
On the Use of Regulator Theory in Neuroscience with Implications for
Robotics
Mireille E. Broucke
Electrical and Computer Engineering, University of Toronto, Toronto, Canada
Keywords:
Regulator Theory, Adaptive Internal Models, Motor Control, Cerebellum.
Abstract:
We survey recent results on the use of regulator theory in neuroscience, particularly to model the contribution
of the cerebellum to motor systems. Based on our study of the slow eye movement systems as well as visuomo-
tor adaptation, several themes emerge, including a promising structural model of the cerebellum, and insights
on how the cerebellum may enable and disable internal models. Implications for robotics are discussed at the
end of the paper.
1 INTRODUCTION
This paper presents an overview of our recent work
on applying regulator theory to problems of neuro-
science, particularly modeling the contribution of the
cerebellum to human motor systems. We present
a structural model that is inspired by the cerebellar
anatomy. This model will be recognizable as an adap-
tive observer. Our working hypothesis is that the pri-
mary function of the cerebellum is disturbance rejec-
tion of exogenous reference and disturbance signals.
This interpretation of cerebellar function places the
internal model principle at front and center (Francis
and Wonham, 1976). The idea to interpret the cere-
bellum in terms of disturbance rejection is not new.
For instance, the first proposal by Stephen Lisberger
on the role of internal models in the cerebellum in
his survey paper (Lisberger, 2009) is consistent with
a role of disturbance rejection. Lisberger describes
internal models as providing “a model of the inertia
of real-world objects in motion”; see also (Cerminara
et al., 2009). What is new is our use of regulator the-
ory to mathematically model the cerebellum.
This paper is informal. We suppress mathematical
details as much as we can, without necessarily com-
promising on completeness. We want to draw con-
nections between different areas that are typically not
compared side by side: for instance, regulator designs
for disturbance rejection with a systems-level model
of the cerebellum; specific models of motor systems
compared with each other; even comparisons between
continuous-time and discrete-time processes both as-
sociated with the cerebellum. In making this sur-
vey, several themes or takeaways emerge, which we
already summarize here for the reader who is inter-
ested in the main points (meanings of specific terms
are found in the main text below):
• The cerebellar architecture resembles that of an
adaptive observer (Kreisselmeier, 1977; Kanel-
lakopoulos and Kokotovic, 1995).
• The nucleo-cortical pathway is in direct corre-
spondence with the internal model principle in
the sense that without this pathway, the inter-
nal model principle would not be satisfied by the
mathematical model.
• The theory suggests that the granular layer filters
of the cerebellum must synchronize on the same
time constants for filtering mossy fiber (MF) in-
puts to the same cerebellar modules.
• Mathematically speaking, there is considerable
flexibility in terms of how MF inputs to the cere-
bellum may be combined or “pre-bundled”.
• The mathematical models suggest that some MF
inputs have the role to ensure that they are not
cancelled out by the cerebellum. This seemingly
contradictory role may potentially lead to misin-
terpretations of the function of certain cerebellar
modules. On the other hand, the collaborative role
between the cerebellum and feedforward signals
is well known.
• The cerebellum may well be the unique brain
structure that is wired to handle the dangerous op-
eration of shutting on and off internal models for
satisfaction of the internal model principle.
• Research on the cerebellum has implications for
robotics.
Broucke, M.
On the Use of Regulator Theory in Neuroscience with Implications for Robotics.
DOI: 10.5220/0010639100110023
In Proceedings of the 18th International Conference on Informatics in Control, Automation and Robotics (ICINCO 2021), pages 11-23
ISBN: 978-989-758-522-7
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
11

2 CEREBELLUM
The locus of internal models in the brain is believed to
be the cerebellum. The cerebellum is a major brain re-
gion positioned at the back of the head, partly covered
by the cerebral cortex, and itself covering the brain-
stem. In 1967 nobel prize winner John Eccles and
his co-authors laid out the microcircuit of the cerebel-
lum (Eccles et al., 1967). Their work showed that the
cerebellum contains relatively few neuron types, and
that it has a laminated structure with a repeating ar-
chitectural pattern pervading each layer or functional
module. Each module has only two input pathways
and a single output pathway (Apps et al., 2018).
The first of two input pathways to the cerebellum
is via the mossy fiber (MF) inputs. The MFs carry a
rich flow of information from sensory inputs as well
as the output of the cerebellum itself. Mossy fiber out-
puts are received by tens of billions of granule cells,
the most common cell type of the brain. The axons
of the granule cells form parallel fibers, which con-
nect with the dendrites of the principal neuron type of
the cerebellum, the Purkinje cells (PCs). Each PC re-
ceives synaptic connections from as many as 200,000
parallel fibers, resulting in a massive fan-in of infor-
mation. The second input pathway to the cerebellum
is via the climbing fibers, which are the axons of cells
from part of the brainstem called the inferior olive.
The climbing fiber input carries a sensory error signal,
and each climbing fiber forms a powerful connection
with a single PC. Climbing fibers are capable to mod-
ify the strength of the synapse between parallel fiber
inputs onto the PCs. The PC axons project to the deep
cerebellar nuclei and the vestibular nuclei, forming
the only output pathway from the cerebellum.
Notable features of the anatomical structure from
a control perspective are:
(i) The cerebellum has a purely feedforward struc-
ture. Information flows from the MF inputs to
granule cells and then via the parallel fibers to
the PCs. The PCs send their outputs to the deep
cerebellar nuclei and vestibular nuclei.
(ii) Each functional module of the cerebellum is
identical to the others and performs the same
neural computation. The only distinction be-
tween modules is in terms of the input and output
connections to other regions of the brain.
(iii) Each functional module of the cerebellum pro-
cesses its own sensory error signal received via
the climbing fiber inputs from a circumscribed
region of the inferior olive. Each module sends
its output to a circumscribed region in the cere-
bellar nuclei or vestibular nuclei.
(iv) The adaptive capability of the cerebellum is pro-
vided by the climbing fiber input which changes
the strength of the synapse between the parallel
fibers and the PCs.
(v) Mossy fibers projecting to a similar region of the
cerebellar cortex encode similar information.
(vi) Each of the deep cerebellar nuclei and the
vestibular nuclei has a projection to the MF in-
puts of the cerebellum. This projection is termed
the nucleo-cortical pathway and is regarded to
provide an efference copy of the motor command
issued by the cerebellum (Ruigrok, 2011; Houck
and Person, 2014).
3 STRUCTURAL MODEL
The features we have highlighted suggest a struc-
tural model associated with a single cerebellar mod-
ule. This model that does not reveal function but ac-
cords with cerebellar structure at a systems level:
˙x = Ax + Bu + Ed
1
(3.1a)
e = Cx + Dd
2
(3.1b)
˙w
1
= F
1
w
1
+ G
1
u
m f ,1
(3.1c)
.
.
.
˙w
k
= F
k
w
k
+ G
k
u
m f ,k
(3.1d)
˙w
k+1
= F
k+1
w
k+1
+ G
k+1
u
im
(3.1e)
ˆw = (w
1
, . . . , w
k+1
) (3.1f)
˙
ˆ
ψ = γe ˆw
T
(3.1g)
u
im
=
ˆ
ψ ˆw (3.1h)
u = u
s
+ u
im
. (3.1i)
Equation (3.1a) represents the open-loop system un-
der regulation by the cerebellum: horizontal eye po-
sition, eye velocity, hand position, hand grip force,
gait, stance, and so forth. Signal e ∈ R is an er-
ror that the cerebellum is tasked with asymptotically
driving to zero. Signal d
1
∈ R is a persistent exoge-
nous disturbance entering at the plant input. Signal
d
2
∈ R is a persistent exogenous disturbance entering
at the error measurement. Distinct MF input signals
are u
m f ,1
, . . . , u
m f ,k
, u
im
∈ R, which arrive by way of
the filters (3.1c)-(3.1e) with corresponding filter states
w
1
, . . . , w
k+1
. We assume each F
i
, i = 1, . . . , k + 1 is
Hurwitz. These filters are in analogy with the lead-lag
filters utilized in (Fujita, 1982) to model the granular
layer, but we allow a more general interpretation here.
The filter (3.1e) is particularly important as it mod-
els the nucleo-cortical pathway. The equation (3.1g)
is the standard least-mean-squares (LMS) parameter
adaptation law to model the modifiable synapses be-
tween parallel fibers and PCs. The signal e in (3.1g) is
ICINCO 2021 - 18th International Conference on Informatics in Control, Automation and Robotics
12

supplied by the climbing fiber input. Parameter γ > 0
is the adaptation rate. The output of the cerebellum is
u
im
, and the motor command is u, which includes u
s
for closed-loop stability, if needed. A control theorist
will recognize the structural model to be an adaptive
observer (Kreisselmeier, 1977; Kanellakopoulos and
Kokotovic, 1995).
4 DISTURBANCE REJECTION
The structural model does not endow the cerebellum
with any particular function except that of filtering
MF inputs with parameter adaptation. Our working
hypothesis is that the primary function of the cerebel-
lum is disturbance rejection, bringing into view regu-
lator theory (Wonham, 1985). In this section we look
at a representative regulator design to solve the dis-
turbance rejection problem so that we can bring out
comparisons with the structural model. The design
utilizes adaptive internal models (Nikiforov, 2004a;
Nikiforov, 2004b).
Consider the open-loop system
˙x = Ax + B(u + d) (4.1a)
˙w = Fw + Gd (4.1b)
d = ψw (4.1c)
e = Cx , (4.1d)
where x ∈ R
n
is the state, w ∈ R
q
is the exosystem
state, u ∈ R is the control input, e ∈ R is the error
to be regulated, and d ∈ R is a persistent, exogenous
disturbance signal entering the plant at the control in-
put. The exosystem (4.1b) provides a model of the
frequency content of the disturbance in terms of a row
vector of unknown parameters ψ ∈ R
1×q
.
Problem 4.1 (Regulator Problem). Consider the sys-
tem (4.1). Find a error feedback regulator
˙x
c
= Fx
c
+ Ge (4.2a)
u = Hx
c
+ Ke , (4.2b)
with regulator state x
c
(t) ∈ R
p
, such that the follow-
ing conditions are met:
(AS) The equilibrium (0, 0) ∈ R
n
× R
p
of the unforced
closed-loop system
˙x = (A + BKC)x + BHx
c
(4.3a)
˙x
c
= Fx
c
+ GCx (4.3b)
is asymptotically stable.
(R) The forced closed-loop system satisfies: for
all initial conditions (x(0), x
c
(0), w(0)), e(t) −→
0. /
The following assumptions are standard in the
regulator theory literature; see (Saberi et al., 2000)
for more background: (A1) (A, B) is controllable.
(A2) (C, A) is observable. (A3) S := F + Gψ has
simple eigenvalues on the imaginary axis. (A4)
det
A − λI B
C 0
6= 0 for all λ ∈ σ(S). (A5) (F, G) is
controllable, F is Hurwitz, and (ψ, S) is w.l.o.g. an
observable pair. (A6) An upper bound on q is known,
but the parameters of (ψ, S) are unknown. (A7) The
parameters (A, B,C) are known. (A8) The measure-
ment is x.
Consider a control input of the form (3.1i). The
first component u
s
is selected for stabilization: choose
u
s
= Kx such that (A + BK) is Hurwitz. The sec-
ond component u
im
is designed to satisfy the inter-
nal model principle (Francis and Wonham, 1976).
Consider an adaptive internal model from (Nikiforov,
2004a):
˙w
0
= Fw
0
+ FNx (4.4a)
˙w
1
= Fw
1
− NAx (4.4b)
˙w
2
= Fw
2
− NBu (4.4c)
ˆw = w
0
+ Nx + w
1
+ w
2
(4.4d)
˙
ˆ
ψ = γ(B
T
Px) ˆw
T
(4.4e)
u
im
= −
ˆ
ψ ˆw, (4.4f)
where N satisfies NB = G,
ˆ
ψ is an estimate of ψ, γ > 0
is the adaptation rate, and P ∈ R
n×n
is the symmet-
ric, positive definite solution of the Lyapunov equa-
tion (A + BK)
T
P + P(A + BK) = −Q, with Q ∈ R
n×n
symmetric and positive definite.
Now we observe
˙
ˆw = Fw
0
+ FNx + N(Ax + Bu + Bd)
+ Fw
1
− NAx + Fw
2
− NBu
= F ˆw + Gd . (4.5)
Thus, ˆw evolves according to the same dynamics as in
(4.1b). Define the estimation error
e
w = w − ˆw. Then
˙
e
w = F
e
w, and since F is Hurwitz,
e
w(t) −→ 0, so ˆw pro-
vides an estimate of the exosystem state w. Further,
(4.4) satisfies the internal model principle by way of
(4.4c). For suppose that
ˆ
ψ = ψ. Substituting u into
(4.4c), we have
˙w
2
= Fw
2
− NB(u
s
+ u
im
)
= (F + Gψ)w
2
+ Gη
2
,
where η
2
= ψ(w
0
+Nx + w
1
)− u
s
. As such, this filter
includes the unstable poles of the exosystem. Finally,
the following result is proved in (Battle and Broucke,
2021).
Theorem 4.1. Consider the system (4.1) satisfying
Assumptions (A1)-(A8), and the regulator (4.4). Sup-
pose A + BK is Hurwitz. Then this regulator solves
the disturbance rejection problem.
On the Use of Regulator Theory in Neuroscience with Implications for Robotics
13
In comparing (4.4) with (3.1), there are three im-
portant points:
(i) The filter (4.4c), which fulfills the requirements
of the internal model principle, corresponds to the
nucleo-cortical pathway in (3.1e).
(ii) The model (4.4) bundles together filter inputs Nx,
Ax, and Bu in (4.4a)-(4.4c) based on prior knowl-
edge of the plant parameters. However, these filter
inputs need not be aggregated in this way. Mathe-
matically speaking, states or other sensory inputs
may arrive as filter inputs according to a number
of patterns or combinations, potentially depend-
ing on the structure of the open-loop system. This
mathematical flexibility, in turn, may imply that
an “unpacking” of MF inputs to the cerebellum is
necessary to determine their constituent compo-
nents, making the modeling problem more chal-
lenging.
(iii) The model (4.4) requires that all the filters (4.4a)-
(4.4c) have synchronized to utilize the same fil-
ter time constants, i.e. F
i
= F
j
. Since the fil-
ters (3.1c)-(3.1e) or (4.4a)-(4.4c) are nominally
intended to model the granular layer of the cere-
bellum, this raises the question of whether the
granular layer is capable of some form of dynamic
synchronization.
While we have identified intriquing analogies be-
tween cerebellar structure and internal model designs
for disturbance rejection from regulator theory, the
comparison is still somewhat superficial. The ocu-
lomotor system, discussed in the next section, pro-
vides more concrete evidence that such analogies can
be fruitful toward model building.
5 OCULOMOTOR SYSTEM
The oculomotor system comprises several eye move-
ment systems: the vestibulo-ocular reflex (VOR), op-
tokinetic system (OKS), the gaze fixation system, the
smooth pursuit system, the vergence system, and the
saccadic system. The oculomotor system provides a
good starting point for studying the cerebellum be-
cause it has a very simple plant (the eyeball), and it
is believed to provide the blueprint for all other motor
systems (Leigh and Zee, 2015).
5.1 VOR, Smooth Pursuit, and Gaze
Holding
In (Broucke, 2020; Broucke, 2021) we presented a
model of the VOR, smooth pursuit, and gaze holding
r − x
h
+
−
Σ
e
IO
B
FTN
u
˙x
h
P
MN
x
C
CF
MF
PC
u
im
u
c
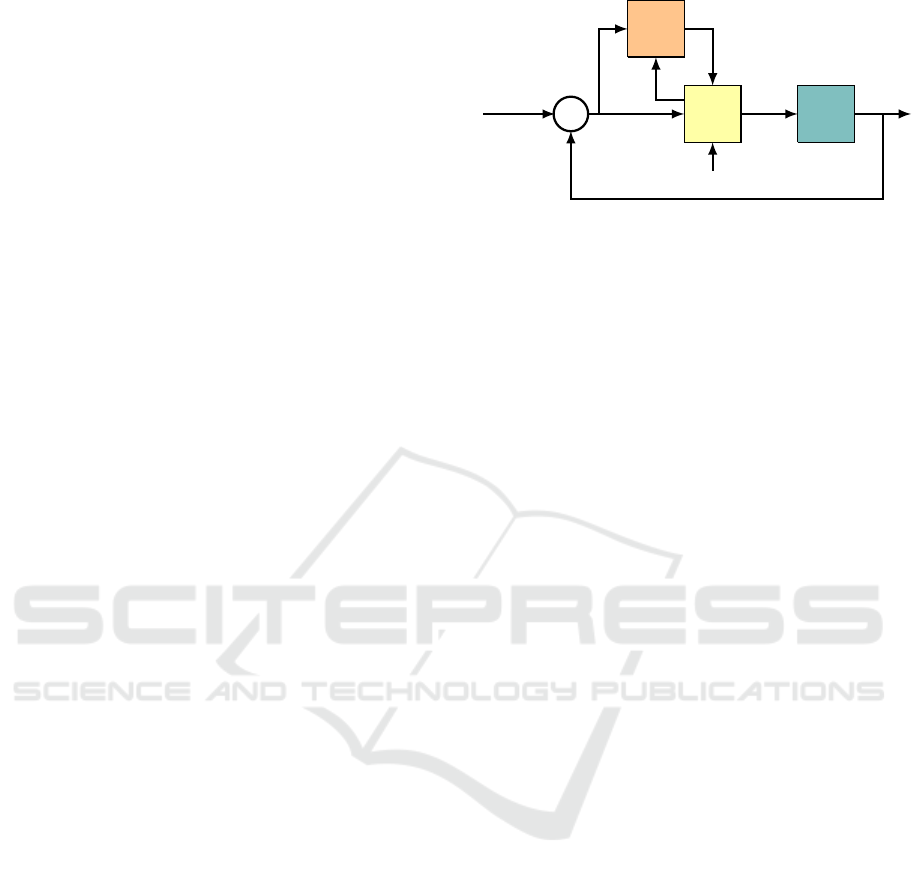
Figure 1: Control architecture for the VOR, gazing holding,
and smooth pursuit systems.
for horizontal motion of one eye by applying an adap-
tive internal model design from (Serrani and Isidori,
2000; Serrani et al., 2001). Let x ∈ R be the horizon-
tal eye angle, x
h
the horizontal head angle, and r the
horizontal angular position of a target. The model is:
˙x = −K
x
x + u (5.1a)
e = r − x − x
h
(5.1b)
˙
ˆx = −K
x
ˆx + u (5.1c)
˙w
1
= Fw
1
+ Gu
s
(5.1d)
˙w
2
= Fw
2
+ Gu
im
(5.1e)
ˆw := w
1
+ w
2
(5.1f)
˙
ˆ
ψ = γe ˆw
T
(5.1g)
u
b
= α
X
ˆx − α
VOR
˙x
h
(5.1h)
u
s
= K
e
e (5.1i)
u
im
=
ˆ
ψ ˆw (5.1j)
u = u
b
+ u
s
+ u
im
. (5.1k)
Equation (5.1a) is the first order model of the oculo-
motor plant (Sylvestre and Cullen, 1999). Equation
(5.1b) is the retinal error, the difference between the
target angle r and the gaze angle x + x
h
. It is this er-
ror that the cerebellum is tasked with driving asymp-
totically to zero. Equation (5.1c) models the brain-
stem neural integrator (Robinson, 1974) which acts
as an observer to provide an estimate ˆx of eye position.
Equations (5.1d)-(5.1g) comprise the adaptive inter-
nal model in the cerebellum. The motor command
u has a component u
b
corresponding to a brainstem-
only pathway for pure feedforward signals, a compo-
nent u
s
= K
e
e to improve closed-loop stability, and
u
im
, the cerebellar output from the PCs.
The model can be compared to the known neural
circuit associated with these eye movement systems
(B
¨
uttner and B
¨
uttner-Ennever, 2006), visualized by a
block diagram in Figure 1. The error signal (5.1b)
is transmitted from the visual cortex to the inferior
olive (IO), where it is relayed to appropriate climbing
fiber inputs (CFs) of the cerebellum (C), specifically,
the module called the floccular complex. This is the
signal e appearing in (5.1g). The cerebellum also re-
ICINCO 2021 - 18th International Conference on Informatics in Control, Automation and Robotics
14
ceives MF inputs from the medial vestibular nuclei
(MVN) in the brainstem (B). These are the MF inputs
in (5.1d)-(5.1d). The sole output of the cerebellum is
u
im
, transmitted via its PCs to floccular target neurons
(FTNs) in the MVN (Ramanchandran and Lisberger,
2008). The MVN also receives a head velocity sig-
nal from the semicircular canals of the ear, signal ˙x
h
in (5.1h), corresponding to the VOR. The parameter
α
VOR
is the VOR gain. The eye position signal α
X
ˆx
corresponds to the projection from the brainstem nu-
cleus prepositus hypoglossi (NPH) to the MNs. The
output of the MVN is sent both to the neural integra-
tor (NI) in the NPH and directly to the oculomotor
neurons (MNs) of the oculomotor plant (P).
As we discussed above, the MF inputs in (5.1d)-
(5.1e) may be bundled in a number of ways with no
effect on the overall behavior. For example, the two
filters could be combined into one, as was done in
(Serrani and Isidori, 2000), for a more parsimonious
model
˙
ˆw = F ˆw + G(u
s
+ u
im
).
This modification affects the choice of parameters be-
ing adapted, but it does not affect overall model be-
havior. Alternatively one could write
˙w
1
= Fw
1
+ FGe
˙w
2
= Fw
2
+ G(u − u
b
)
ˆw := (w
1
, w
2
)
The feedforward signals in u
b
are now arriving as MF
inputs to be subtracted from the overall motor com-
mand. This subtraction of feedforward signals is re-
quired so that their effect is not cancelled by the cere-
bellum (the cerebellum provides a top-up to the ac-
tion of feedforward signals). Depending on the origin
of the constituent components of the motor command
u in the brain, such an explicit subtraction of certain
MF inputs may arise, if not for the floccular complex,
possibly in another cerebellar module. Such a situ-
ation would certainly cloud an understanding of the
role of certain MF inputs to the cerebellum.
The model (5.1) recovers the standard lesion,
behavioral, and neurological experiments associated
with the VOR, gaze holding, and smooth pursuit; see
(Broucke, 2020; Broucke, 2021). Here we discuss
two interesting experiments which highlight the spe-
cial capabilities of the cerebellum.
A first experiment called the error clamp explores
the role of the error signal using a technique called
retinal stabilization (Barnes et al., 1995; Morris and
Lisberger, 1987; Stone and Lisberger, 1990). A mon-
key is trained to track a visual target moving hor-
izontally at constant speed. After reaching steady-
state, the error is optically clamped at zero using an
experimental apparatus that centers the target image
directly on the fovea. In experiments it is observed
that, despite zero error, the eye continues to track the
target for some time after. Neuroscientists postulate
so called extraretinal signals drive the smooth pur-
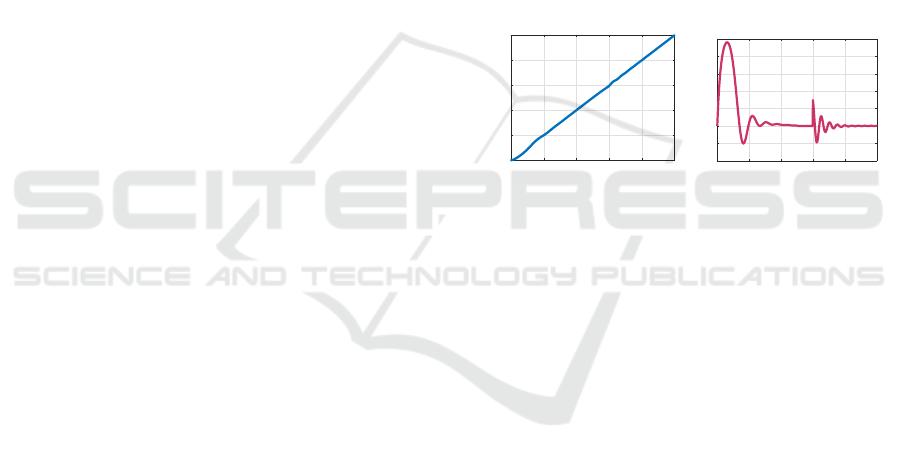
suit system. Figure 2 depicts the error clamp with
our model, showing on the left that the eye contin-
ues to track the target despite the measurement being
clamped at e ≡ 0 during the time interval t ∈ [5, 6].
The right figure shows the (physical) error, showing
that tracking is not robust during the time interval that
the measurement is clamped. In the second exper-
iment called target blanking, a horizontally moving
target is temporarily occluded, yet the eye continues
to track the target (Cerminara et al., 2009; Churchland
et al., 2003); researchers have postulated the brain has
an internal model of the motion of the target. In-
deed, direct measurement of the appropriate PCs of
the cerebellum shows that they remain active during
the time that the target is occluded.
0 2 4 6 8 10
Time (secs)
0
10
20
30
40
50
Eye Angle (deg)
0 2 4 6 8 10
Time (secs)
-0.4
-0.2
0
0.2
0.4
0.6
0.8
1
Error
Figure 2: Smooth pursuit with an error clamp during t ∈
[5, 6]s. The eye angle x on the left and the error e on the
right.
Let’s consider the meaning of these experiments
in terms of (5.1). In the first experiment, the error is
clamped at zero, so we can set e ≡ 0 to obtain
˙w
1
= Fw
1
˙w
2
= Fw
2
+ Gu
im
ˆw := w
1
+ w
2
.
Since F is Hurwitz, the first filter state w
1
in steady-
state is zero. Then the second filter evolves according
to ˙w
2
= (F + Gψ)w
2
, where F + Gψ is marginally
stable by (A3) (and assuming parameters have con-
verged). Clearly w
2
provides the drive for continued
pursuit during the error clamp, and it depends on the
nucleo-cortical pathway. However, in the second ex-
periment there is no error signal while the target is
occluded. It would certainly be paradoxical for the
cerebellum to continue to supply (on its own) a drive
for pursuit when there is no sensory error. We pos-
tulate that a higher brain center gates the activity of
the nucleo-cortical pathway. When there is no error
signal and/or the subject is not interested in an exter-
nal stimulus, then the pathway is disabled in order to
On the Use of Regulator Theory in Neuroscience with Implications for Robotics
15

arrive at the stable model
˙w
1
= Fw
1
˙w
2
= Fw
2
ˆw := w
1
+ w
2
, ,
in which all filter states gradually decay to a quies-
cent level of activity. However, when the error signal
is temporarily dropped but the subject remains inter-
ested in the stimulus (e.g. a lion pursuing prey), then
the nucleo-cortical pathway is not disabled. Finally,
these experiments raise intriguing questions for reg-
ulator theory. Are there engineering contexts when
a regulator should be capable to distinguish no error
measurement from a zero error measurement?
5.2 Optokinetic System
In the previous section we considered a model of a
part of the cerebellum, the floccular complex (FC),
involved in regulation of the vestibulo-ocular reflex,
smooth pursuit, and gazing hold eye movement sys-
tems. This section discusses a second functional mod-
ule of the cerebellum, the nodulus-uvula (NU) which
is responsible for regulating the optokinetic system.
The optokinetic system is an eye movement sys-
tem to stabilize vision on a full-field moving visual
surround. This eye movement system contrasts with
the eye movement systems of the previous section
whose goal is to stabilize an object on the fovea. How
the optokinetic system interacts with the other eye
movement systems is of great interest scientifically,
but also theoretically from the perspective of control
theory: can parallel adaptive internal models work
collaboratively to regulate the same error? Or does the
brain utilize a switching mechanism to switch from
one adaptive internal model to the other, reminiscent
of switched system architectures for adaptive control
(Narendra and Annaswamy, 1989)?
Pioneering experimental work in the 1970’s on the
optokinetic system (Cohen et al., 1977; Raphan et al.,
1979; Waespe and Henn, 1978a; Waespe and Henn,
1978b) lead to the discovery of the velocity storage
mechanism (VSM), a behavior in which eye velocity
is stored while following a constant velocity visual
surround, even with intervening saccades (a fast reset
of eye position) in a behavior called nystagmus. A
striking feature of the VSM is that it partially meets
the requirements of the internal model principle, as if
evolution made a first attempt at architecting a neural
internal model.
In (Battle and Broucke, 2021) we proposed a
model of the optokinetic system given by
˙x
1
= x
2
(5.2a)
˙x
2
= α
2
(−x
2
− K
x
x
1
+ u) (5.2b)
e = ˙x
w
− ˙x
h
− x
2
(5.2c)
˙
ˆx = −K
x
ˆx + u (5.2d)
˙v = −K
v
v + K
v
e (5.2e)
˙w
0
= Fw
0
+ FGe (5.2f)
˙w
1
= Fw
1
− Ge (5.2g)
˙w
2
= Fw
2
− Gu
im
(5.2h)
˙w
3
= Fw
3
− G ˆx (5.2i)
˙w
4
= Fw
4
− G ˙x
h
(5.2j)
˙w
5
= Fw
5
− Gv (5.2k)
ˆw = (w
0
+ Ge, w
1
, w
2
, w
3
, w
4
, w
5
) (5.2l)
˙
ˆ
ψ = γe ˆw
T
(5.2m)
u
im
=
ˆ
ψ ˆw (5.2n)
u
b
= α
X
ˆx − α
VOR
˙x
h
+ α
OK
e + α
V
v (5.2o)
u = u
b
+ u
im
. (5.2p)
We utilized a second-order model of the oculomo-
tor plant in (5.2a)-(5.2b), with x
1
the horizontal eye
angle and x
2
the eye angular velocity, because the op-
tokinetic system stabilizes eye velocity, not eye posi-
tion. The error signal e in (5.2c) to be regulated by the
cerebellum is the retinal slip velocity, the difference
between the horizontal angular velocity of the visual
field ˙x
w
and the gaze velocity x
2
+ ˙x
h
. A non-zero
˙x
w
is induced in experiments when a subject is seated
inside a rotating optical drum. The brainstem neural
integrator again appears in (5.2d). Equation (5.2e) is
the velocity storage integrator of the optokinetic sys-
tem (Cohen et al., 1977). The motor command u is
now regarded as an acceleration input; v is the state
of the velocity storage integrator; α
OK
e captures the
drive provided by the optokinetic reflex, where α
OK
is
the called the optokinetic gain; the vestibulo-ocular
reflex is modeled by α
VOR
˙x
h
, as before. The term α
V
v
captures the drive provided by the velocity storage in-
tegrator. Finally, we mention that there is no stabi-
lizing feedback u
s
in this model because the velocity
dynamics of the oculomotor plant are already highly
stable.
In comparing this model to the structural model
(3.1), we observe the additional filters (5.2f)-(5.2k)
driven by feedforward signals ˆx, ˙x
h
, and v. Mathe-
matically speaking, it can be shown that if these sig-
nals are not included as MF inputs, then they would
be cancelled or rejected by the activity of the nodu-
lus/uvula as predicted by the model. Thus, a pattern
we have already highlighted on the variable roles of
certain MF inputs is reinforced again with this model:
mathematically speaking, MF inputs may either ap-
ICINCO 2021 - 18th International Conference on Informatics in Control, Automation and Robotics
16

pear because they are directly involved in disturbance
estimation or they appear to avoid being cancelled by
the cerebellum.
The model (5.2) is consistent with the neural cir-
cuit, and it recovers five standard behaviors of the op-
tokinetic system: optokinetic nystagmus (OKN); op-
tokinetic after-nystagmus (OKAN I); OKAN suppres-
sion; OKN suppression; and OKAN II. For instance,
OKN is an eye movement in which the eye tracks the
velocity of a (full-field) moving visual surround dur-
ing the so-called slow phase, followed by a saccade to
rapidly reset the eye position to zero in the fast phase
(Cohen et al., 1977; Raphan et al., 1979). OKAN I is
a behavior following OKN when the lights are turned
off. During OKAN I nystagmus continues in the same
direction as OKN, even though there is no visual stim-
ulation (Cohen et al., 1977; B
¨
uttner et al., 1976).
Figure 3 shows simulation results for OKN and
OKAN I using our model, with the optokinetic drum
rotating at a constant velocity of 60 deg/s for 60s. The
initial jump in slow phase eye velocity is attributable
to the large retinal slip velocity at the onset of the
experiment and the charging of the VSM. The non-
zero steady-state error during OKN is observed be-
cause the NU internal model is “untrained”. Once the
lights are extinguished at t = 60s, visual signals are no
longer present and the cerebellum is effectively inac-
tive, the signal e is unavailable, and u
im
= 0 (based on
gating the nucleo-cortical pathway). This causes the
slow-phase eye velocity to rely on the dynamics from
the VSM, which slowly dissipates its stored velocity,
creating OKAN I.
If the subject is involved in repeated trials of the
same experiment eliciting OKN and OKAN I, the
NU internal model is “trained” over time. Conse-
quently, the OKN steady-state slow-phase eye veloc-
ity increases (Miki et al., 2020, Fig 1); the OKAN
I time constant decreases (Cohen et al., 1977, Fig
7); and the OKAN I duration decreases (Waespe and
Henn, 1978b, Fig 2, 3). These results are shown on
the right of Figure 3.
0 50 100 150 200
Time (secs)
0
10
20
30
40
50
60
Stimulus and Eye Velocities (deg/s)
0 50 100 150 200
Time (secs)
0
10
20
30
40
50
60
Stimulus and Eye Velocities (deg/s)
Figure 3: Untrained (left) and trained (right) OKN and
OKAN I.
This section has demonstrated that a disturbance
rejection interpretation of cerebellar function can be
propitious to arrive at plausible models for one mo-
tor system: the oculomotor system. However, one
may also utilize regulator theory to understand other
adaptive behaviors that are best modeled as discrete,
repetitive processes. Perhaps the most widely studied
adaptive, discrete process is visuomotor adaptation,
considered in the next section.
6 VISUOMOTOR ADAPTATION
Visuomotor adaptation is a subconscious, “machine-
like” brain process taking place over repetitive trials
and elicited by a visual error closely following the
execution of a movement. Visuomotor adaptation is
intended to calibrate over a lifetime the mapping be-
tween what is seen and how to move. As a means
to expose the underlying computations of this brain
process, neuroscientists create experiments that ar-
tificially perturb what is seen by the subject during
movement. Examples include saccades with an inter-
saccadic step of the target (Kojima et al., 2004); the
visuomotor rotation experiment with fast arm reaches
(Smith et al., 2006; Shadmehr and Wise, 2005); and
throwing darts while looking through prism glasses
(Martin et al., 1996).
Visuomotor adaptation experiments consist of
repetitive trials of a certain movement such as a sac-
cade or arm reach. The trials are classified by type,
and sequences of blocks of trials of specific types are
utilized to elicit so-called dynamic behaviors of adap-
tation. A baseline (B) block familiarizes the subject
with the experimental aparatus under unperturbed,
normal conditions. A learning (L) block occurs after
a baseline block when a perturbation or disturbance is
introduced. A washout (W) block follows a learning
block when the perturbation is removed. An unlearn-
ing (U) block follows a learning block when the per-
turbation changes in sign but not magnitute relative to
the learning block. A relearning (R) block is a second
learning block with the same perturbation. A down-
scaling block (D) is a second learning block in which
the perturbation is set to a fraction of its value in the
first learning block. A no-visual-feedback (N) block
is a block of trials in which no error measurement is
presented to the subject. An error clamp (C) block
is a block of trials when the error measurement pre-
sented to the subject is clamped artificially to a value
unrelated to the subject’s movements. When blocks
of trials are sequenced in a particular order and with a
particular number of trials in each block, then several
phenomena emerge in experiments:
• Savings is a behavior in which learning is sped up
in the second learning block relative to the first
one.
On the Use of Regulator Theory in Neuroscience with Implications for Robotics
17
• Reduced savings is a behavior in which savings is
reduced by inserting a washout block of trials after
the unlearning block. After the washout block,
relearning does not proceed as rapidly as in the
savings experiment.
• Anterograde interference is a behavior in which
a previously learned task reduces the rate of sub-
sequent learning of a different (and usually oppo-
site) task.
• Rapid unlearning is a behavior in which the rate
of unlearning is faster than the rate of initial learn-
ing, if the number of trials in the learning block is
small.
• Rapid downscaling is a behavior in which the rate
of learning in a secondary learning block is faster
when the rotation is set to a fraction of its value in
the initial learning block.
• Spontaneous recovery is a behavior observed dur-
ing the washout block of a BLUW experiment
in which the response partially “rebounds” to its
value at the end of the learning block rather than
converging monotonically to zero.
0 100 200 300
Trial
-40
-20
0
20
40
Disturbance
0 100 200 300
Trial
0
10
20
30
x(k)
0 10 20 30
Trial
0
5
10
15
20
25
x(k)
Learning
Relearning
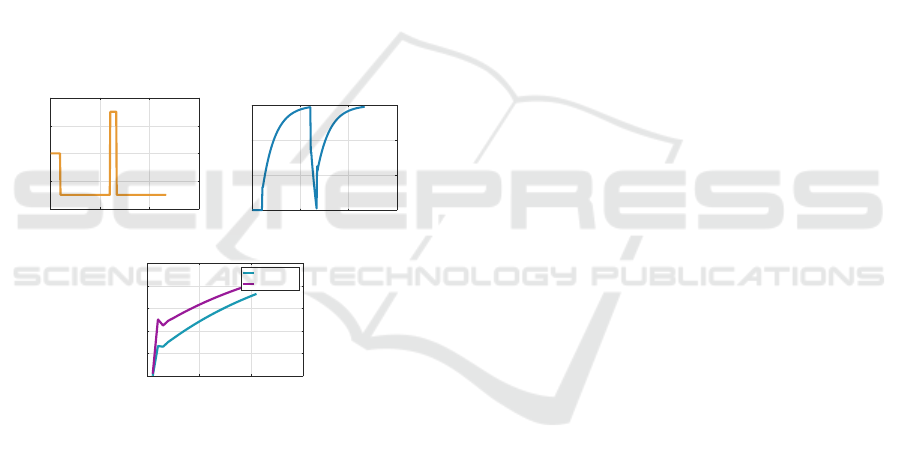
Figure 4: Savings in a BLUR experiment. In the bottom
figure x(k) during the learning block is plotted in blue su-
perimposed with a horizontally shifted version of x(k) dur-
ing the relearning block in purple. The purple curve is larger
than the blue curve corresponding to faster learning in the
relearning block.
We used regulator theory to develop a model of
visuomotor adaptation in (Gawad and Broucke, 2020;
Hafez et al., 2021) with the goal to recover the six
standard behaviors of visuomotor adaptation. The
model was based on three assumptions. First, we
focused on motor adaptation tasks involving one de-
gree of freedom of movement; for instance, horizon-
tal movement of the eye, hand angle relative to a ref-
erence angle in a horizontal plane, forward (coronal)
inclination of the body relative to a vertical reference,
the horizontal angle of a dart thrown by a subject, and
so forth. Second, we assumed the open-loop model is
linear time-invariant. Third, we focused on constant
disturbances, as currently there is a dearth of experi-
ments with non-constant disturbances.
Let integer k be the trial number; x(k) is the state
of a single degree of freedom of the body at the end
of the k-th trial; d(k) is an additive disturbance in the
measurement during the k-th trial; and e(k) is the error
between a measurement y(k) = x(k) + d(k) observed
by the subject at the end of the k-th trial and a ref-
erence r(k). Our discrete-time model of visuomotor
adaptation is:
x(k + 1) = Ax(k) + Bu(k) (6.1a)
e(k) = r(k) − d(k) − x(k) (6.1b)
w
0
(k + 1) = Fw
0
(k) + FGe(k) (6.1c)
w
1
(k + 1) = Fw
1
(k) − Ge(k) (6.1d)
w
2
(k + 1) = Fw
2
(k) − Gu(k) (6.1e)
ˆw(k) = (w
0
(k) + Ge(k), w
1
(k), w
2
(k)) (6.1f)
u(k) = Ke(k) + K
w
ψ ˆw(k). (6.1g)
The open-loop system model (6.1a) provides a high-
level, abstract description of the quantitative change
over successive trials of a single degree of freedom of
the body. The term Ax(k) models a retention or mem-
ory mechanism of the state in the previous trial. We
assume the filters (6.1c)-(6.1e) are stable; that is, F is
Schur stable. We have not written a parameter adap-
tation law for unknown parameters ψ (although one
may do so) since experiments show that the parame-
ters vary extremely slowly; see (Gawad and Broucke,
2020). The controller u has the same components
as before: u
s
(k) = Ke(k) is to improve closed-loop
stability, while u
im
= K
w
ψ ˆw(k) is the component to
satisfy the internal model principle. The parameter
0 < K
w
< 1 is explained below.
Figures 4 shows a simulation for a BLUR exper-
iment to elicit savings. The top left figure shows the
disturbance value d(k) as a function of k, and the top
right figure shows x(k). The bottom figure shows x(k)
during the learning block superimposed with x(k) dur-
ing the relearning block. We see that relearning is
faster than learning, demonstrating that savings have
indeed occurred in the relearning block.
Visuomotor adaptation experiments analogous to
the error clamp and target blanking experiments for
the smooth pursuit system have also been performed.
These provide dramatic evidence of the brain’s capa-
bility to enable or disable internal models. For in-
stance, many experimental studies of the form BLN
have been conducted on the effect of removing the vi-
sual error in an N block following a learning block
(Galea et al., 2011; Kitago et al., 2013; Bond and
Taylor, 2015). The major finding is that during the N
block, x(k) slowly returns to a nominal reference po-
ICINCO 2021 - 18th International Conference on Informatics in Control, Automation and Robotics
18
sition. Further, Figure 2 of (Kitago et al., 2013) shows
that the rate of decay is faster in a W block than an N
block.
As we already discussed for the oculomotor sys-
tem, when there is no error measurement, we must
remove the signal e(k) from every filter input in (6.1).
To disable the internal model, it is also necessary to
disable the efference copy u(k) in (6.1e). The re-
sulting internal model will consist of filters that are
all stable, and therefore the response x(k) will grad-
ually return to a zero reference value. In summary,
if we assume that visuomotor adaptation operates in
a manner that is consistent with the oculomotor sys-
tem, then gating the nucleo-cortical pathway provides
an explanation for how internal models can be en-
abled or disabled in visuomotor adaptation. Figure 5
shows the results for (6.1) for a BLN experiment us-
ing this method to disable the internal model during
the N block.
0 20 40 60 80
Trial
0
10
20
30
40
x(k)
N
W
Figure 5: BLN experiment v.s. BLW experiment.
A number of error clamp experiments of the form
BLC(µ) have been reported including (Smith et al.,
2006; Kitago et al., 2013; Shmuelof et al., 2012;
Vaswani et al., 2015). In these experiments a sub-
ject makes fast arm reaches to a target on a computer
screen while observing a cursor intended to represent
the hand position at the end of a reach. A disturbance
d(k) is introduced during the learning blocks so that
the observed cursor angle is y(k) = x(k) + d(k). Dur-
ing error clamp blocks, the error observed by the sub-
ject is clamped at a constant value e(k) ≡ e. Figure 2
of (Vaswani et al., 2015) reported results with various
statistics on the error clamp value. These experiments
further expose interesting on/off behavior of internal
models associated with visuomotor adaptation. In a
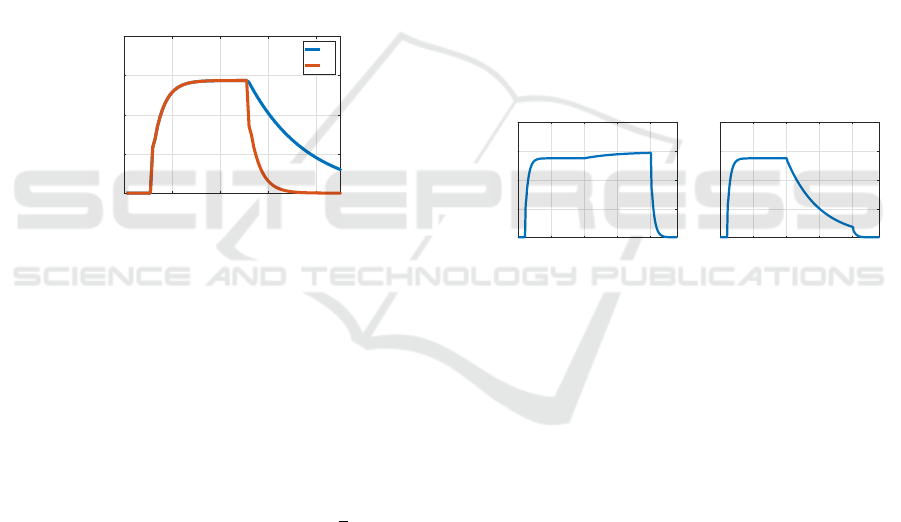
C(−2.7) block with e(k) ≡ −2.7, it is observed that
the hand angle remains close to its value at end of the
learning block. In a C(0) block with e(k) ≡ 0, the
hand angle returned to zero at a slow rate. Figure 6
shows the behavior of our model in a BLC(−2.7) ex-
periment. We observe the hand angle remains close
to 30
◦
, its value at the end of the learning block, as re-
ported in (Vaswani et al., 2015). By comparison, the
right figure shows a BLC(0) experiment. Now the
hand angle slowly returns to zero.
The behavior in Figure 6 is nothing like what a
control theorist expects of an internal model. We ex-
pect that when e(k) is clamped at zero, the behavior
is as in the left of the figure, but when e(k) is clamped
at a non-zero value, the internal model is unstable.
Instead, the experiments demonstate that the human
brain has made a “hedge” on the internal model prin-
ciple: zero error signals induce a return to a quiescent
state, while a persistent, small, non-zero error is nec-
essary to keep the internal model active. We have used
the parameter 0 < K
w
< 1 to quantitatively charac-
terize this hedge; however, this single parameter cer-
tainly does not complete the story. There are likely
deeper meanings behind this curious phenomenon.
We summarize by saying that the preponderance
of experimental evidence may well point to the idea
that the sole reason for the special wiring of the cere-
bellum, particularly the nucleo-cortical pathway, is to
implement the delicate operation of enabling and dis-
abling internal models without inducing abrupt or un-
stable behavior in the subject.
0 50 100 150 200
Trial
0
10
20
30
40
x(k)
0 50 100 150 200
Trial
0
10
20
30
40
x(k)
Figure 6: BLC experiment, comparing C(-2.7) and C(0)
blocks.
7 IMPLICATIONS FOR
ROBOTICS
We have given evidence that there is value to study the
cerebellum from the perspective of disturbance rejec-
tion and to utilize regulator theory to derive models.
We argue here that the study of the cerebellum us-
ing regulator theory has implications for robotics. We
make our case using an example of a robot learning a
new tool. The discussion is informal, as the primary
aim is to stimulate new ideas rather than to prove cor-
rectness of fully developed algorithms, etc.
Consider a robot equipped with an arm and
foveated, movable cameras resembling the function-
ality of the human eye. The robot is capable to per-
form rapid reaching movements with its arm using
feedforward, pre-learned commands. The robot is
tasked with performing such rapid movements while
manipulating a new handheld tool - for example a
brush with a long handle to remove brambles from
On the Use of Regulator Theory in Neuroscience with Implications for Robotics
19

a dog. We pose the problem of training the robot
to learn a new tool as a disturbance rejection prob-
lem. For simplicity, consider one degree of freedom
of movement, say the final horizontal angle of the
robot’s end effector at the end of a reach. We make
the following assumptions:
Assumption 7.1.
• N stationary targets are randomly positioned in
the robot’s visual field but sufficiently separated
that the robot is able to measure a distinct error
between the arm (or the tool) end effector and any
foveated target. Let r(i) denote the horizontal an-
gular position of the ith target for i = 1, . .. , N.
• Each target i has associated to it a feedforward
(non-error-based) motor command denoted u
f ,i
that was acquired through prior experience dur-
ing nominal behavior (without using tools). We
assume u
f ,i
drives the robot end-effector directly
to target position r(i) with negligible error under
nominal conditions.
• The visual field is partitioned into sectors called
adaptation fields. Each adaptation field has as-
sociated to it an adaptive internal model. For
simplicity we assume there is only one target per
adaptation field.
• The robot has the efficacy to foveate any target
at the end of a reach, at will. The index of the
target that is foveated at the end of the kth reach is
m(k) ∈ {1, . . .,N}.
• The robot has the efficacy to choose any target for
the next reach, at will. The index of the target for
the (k + 1)th reach is t(k) ∈ {1, . . . , N}.
Let x(k) represent the robot end-effector horizon-
tal angle at the end of the kth reach, and d(k) is the
constant angular offset introduced by the tool. The
open-loop model is:
x(k + 1) = u(k)
e(k) = r(m(k)) − x(k) − d(k).
The first equation says the robot is capable to move
to any commanded horizontal angular position (the
true nonlinear robot model may require a prelimi-
nary feedback linearization step to achieve this simple
form). The second equation defines the error mea-
sured at the end of the kth reach, the angular displace-
ment between the m(k)th target and the end of the
tool. Because it is highly expensive to process full-
field visual information, the robot only records error
measurements for targets it has foveated on. Further,
we assume that the trigger signal to update any inter-
nal model is its own error signal. In other words, the
robot’s gaze at the end of the reach determines the in-
ternal model to be updated. The internal model update
for a target that has been foveated is:
w
0,m
(k + 1) = Fw
0,m
(k) + FGe(k)
w
2,m
(k + 1) = Fw
2,m
(k) + G(u(k) − u
f ,m
)
ˆw
m
(k) = w
0,m
(k) + Ge(k) − w
2,m
(k) ,
where m = m(k) is as above. All other internal models
with i 6= m(k) have an update of the form:
w
0,i
(k + 1) = F
n
w
0,i
(k)
w
2,i
(k + 1) = F
n
w
2,i
(k)
ˆw
i
(k) = w
0,i
(k, i) − w
2,i
(k)
This second update is a proxy for “no update”. We set
F
n
= 0.999, meaning the internal model i very slowly
dissipates its state until the next update when m(k) =
i. Finally the motor command is
u(k) = u
f ,t
+ ψ ˆw
t
(k) ,
where t = t(k) is as above, and ψ represents unknown
parameters (that have been adapted apriori, hence the
adaptation process is omitted). Several observations
are in order.
• We have subtracted off the feedforward command
u
f ,m
from the motor command u(k) in analogy
with our discussion for the oculomotor system.
Recall that this intervention was required for the
slow eye movement systems to ensure the cere-
bellum does not cancel the effect of useful feed-
forward signals. Internal models are intended to
work synergistically with feedforward signals, a
notion well expounded particularly with regard to
the VOR (Ito, 1984).
• The internal model output that appears in the mo-
tor command is dictated by the choice of feed-
forward command, which is itself subject to the
robot’s will. That is, feedforward commands and
their associated internal model output are always
paired.
• Our model dissociates the updating of an internal
model from the ensuing motor command. That
is, some internal model updates are unobservable,
possibly only to be revealed as aftereffects once
the experiment is concluded.
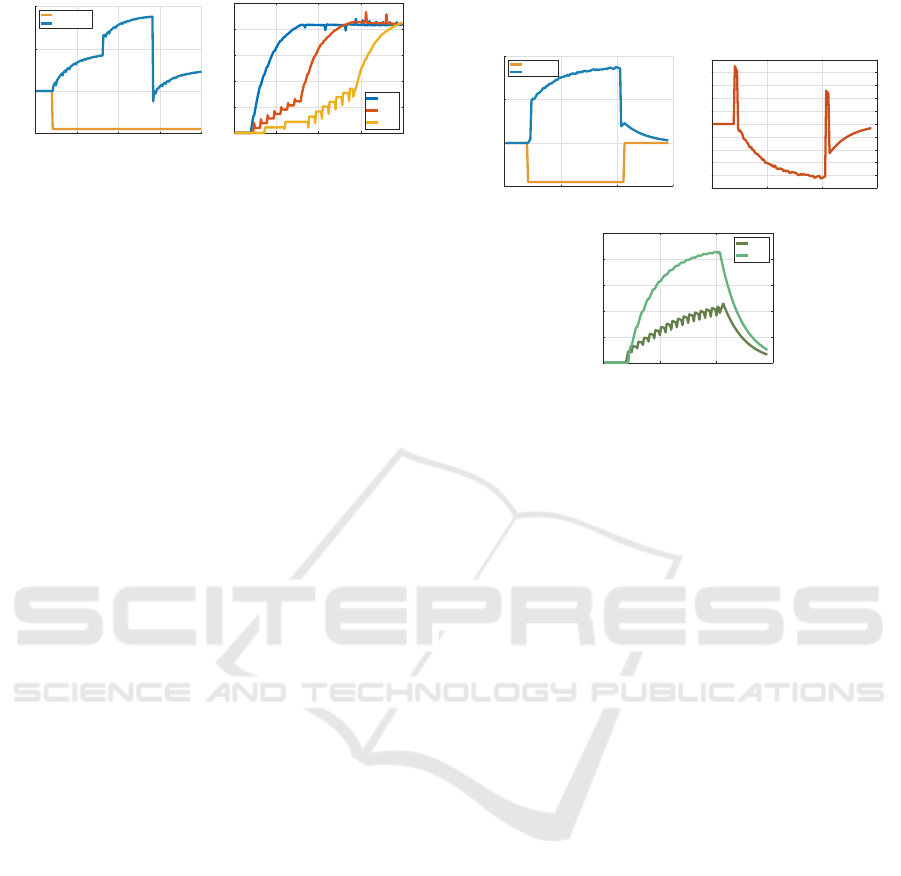
Simulation results using this model are shown in
Figure 7. There are three targets with horizontal an-
gular positions r(1) = 0, r(2) = 45, and r(3) = −20.
The handheld tool causes a disturbance in the arm po-
sition by an amount of −45 degrees. After a baseline
block of 20 trials with no tool, the robot picks up the
tool and reaches for target 1 for 60 trials, then target 2
for 60 trials, and target 3 for the last 60 trials. While
the robot reaches for target 1, it glances at target 2 at
the end of every 8th reach and at target 3 at the end
of every 12th reach. We see in the right figure that
ICINCO 2021 - 18th International Conference on Informatics in Control, Automation and Robotics
20
0 50 100 150 200
Trial
-50
0
50
100
Disturbance
Arm Angle
0 50 100 150 200
Trial
0
10
20
30
40
50
what
1
what
2
what
3
Figure 7: A robot arm reaching for each of three targets,
while occasionally glancing at the other two. The left figure
shows the arm angle and the right figure shows ˆw
i
(k) for the
three internal models.
the internal models begin “charging up” as they col-
lectively estimate the disturbance induced by holding
the tool. By the time the robot reaches for the second
target, the first internal model is almost fully charged.
Only infrequent glances are needed to retain a fresh
estimate of the disturbance by this internal model. Fi-
nally, by the end of the experiment, all internal models
have approached a consensus value on the disturbance
induced by holding a tool. The robot may save the
motor memory, so if this tool is encountered again,
the revised motor commands may be immediately re-
called.
The model we have presented for simple tool
learning was not an abstract exercise, but has been
inspired by our study of visuomotor experiments with
human subjects. To bring home this point, we ap-
ply our model to a well-known experiment on feed-
forward (so-called explicit) strategies in visuomotor
adaptation (Mazzoni and Krakauer, 2006). A subject
is presented with two targets separated by a fixed an-
gle of 45 degrees. The subject is instructed to aim for
the second target at r(2) = 45 degrees while observ-
ing a cursor position on a computer screen displayed
at the end of each reach. The cursor position has been
rotated by d(k) = −45 degrees from the true hand an-
gle, so that by aiming for the second target, the subject
is able to make the error between the cursor and a first
target at r(1) = 0 degrees be close to zero.
We can simulate this experiment using our learn-
ing model. We assume the subject aims for the second
target in all trials, so t(k) = 2, according to the instruc-
tions of the experimenter. However, the subject occas-
sionally shifts the gaze at the end of a reach to the first
target (Rand and Rentsch, 2015; de Brouwer et al.,
2018). Suppose the subject attends to the first target at
the end of 20 percent of trials and to the second target
in 80 percent of trials. Simulation results are shown in
Figure 8. The top figures show qualitatively the same
results as obtained in (Mazzoni and Krakauer, 2006).
The bottom figure shows the response of the internal
models - both are estimating −d(k) = 45 (the minus
sign is an artifact of our choice of parameters, and is
not significant). The second internal model is faster
because it experiences more frequent updates.
0 50 100 150
Trial
-50
0
50
100
Disturbance
Hand Angle
0 50 100 150
Trial
-50
-40
-30
-20
-10
0
10
20
30
40
50
Error
0 50 100 150
Trial
0
10
20
30
40
50
what
1
what
2
Figure 8: Mazzoni and Krakauer’s experiment. The top left
figure displays the disturbance d(k) and the hand angle x(k)
as a function of the trial number k. The top right figure
shows the error e(k) with respect to the first target only. The
bottom figure shows the internal model states ˆw
1
(k), ˆw
2
(k)
as a function of k.
8 CONCLUSION
This paper has presented an overview of results on the
use of regulator theory to interpret and model the con-
tribution of the cerebellum to motor systems. We con-
sidered the slow eye movement systems: the VOR,
gaze holding, and smooth pursuit, as well as the op-
tokinetic system. We found that using regulator the-
ory one could derive models that are consistent with
the known neural circuits and also recover the stan-
dard experimental results for those motor systems.
We also surveyed results for visuomotor adaptation;
despite the fact that the models are discrete-time dif-
ference equations rather than differential equations, a
nearly identical methodology as in continuous time
could be employed to derive a model that recovers
many of the standard experimental results.
The paper has several takeaways on the form of
the resulting internal models, and what they may tell
us about the cerebellum. A recurring pattern in the
motor systems we examined is that the cerebellum
must be endowed with a special capability to enable
and disable internal models without causing damage
to the body. We have identified the nucleo-cortical
pathway as a possible mechanism to implement this
capability.
Finally, this paper has argued that an approach to
modeling the cerebellum based on regulator theory
can well serve a research agenda to develop humanoid
robots that possess cerebellar-like intelligences.
On the Use of Regulator Theory in Neuroscience with Implications for Robotics
21

REFERENCES
Apps, R., Hawkes, R., and et. al., S. A. (2018). Cerebellar
modules and their role as operational cerebellar pro-
cessing units. Cerebellum, 17:654–682.
Barnes, G., Goodbody, S., and Collins, S. (1995). Volitional
control of anticipatory ocular pursuit responses under
stabilized image conditions in humans. Experimental
Brain Research, 106:301–317.
Battle, E. and Broucke, M. (2021). Adaptive internal mod-
els in the optokinetic system. In IEEE Conference on
Decision and Control. Submitted.
Bond, K. and Taylor, J. (2015). Flexible explicit but
rigid implicit learning in a visuomotor adaptation task.
Journal of Neurophysiology, 113:3836–3849.
Broucke, M. E. (2020). Model of the oculomotor sys-
tem based on adaptive internal models. IFAC-
PapersOnLine, 53(2):16430–16437. 21th IFAC World
Congress.
Broucke, M. E. (2021). Adaptive internal model theory
of the oculomotor system and the cerebellum. IEEE
Transactions on Automatic Control.
B
¨
uttner, U. and B
¨
uttner-Ennever, J. (2006). Present con-
cepts of oculomotor organization. Prog. Brain Re-
search, 151:1–42.
B
¨
uttner, U., Waespe, W., and Henn, V. (1976). Duration and
direction of optokinetic after-nystagmus as a function
of stimulus exposure time in the monkey. Arch. Psy-
chiat. Nervenkr., 222:281–291.
Cerminara, N., Apps, R., and Marple-Horvat, D. (2009). An
internal model of a moving visual target in the lateral
cerebellum. J. Physiology, 587(2):429–442.
Churchland, M., Chou, I., and Lisberger, S. (2003). Ev-
idence for object permanence in the smooth-pursuit
eye movements of monkeys. Journal of Neurophysi-
ology, 90:2205–2218.
Cohen, B., Matsuo, V., and Raphan, T. (1977). Quantitative
analysis of the velocity characteristics of optokinetic
mystagmus and optokinetic after-nystagmus. J. Phys-
iology, 270:321–344.
de Brouwer, A., Albaghdadi, M., Flanagan, J., and Gallivan,
J. (2018). Using gaze behavior to parcellate the ex-
plicit and implicit contributions to visuomotor learn-
ing. Journal of Neurophysiology, 120:1602–1615.
Eccles, J., Ito, M., and Szentagothai, J. (1967). The Cere-
bellum as a Neuronal Machine. Springer.
Francis, B. and Wonham, W. (1976). The internal model
principle of control theory. Automatica, 12:457–465.
Fujita, M. (1982). Adaptive filter model of the cerebellum.
Biological Cybernetics, 45:195–206.
Galea, J., Vazquez, A., Pasricha, N., de Xivry, J., and Cel-
nik, P. (2011). Dissociating the roles of the cerebellum
and motor cortex during adaptive learning: the motor
cortex retains what the cerebellum learns. Cerebral
Cortex, 21:1761—-1770.
Gawad, A. A. and Broucke, M. E. (2020). Visuomotor adap-
tation is a disturbance rejection problem. In IEEE
Conference on Decision and Control, pages 3895–
3900.
Hafez, M., Uzeda, E., and Broucke, M. (2021). Discrete-
time output regulation and visuomotor adaptation.
Letters of the Control Systems Society. Submitted.
Houck, B. D. and Person, A. L. (2014). Cerebellar loops:
a review of the nucleocortical pathway. Cerebellum,
13:378–385.
Ito, M. (1984). The Cerebellum and Neural Control. Raven
Press.
Kanellakopoulos, M. K. I. and Kokotovic, P. (1995).
Nonlinear and Adaptive Control Design. Wiley-
Interscience.
Kitago, T., Ryan, S., Mazzoni, P., Krakauer, J., and Haith,
A. (2013). Unlearning versus savings in visuomotor
adaptation: comparing effects of washout, passage of
time, and removal of errors on motor memory. Fron-
tiers in Human Neuroscience, 7(307).
Kojima, Y., Iwamoto, Y., and Yoshida, K. (2004). Memory
of learning facilitates saccadic adaptation in the mon-
key. Journal of Neuroscience, 24(34):7531–7539.
Kreisselmeier, G. (1977). Adaptive observers with expo-
nential rate of convergence. IEEE Transactions on
Automatic Control, 22(1):2–8.
Leigh, R. J. and Zee, D. S. (2015). The Neurology of Eye
Movements. Oxford University Press, 5th ed.
Lisberger, S. (2009). Internal models of eye movement in
the floccular complex of the monkey cerebellum. Neu-
roscience, 162(3):763–776.
Martin, T., Keating, J., Goodkin, H., Bastian, A., and
Thach, W. (1996). Throwing while looking through
prisms ii. specificity and storage of multiple gaze-
throw calibrations. Brain, 119:1199 – 1211.
Mazzoni, P. and Krakauer, J. (2006). An implicit plan over-
rides an explicit strategy during visuomotor adapta-
tion. Journal of Neuroscience, 26:3642–3645.
Miki, S., Urase, K., Baker, R., and Hirata, Y. (2020). Ve-
locity storage mechanism drives a cerebellar clock for
predictive eye velocity control. Nature Science Re-
ports, 10(6944).
Morris, E. and Lisberger, S. (1987). Different responses to
small visual errors during initiation and maintenance
of smooth-pursuit eye movements in monkeys. Jour-
nal of Neurophysiology, 58(6):1351–1369.
Narendra, K. and Annaswamy, A. (1989). Stable Adaptive
Systems. Dover Publications.
Nikiforov, V. O. (2004a). Observers of external determin-
istic disturbances i. objects with known parameters.
Automation and Remote Control, 65(10):1531–1541.
Nikiforov, V. O. (2004b). Observers of external determinis-
tic disturbances ii. objects with unknown parameters.
Automation and Remote Control, 65(11):1724–1732.
Ramanchandran, R. and Lisberger, S. (2008). Neural sub-
strate of modified and unmodified pathways for learn-
ing in monkey vestibuloocular reflex. J. Neurophysi-
ology, 100:1868–1878.
Rand, M. and Rentsch, S. (2015). Gaze locations affect ex-
plicit process but not implicit process during visuomo-
tor adaptation. Journal of Neurophysiology, 113:88–
99.
ICINCO 2021 - 18th International Conference on Informatics in Control, Automation and Robotics
22

Raphan, T., Matsuo, V., and Cohen, B. (1979). Velocity
storage in the vestibulo-ocular reflex arc (vor). Exper-
imental Brain Research, 35:229–248.
Robinson, D. A. (1974). The effect of cerebellectomy on
the cat’s vestibulo-ocular integrator. Brain Research,
71(2):195–207.
Ruigrok, T. (2011). Ins and outs of cerebellar modules.
Cerebellum, 10:464–474.
Saberi, A., Stoorvogel, A., and Sannuti, P. (2000). Con-
trol of Linear Systems with Regulation and Input Con-
straints. Springer.
Serrani, A. and Isidori, A. (2000). Semiglobal nonlinear
output regulation with adaptive internal model. IEEE
Conference on Decision and Control, pages 1649–
1654.
Serrani, A., Isidori, A., and Marconi, L. (2001). Semi-
global nonlinear output regulation with adaptive inter-
nal model. IEEE Transactions on Automatic Control,
46(8):1178–1194.
Shadmehr, R. and Wise, S. (2005). The Computational Neu-
robiology of Reaching and Pointing. MIT Press.
Shmuelof, L., Hang, V., Haith, A., Dekicki, R., Mazzoni, P.,
and Krakauer, J. (2012). Overcoming motor forgetting
through reinforcement of learned actions. Journal of
Neuroscience, 32(42):14617–14621.
Smith, M., Ghazizadeh, A., and Shadmehr, R. (2006). In-
teracting adaptive processes with different timescales
underlie short-term motor learning. PLOS Computa-
tional Biology, 4(6).
Stone, L. S. and Lisberger, S. G. (1990). Visual responses
of purkinje cells in the cerebellar flocculus during
smooth-pursuit eye movements in monkeys. i. sim-
ple spikes. Journal of Neurophysiology, 63(5):1241–
1261.
Sylvestre, P. A. and Cullen, K. E. (1999). Quantitative anal-
ysis of abducens neuron discharge dynamics during
saccadic and slow eye movements. Journal of Neuro-
physiology, 82(5):2612–2632.
Vaswani, P., Shmuelof, L., Haith, A., Deknicki, R., Huang,
V., Mazzoni, P., Shadmehr, R., and Krakauer, J.
(2015). Persistent residual errors in motor adaptation
tasks: reversion to baseline and exploratory escape.
Journal of Neuroscience, 35(17):6969–6977.
Waespe, W. and Henn, V. (1978a). Conflicting visual-
vestibular stimulation and vestibular nucleus activ-
ity in alert monkeys. Experimental Brain Research,
33:203–211.
Waespe, W. and Henn, V. (1978b). Reciprocal changes
in primary and secondary optokinetic after-nystagmus
(okan) produced by repetitive optokinetic stimula-
tion in the monkey. Archiv Psychiatrie und Ner-
venkrankheiten, 225:23–30.
Wonham, W. M. (1985). Linear Multivariable Control: a
Geometric Approach. Springer-Verlag.
On the Use of Regulator Theory in Neuroscience with Implications for Robotics
23
