section 2
presents some related works on the digitization of
Clinical Practice Guidelines. To describe the
background, we divided Section 3 into two sub-
1
CPGs are sets of systematic statements which provide
health professionals and patients with a basis on which to take
sections: Section 3.1 details the model driven
engineering paradigm, and Section 3.2 briefly
describes the project’s genetic background. Section 4
explains the GIMO-PD platform, including its 5
functional modules: the clinical guide management
module (Section 4.1); the decision-making module
(Section 4.2); the motor control module (Section 4.3);
the neuroimaging module (Section 4.4); and the
genetic analysis module (Section 4.5). Finally,
Section 5 presents the main conclusions and sets out
some strategic considerations regarding future lines
of research.
2 RELATED WORKS
This section describes some works related to the
digitization of Clinical Practice Guidelines to
improve the treatment of patients with specific
diseases. No proposals specifically designed to
improve the treatment of patients with PD could be
found, but the works described here are nevertheless
interesting as they provide an idea of the current state
of the art in the digitization of Clinical Practice
Guidelines in general.
Laleci et al. (Laleci Erturkmen et al., 2019)
presented and implemented a semi-automatic care
plan management tool integrated with clinical
decision support services. The tool seamlessly
accessed and assessed patients’ Electronic Health
Records (EHRs) to suggest personalised
recommendations for individually customized care
plans.
Jimenez-Molina et al. (Jimenez-Molina et al.,
2018) proposed a framework for the development of
chronic disease support systems and applications as a
solution to shortcomings in the integration of
applications and existing healthcare systems, the
reusability of technical knowledge in the creation of
new systems and the use of gathered data in the
generation of new knowledge.
El-Sappagh et al. (El-Sappagh et al., 2018)
proposed a semantically fuzzy, rule-based system
framework for diabetes diagnosis using multiple
aspects of knowledge—fuzzy inference, an
ontological reasoning process, and a fuzzy analytical
hierarchy process—to provide a more intuitive,
dynamic, accurate design.
Aborokabah et al. (Aborokbah et al., 2018)
proposed a context-aware clinical decision support
model for heart failure risk prediction. The proposed
decisions about the healthcare responses most appropriate in
specific clinical circumstances (Field & Lohr, 1990).
WEBIST 2021 - 17th International Conference on Web Information Systems and Technologies
268
model was evaluated using a dataset of potential heart
failure patients with metrics including prediction
accuracy, sensitivity, specificity and receiving
operating characteristic.
Afzal et al. (Afzal et al., 2017) proposed an
automated knowledge acquisition methodology with
a comprehensible knowledge model for cancer
treatment based directly on information in existing
cancer treatment documents. This methodology is
supported by software tools and is helpful in finding
hidden knowledge in clinical documents. It is also
generalizable to other domains as a means of assisting
clinicians in decision making and education.
Pombo et al. (Pombo et al., 2016) presented a
Clinical Decision Support System (CDSS) based on
data imputation principles for pain evaluation. The
system produced tailored alarms, reports and clinical
guidance based on collected patient-reported data.
After analyzing previous related works, we can
identify some specific contributions of our paper: (1)
The application and validation of the technological
solution in a poorly treated disease through the
digitization of clinical guidelines (that is, PD); (2)
Previous works are focused on the follow-up of
patients and their treatments, while GIMO-PD
integrates the use of neuroimaging techniques, mobile
technologies for the detection of motor symptoms,
genetic and pharmacological information; (3) The use
of MDE-based mechanisms to systematize the
technological development of the platform.
3 BACKGROUNDS
3.1 Model Driven Paradigm
In the context of GIMO-PD, the objective of the
model-driven engineering (MDE) paradigm was to
improve the automation and digitization of the
clinical practice guidelines associated with the
treatment of Parkinson's disease.
The MDE paradigm (Schmidt, 2006) emerged in
response to the complexity of software systems,
making it possible to express the concepts of the
problem domain in an effective manner. The paradigm
defines models and establishes transformation rules
based on those models to generate new, more
technologically oriented models. These mechanisms
are intended to increase automation during the
software development life cycle.
To implement this new paradigm in real projects,
standardization was necessary. OMG standardized the
use of the MDE paradigm using Model Driven
Architecture (MDA, 2003). MDA defines
transformation rules between models until source code
or another model with the characteristics of a particular
technology are obtained. It is based on the following
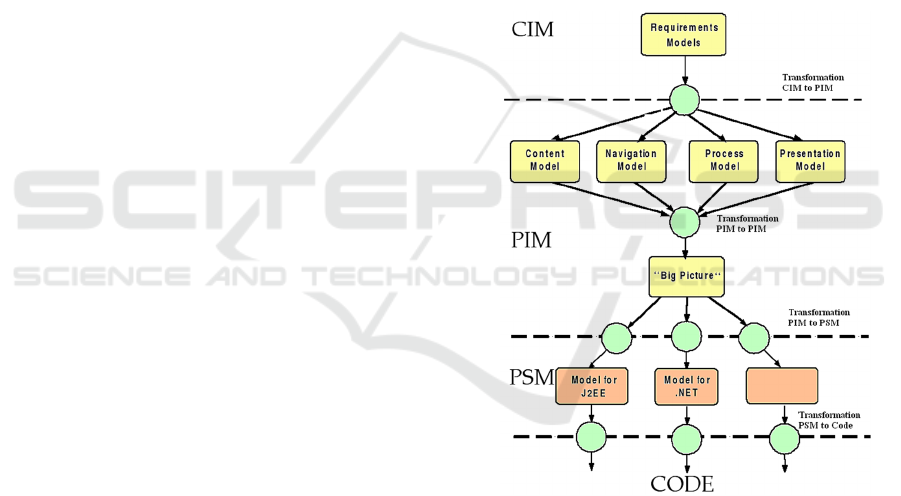
four types of levels or models as shown in Figure 1:
- ICM (Independent Computing Model). This is
considered the highest and the most abstract
level of business model.
- PIM (Platform Independent Model). This
represents the business process and system
structure model. These models are not related
to any one specific technology.
- PSM (Platform Specific Model). This is
specifically related to the platform where the
system is to be implemented: for example,
operating systems, programming languages or
middleware platforms.
- Source code. This refers to the appropriate
coding and implementation of the system.
Figure 1: Model-Driven Engineering.
MDE has big advantages for software
development. It provides specific, relevant results in
software projects. The systematic generation of
models based on previous models assures traceability
through levels and can potentially cut down
development time. If suitable tools were defined, this
process could also even be automatic.
3.2 Genetic Background
GIMO-PD proposes the joint integration of
information from different biological biomarkers
GIMO-PD: Towards a Health Technology Proposal for Improving the Personalized Treatment of Parkinson’s Disease Patients
269

(genetic and neuroimaging) with movement analysis
and the digitalization of clinical guidelines, making it
possible to apply personalized medicine models to PD
patients.
Genetics can impact patient profiles through drug-
gene factors and genetic risk factors:
1) Drug-gene factors. Genes can modulate a
person’s response to drugs, and the study of this
interaction is called pharmacogenomics (Grant,
2001). Studies have been carried out in recent
years into the benefits of certain drugs in the
treatment of PD. Some, for example, focussed
on correlating the clinical responses of patients
who had received different doses of the drug
levodopa with their activity (Bialecka et al.,
2008; Cheshire et al., 2013). These studies also
identified a relationship between clinical
responses and the SLC6A3 gene that encodes
the dopamine transporter. That same gene is
related to levadopa absorption. A
comprehensive review of pharmacogenetics
pertaining to PD can be found in the literature
(Kurzawski et al., 2015; Politi et al., 2018).
2) Genetic risk factors. Genes can also predispose
patients to certain motor and non-motor
symptoms. To adapt the patient's treatment and
improve her quality of life, these genetic factors
must therefore be taken into account. The
genetic risk factors associated with symptoms of
Parkinson's disease have been identified in
different research papers. They include
increased risk of cognitive impairment (Foltynie
et al., 2009), risk of visual hallucinations
(Redenšek et al., 2019), and risk of severe
movement control disorders (Napier et al., 2015;
Redenšek et al., 2019).
4 GIMO-PD ARCHITECTURE
The GIMO-PD platform will have five functional
modules with which to achieve its objectives (see
Section 1):
1. Clinical Practice Guidelines management
model. This covers the definition, execution
and monitoring of clinical guidelines, and
their integration with external systems.
2. Decision-making module. This module
considers combination of the information
collected from the neuroimaging, genetic
analysis, and motor control modules to
establish a clinical recommendation.
3. Motor control module. This includes
technologies based on wearable devices for
detecting symptoms and motor disorders.
4. Neuroimaging module. This analyses DAT-
SPECT images and provides quantitative,
objective information about the patient's
condition.
5. Genetic analysis module. This proposes the
integration of information from different
biological biomarkers.
The above modules will be integrated into a
software platform based on Cloud-computing to host,
exchange and process all genetic, neuroimaging and
motor monitoring information. Figure 2 shows the
architecture of the GIMO-PD platform.
The core of this platform is the clinical guidelines
management module, which will provide
recommendations to be followed by health
professionals. These recommendations will be
established after analysing the genetic, neurological,
and motor variables of the patient. The analysis will
be carried out in conjunction with the Clinical
Decision Support System (CDSS). It will include
machine learning algorithms trained with
experimental clinical data.
The project’s genetic and neurological analysis
will include a chemical component to determine
which genetic variants described in the literature
influence the evolution and treatment of the disease.
The genetic variables identified are then included in
the CDSS. The patient’s pathological situation will
also be evaluated by neuroimage analysis.
With regard to the management of motor and non-
motor markers, the GIMO-PD platform will include
mechanisms to monitor these aspects. On the one
hand, it will include the design and development of a
wearable bracelet with sensors to monitor the
patient's motor function and detect motor
complications. On the other, non-motor markers will
be periodically checked with short scales and
validated using the patient's smartphone.
The objective of each module is explained in
detail below.
4.1 Clinical Practice Guidelines
Management Model
This GIMO-PD module will run the CPG
management life cycle. This includes the definition,
execution and monitoring of clinical guidelines, and
their integration with external systems. To achieve
these goals, the module has three main sub-modules:
WEBIST 2021 - 17th International Conference on Web Information Systems and Technologies
270
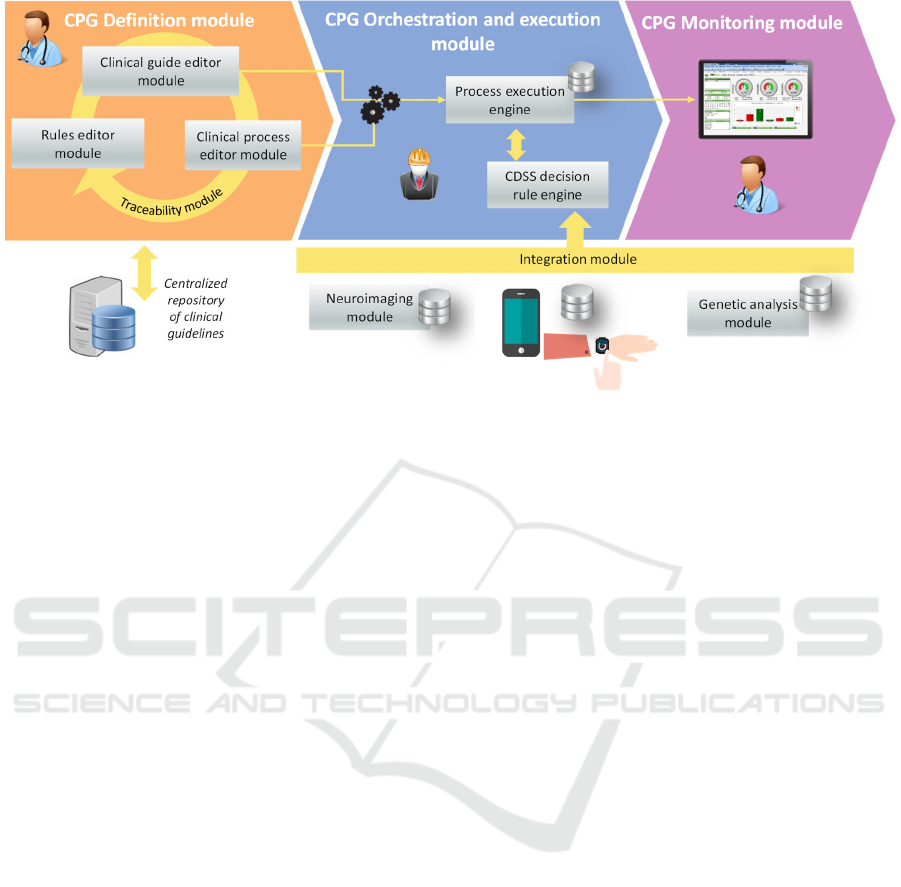
Figure 2: GIMO-PD: towards to model-driven software architecture.
1. Definition sub-module. This will contain
model-driven mechanisms (based on MDE
principles; see Section 3.1) for defining static
CPG models. For this purpose, a set of
domain-specific metamodels and languages
will have been designed to describe activity
flows, clinical recommendations, clinical
variables, decision rules, etc. All these aspects
are essential to define any clinical guideline.
This sub-module will also include a set of
transformation rules to obtain an executable
version from a previously defined static
model. The executable version can then be
deployed in the execution sub-module.
2. Execution and integration sub-module. This
sub-module will comprise a process engine for
executing the static CPD models defined in the
previous sub-module. It could be considered
the core of GIMO-PD because it will be
responsible for orchestrating communications
with the other modules on the GIMO-PD
platform. It will also provide the user entry
point to the platform.
3. Monitoring sub-module. This sub-module will
monitor the healthcare professional’s
performance and task flow. For this purpose,
the platform will define key performance
indicators related to running instances,
average running time, etc.
4.2 Decision-Making Module (CDSS
Decision Rule Engine)
Clinical practice guidelines provide protocols for
establishing quality diagnoses and treatments.
Although these guidelines are usually quite broad,
however, they are descriptions that do not fully cover
all the casuistry associated with evaluating clinical
variables to make an optimal decision. This module
will complement the clinical guideline execution
module by providing additional functions for
improving evaluation and decision-making.
In this module, the GIMO-PD platform will
include a Clinical Decision Support System (CDSS).
CDSSs are decision systems that provide specific
recommendations based on the knowledge model that
feeds them (Liu et al., 2006).
The CDSS collects information from the
neuroimaging, genetic analysis, and motor control
modules. Once the combination of this information
has been considered, a clinical recommendation can
be established.
GIMO-PD’s clinical decision module will be
designed and developed considering two design
techniques: (1) Rule Based Reasoning (RBR) (Shoaip
et al., 2019), which establishes a set of clinical rules
considering specific clinical criteria and
recommendations previously defined in CPGs ; and
(2) Case Based Reasoning (CBR) (Li et al., 2018),
which will be capable of automatically generating
clinical rules after analysing prior knowledge stored,
for example, in knowledge bases.
The module’s hybrid design is due to the intrinsic
characteristics of the GIMO-PD project, in which
most of the on-park data comes from previously
diagnosed and treated cases. Diagnosis and treatment
of Parkinson's disease requires the analysis both of
patient information and of historical information
(based on previously populated knowledge bases). In
this regard, the application of CBR techniques makes
GIMO-PD: Towards a Health Technology Proposal for Improving the Personalized Treatment of Parkinson’s Disease Patients
271

it possible to automatically generate rules, thus
complementing the rules defined by the clinical
practice guide using RBR techniques.
4.3 Motor Control Module
Tremor is a primary symptom and one of the most
disabling general symptoms of Parkinson's disease
(Ruonala et al., 2013). In fact, it is one of the aspects
most evaluated by health professionals to determine
the progression of the disease. The monitoring and
evaluation of motor symptoms in PD is mainly based
on historical information and neurological
examinations (usually biannual). These methods have
many drawbacks: (1) the data may be subjective,
because it depends on the patient's memory and
perception of his own symptoms (and his ability to
identify symptoms and medical terminology); and (2)
the data are highly dependent on the experience of the
healthcare professional.
Many research articles have analysed
parkinsonian gait to try to detect movement disorders
(Cifuentes et al., 2010). However, there is a
significant handicap. These movements are disturbed
by other factors (lack of balance, trunk bent forward,
stiffness, tremor, etc.) which are not isolated and
usually cause a high rate of false positives and
negatives. This makes it difficult to determine exactly
what movement the patient is making at any given
moment. It is also important to mention that these
motor symptoms depend on each patient and their
degree of illness. The study of a PD patient’s gait is
therefore a field that still requires protracted research
if useful results are to be obtained.
In this context, GIMO-PD presents a
technological proposal for identifying motor
disorders in patients with Parkinson's disease. The
platform will include technologies based on
wearable devices for the detection of symptoms and
motor disorders. The wearable nature of these
devices facilitates their continuous, non-invasive
use to capture kinematic information through
inertial sensors, and also through lifelong learning
techniques.
2
Single-photon emission computed tomography with
ioflupane (123I), also known as 123I-FP-CIT SPECT, is the
most widely used complementary test in this type of
diagnosis. (Olivares Romero & Arjona Padillo, 2013).
3
The dopamine transporter (DAT) is responsible for
clearing dopamine from the synaptic cleft after its release.
4.4 Neuroimaging Module
Image quantification techniques are common in
medical research, but their complexity has historically
prevented their effective use in clinical practice.
As a solution to this problem, GIMO-PD proposes
integrating neuroimage analysis and clinical practice.
For this purpose, this module will include
functionalities and machine learning algorithms for
analysing SPECT (single photon emission computed
tomography) images with the [123I] FP-CIT
2
technique. This technique makes it possible to
visualize DAT
3
(Dopamine Active Transporter)
activity and detect presynaptic dopaminergic deficit.
This is useful in the early diagnosis of Parkinson's
disease and also in differentiating the disease from
other nondegenerative parkinsonian disorders.
DAT-SPECT image analysis provides
quantitative, objective information on the patient's
condition, which can then easily be compared with
historical patient data and clinical knowledge bases.
By comparing data in this way, the clinical specialist
can specify the patient's situation more accurately.
4.5 Genetic Analysis Module
In GIMO-PD, a personalized medicine model will be
applied to PD, proposing the integration of
information from different biological biomarkers,
both genetic and neuroimaging. As explained earlier
in Section 3.2, genetics play a key role in defining
patient profiles in two areas: drug-gene interaction
and genetic risk factors.
GIMO-PD will offer a catalogue of genetic
biomarkers identified in the literature as being relevant
to patients’ responses to treatment (pharmacogenetic
interaction), the evolution of Parkinson's disease, and
the appearance of certain symptoms (genetic risk
factors). These biomarkers are useful for prognosis and
as predictors of response to treatment.
5 CONCLUSIONS AND FUTURE
RESEARCH
Parkinson's disease is the second most common
neurodegenerative disease, and its pharmacological
Imaging DAT availability measures dopamine terminal
functionality and provides a method for detecting states of
striatal dopamine deficiency in idiopathic Parkinson’s
disease and atypical neurodegenerative parkinsonian
disorders such as multiple system atrophy and progressive
supranuclear palsy (Brooks, 2010).
WEBIST 2021 - 17th International Conference on Web Information Systems and Technologies
272

treatment usually has undesired effects (motor
fluctuations, dyskinesias and other motor alterations).
This paper details a software platform for
improving clinical decision-making and providing
individual Parkinson’s disease patients with the
treatment most appropriate to their own personal
characteristics. This platform is going to be named
GIMO-PD: a project for applying a personalized
medicine model to Parkinson's disease. To achieve
this objective, GIMO-PD will integrate information
from different data sources: biological biomarkers
(both genetic and image), analysis of movement
disorders observed while monitoring patients in real
time, and clinical information from clinical practice
guidelines for the treatment of Parkinson's disease.
Regarding future lines of work, this project can be
expanded in several ways. One area of study would
be to look at new functionalities of the GIMO-PD
platform and the monitoring of more parameters
when analysing patient movement disorders. The
project might also be extended to address other
diseases, taking into account i) different parameters
when monitoring patients and ii) the
recommendations of different clinical guidelines
specific to other diseases.
ACKNOWLEDGEMENTS
This research is framed in the GIMO-PD (RTC2019-
007150-1) project of the Spanish Ministry of
Economy and Competitiveness, which is financed by
European funds. In addition, this article is funded by:
the NICO project (PID2019-105455GB-C31) of the
Spanish Ministry of Economy and Competitiveness:
the TRoPA (Early Testing in Medical Robotics
Process Automation) project (CEI-12) of the
Andalusian Ministry of Economy, knowledge,
companies and university; and Aid for the
Consolidation of Groups of the Junta de Andalucía
(2021-TIC021). Finally, GIMO-PD was carried out
by researchers from the University of Seville, from
the FISEVI foundation, and from the Madrija and
Soltel companies.
REFERENCES
Aborokbah, M. M., Al-Mutairi, S., Sangaiah, A. K., &
Samuel, O. W. (2018). Adaptive context aware decision
computing paradigm for intensive health care delivery
in smart cities - A case analysis. Sustainable Cities and
Society, 41 (May 2017), 919–924. https://doi.org/
10.1016/j.scs.2017.09.004
Afzal, M., Hussain, M., Ali Khan, W., Ali, T., Lee, S., Huh,
E. N., Farooq Ahmad, H., Jamshed, A., Iqbal, H., Irfan,
M., & Abbas Hydari, M. (2017). Comprehensible
knowledge model creation for cancer treatment
decision making. Computers in Biology and Medicine,
82(July 2016), 119–129. https://doi.org/10.1016/
j.compbiomed.2017.01.010
Bialecka, M., Kurzawski, M., Klodowska-Duda, G., Opala,
G., Tan, E.-K., & Drozdzik, M. (2008). The association
of functional catechol-O-methyltransferase haplotypes
with risk of Parkinson’s disease, levodopa treatment
response, and complications. Pharmacogenetics and
Genomics, 18(9). https://journals.lww.com/jpharmaco
genetics/Fulltext/2008/09000/The_association_of_fun
ctional.8.aspx
Brooks, D. J. (2010). Imaging dopamine transporters in
Parkinson’s disease. Biomarkers in Medicine, 4(5),
651–660. https://doi.org/10.2217/bmm.10.86
Cheshire, P., Bertram, K., Ling, H., O’Sullivan, S. S.,
Halliday, G., McLean, C., Bras, J., Foltynie, T., Storey,
E., & Williams, D. R. (2013). Influence of single
nucleotide polymorphisms in COMT, MAO-A and
BDNF genes on dyskinesias and levodopa use in
Parkinson’s disease. Neurodegenerative Diseases,
13(1), 24–28. https://doi.org/10.1159/000351097
Cifuentes, C., Martínez, F., & Romero, E. (2010). Análisis
teórico y computacional de la marcha normal y
patológica: una revisión. Revista Med, 18(2), 182.
https://doi.org/10.18359/rmed.1311
Dorsey, E. R., Constantinescu, R., Thompson, J. P., Biglan,
K. M., Holloway, R. G., Kieburtz, K., Marshall, F. J.,
Ravina, B. M., Schifitto, G., Siderowf, A., & Tanner, C.
M. (2007). Projected number of people with Parkinson
disease in the most populous nations, 2005 through
2030. Neurology, 68(5). https://doi.org/10.1212/
01.wnl.0000247740.47667.03
El-Sappagh, S., Alonso, J. M., Ali, F., Ali, A., Jang, J. H.,
& Kwak, K. S. (2018). An ontology-based interpretable
fuzzy decision support system for diabetes diagnosis.
IEEE Access, 6, 37371–37394. https://doi.org/10.1109/
ACCESS.2018.2852004
Espay, A. J., Bonato, P., Nahab, F. B., Maetzler, W., Dean,
J. M., Klucken, J., Eskofier, B. M., Merola, A., Horak,
F., Lang, A. E., Reilmann, R., Giuffrida, J., Nieuwboer,
A., Horne, M., Little, M. A., Litvan, I., Simuni, T.,
Dorsey, E. R., Burack, M. A., … Papapetropoulos, S.
(2016). Technology in Parkinson’s disease: Challenges
and opportunities. Movement Disorders : Official
Journal of the Movement Disorder Society, 31(9),
1272–1282. https://doi.org/10.1002/mds.26642
Field, M. J., & Lohr, K. N. (1990). Clinical Practice
Guidelines: Directions for a New Program. Committee
to Advise the Public Health Service on Clinical
Practice. National Academies Press. http://ebook
central.proquest.com/lib/uses/detail.action?docID=337
7121
Foltynie, T., Cheeran, B., Williams-Gray, C. H., Edwards,
M. J., Schneider, S. A., Weinberger, D., Rothwell, J. C.,
Barker, R. A., & Bhatia, K. P. (2009). BDNF val66met
influences time to onset of levodopa induced dyskinesia
GIMO-PD: Towards a Health Technology Proposal for Improving the Personalized Treatment of Parkinson’s Disease Patients
273

in Parkinson{\textquoteright}s disease. Journal of
Neurology, Neurosurgery \& Psychiatry, 80(2), 141–
144. https://doi.org/10.1136/jnnp.2008.154294
Grant, S. F. A. (2001). Pharmacogenetics and
pharmacogenomics: tailored drug therapy for the 21st
century. Trends in Pharmacological Sciences, 22(1), 3–
4. https://doi.org/https://doi.org/10.1016/S0165-6147
(00)01606-0
Jankovic, J. (2005). Motor fluctuations and dyskinesias in
Parkinson’s disease: Clinical manifestations.
Movement Disorders, 20(SUPPL. 11). https://doi.org/
10.1002/mds.20458
Jimenez-Molina, A., Gaete-Villegas, J., & Fuentes, J.
(2018). ProFUSO: Business process and ontology-
based framework to develop ubiquitous computing
support systems for chronic patients’ management.
Journal of Biomedical Informatics, 82(April), 106–127.
https://doi.org/10.1016/j.jbi.2018.04.001
Kurzawski, M., Białecka, M., & Droździk, M. (2015).
Pharmacogenetic considerations in the treatment of
Parkinson’s disease. Neurodegenerative Disease
Management, 5(1), 27–35. https://doi.org/10.2217/
nmt.14.38
Laleci Erturkmen, G. B., Yuksel, M., Sarigul, B., Arvanitis,
T. N., Lindman, P., Chen, R., Zhao, L., Sadou, E.,
Bouaud, J., Traore, L., Teoman, A., Lim Choi Keung,
S. N., Despotou, G., de Manuel, E., Verdoy, D., de Blas,
A., Gonzalez, N., Lilja, M., von Tottleben, M., …
Kalra, D. (2019). A Collaborative Platform for
Management of Chronic Diseases via Guideline-Driven
Individualized Care Plans. Computational and
Structural Biotechnology Journal, 17, 869–885.
https://doi.org/10.1016/j.csbj.2019.06.003
Li, O., Liu, H., Chen, C., & Rudin, C. (2018). Deep
Learning for Case-Based Reasoning Through
Prototypes: A Neural Network That Explains Its
Predictions. Proceedings of the AAAI Conference on
Artificial Intelligence, 32(1 SE-AAAI Technical Track:
Machine Learning). https://ojs.aaai.org/index.php/
AAAI/article/view/11771
Liu, J., Wyatt, J. C., & Altman, D. G. (2006). Decision tools
in health care: Focus on the problem, not the solution.
BMC Medical Informatics and Decision Making, 6, 1–
7. https://doi.org/10.1186/1472-6947-6-4
MDA. (2003). MDA Guide v1.0.1. June.
https://www.omg.org/news/meetings/workshops/UML
_2003_Manual/00-2_MDA_Guide_v1.0.1.pdf
Napier, T. C., Corvol, J.-C., Grace, A. A., Roitman, J. D.,
Rowe, J., Voon, V., & Strafella, A. P. (2015). Linking
neuroscience with modern concepts of impulse control
disorders in Parkinson’s disease. Movement Disorders,
30(2), 141–149. https://doi.org/https://doi.org/10.10
02/mds.26068
Olesen, J., Gustavsson, A., Svensson, M., & Jo, B. (2012).
The economic cost of brain disorders in Europe. 155–
162. https://doi.org/10.1111/j.1468-1331.2011.03590.x
Olivares Romero, J., & Arjona Padillo, A. (2013).
Diagnostic accuracy of 123I-FP-CIT SPECT in
diagnosing drug-induced parkinsonism: A prospective
study.
Neurología (English Edition), 28(5), 276–282.
https://doi.org/https://doi.org/10.1016/j.nrleng.2012.05
.007
Poewe, W., Seppi, K., Tanner, C. M., Halliday, G. M.,
Brundin, P., Volkmann, J., Schrag, A.-E., & Lang, A.
E. (2017). Parkinson disease. Nature Reviews. Disease
Primers, 3, 17013. https://doi.org/10.1038/nrdp.201
7.13
Politi, C., Ciccacci, C., Novelli, G., & Borgiani, P. (2018).
Genetics and Treatment Response in Parkinson’s
Disease: An Update on Pharmacogenetic Studies.
NeuroMolecular Medicine, 20(1), 1–17. https://doi.org/
10.1007/s12017-017-8473-7
Pombo, N., Rebelo, P., Araújo, P., & Viana, J. (2016).
Design and evaluation of a decision support system for
pain management based on data imputation and
statistical models. Measurement: Journal of the
International Measurement Confederation, 93, 480–
489.
https://doi.org/10.1016/j.measurement.2016.07.009
Redenšek, S., Flisar, D., Kojović, M., Gregorič
Kramberger, M., Georgiev, D., Pirtošek, Z., Trošt, M.,
& Dolžan, V. (2019). Dopaminergic Pathway Genes
Influence Adverse Events Related to Dopaminergic
Treatment in Parkinson’s Disease. Frontiers in
Pharmacology, 10, 8. https://doi.org/10.3389/fphar.20
19.00008
Ruonala, V., Meigal, A., Rissanen, S. M., Airaksinen, O.,
Kankaanpaa, M., & Karjalainen, P. A. (2013). EMG
signal morphology in essential tremor and Parkinson’s
disease. Annual International Conference of the IEEE
Engineering in Medicine and Biology Society. IEEE
Engineering in Medicine and Biology Society. Annual
International Conference, 2013, 5765–5768.
https://doi.org/10.1109/EMBC.2013.6610861
Schmidt, D. C. (2006). Model-Driven Engineering Douglas
C. Schmidt Vanderbilt University Model-driven.
Historia, 39(2), 2–9. http://www.computer.org/portal/
site/computer/menuitem.e533b16739f5...
Shoaip, N., El-Sappagh, S., Barakat, S., & Elmogy, M.
(2019). Chapter 4 - Reasoning methodologies in
clinical decision support systems: A literature review.
In N. Dey, A. S. Ashour, S. J. Fong, & S. Borra (Eds.),
U-Healthcare Monitoring Systems (pp. 61–87).
Academic Press. https://doi.org/https://doi.org/10.10
16/B978-0-12-815370-3.00004-9
WEBIST 2021 - 17th International Conference on Web Information Systems and Technologies
274
