
Extending the Fast Healthcare Interoperability Resources (FHIR)
with Meta Resources
Timoteus Ziminski
1a
, Steven Demurjian
1b
and Thomas Agresta
2c
1
Department of Computer Science and Engineering, University of Connecticut, 371 Fairfield Way, Storrs, U.S.A.
2
Department of Family Medicine, UConn Health, 263 Farmington Avenue, Farmington, U.S.A.
{Timoteus.Ziminski, Steven.Demurjian, Thomas.Agresta }@uconn.edu
Keywords: Data Design, FHIR, Interoperability, Medication Reconciliation, Patterns.
Abstract: The Fast Healthcare Interoperability Resources (FHIR) from the international Health Language Seven (HL7)
organization has been mandated by the United States Office of National Coordinator to promote the secure
exchange of healthcare data for patients through the use of cloud-based APIs. FHIR reformulated the HL7
XML standard by defining 135+ resources that conceptualize the different aspects of healthcare data such as
patients, practitioners, organizations, services, appointments, encounters, diagnostic data, and medications.
Developers of healthcare applications select a subset of the resources that are required to solve their problems.
However, the standard provides no way to effectively organize a subset of resources into a higher-level
construct similar to software design patterns. This paper leverages the design pattern concept to extend the
FHIR standard by defining meta resources that are a conceptual construct that clearly defines the involved
resources and their interactions into one unified artifact. To illustrate the concepts of this paper, we use a
mobile health application for medication reconciliation that integrates information from multiple electronic
health records. We leverage FHIR extension mechanisms such as profiles and Bundle resources to integrate
the meta resource into the resource contextualization layer of the FHIR standard.
1 INTRODUCTION
Electronic health records (EHRs) have reached a
market saturation nearing 92% (Heath, 2016) but
cross-institutional data-sharing is still challenging for
stakeholders (e.g., medical providers such as
physicians, hospitals, clinics, labs, etc.) and fails to be
fully supported by health information exchange
(HIE). Particularly when clinical providers use
different health information technology (HITs)
systems in geographically separate locations. The
demand for interoperable HIE that allows multiple
HITs (e.g., EHRs, e-prescribing systems, pharmacy
information systems, patient portals, etc.) to interact
with one another for healthcare data sharing between
providers is growing rapidly (De Pietro & Francetic,
2018). Increasingly, this exchange is being facilitated
utilizing Fast Healthcare Interoperability Resources
(FHIR) (FHIR, 2021), an HIE standard introduced in
2014 to promote secure sharing of healthcare
a
https://orcid.org/0000-0001-8259-1438
b
https://orcid.org/0000-0003-0654-9910
c
https://orcid.org/0000-0002-6062-067X
data. The standard has passed through an initial
maturity level and is currently widely endorsed and
accepted by HIE stakeholders, policymakers, and
developers of healthcare application systems. FHIR is
mandated by the Office of National Coordinator for
Health Information (ONC, 2021) for making data
available via APIs to patients and for data sharing.
In support of HIE, we have done prior work
(Ziminski et al., 2015) on different software
architectural alternatives and proposed a hybrid HIE
architecture that leverages paradigms that include
service-oriented architecture, grid computing,
publish/subscribe paradigm, and data warehousing to
allow the HITs of stakeholders to be integrated to ease
collaboration among medical providers. Our follow-
up work (Ziminski et al., 2020) extended that effort
to detail an architectural solution for HIE using FHIR
to integrate HITs to facilitate collaboration among
medical providers. Our focus in this paper is to
explore the interoperability at a coarser granularity
Ziminski, T., Demurjian, S. and Agresta, T.
Extending the Fast Healthcare Interoperability Resources (FHIR) with Meta Resources.
DOI: 10.5220/0010546501670176
In Proceedings of the 16th International Conference on Software Technologies (ICSOFT 2021), pages 167-176
ISBN: 978-989-758-523-4
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
167

level that considers the way that the architectures in
our prior work use FHIR for HIE more effectively.
The FHIR standard has 135+ resources, which are
data elements to capture all types of healthcare data
organized into different layers. For example, the base
resource layer contains patients, practitioners, and
family relationships, organizations, services,
appointments, and encounters. The clinical resources
are for a patient’s health history, diagnostic data,
medications, care provision, and request/response
communication. Further resources are organized in
the foundation, financial, and specialized layers.
One of the limitations of the FHIR standard is that
it is left up to the discretion of each designer and
developer to individually choose a set of resources
that is used to solve a particular problem. However,
at a design level, there is no way to effectively
organize the subset of FHIR resources required for a
particular problem in any way that would promote
reuse. One of the classic software engineering design
and development approaches is design patterns which
were pioneered in (Gamma, 1995) and came into
widespread use. Design patterns originated in an
investigatory process when software engineers and
developers realized that they were constantly copying
and reimplementing similar code in different projects.
For example, the Observer pattern is utilized to define
a one-to-many relationship between objects. The
Model View Controller pattern has: a model to
capture business rules for accessing and updating
data, a view that renders the contents of the model for
the presentation of the stored data, and the controller
that translates the view into actions such as button
clicks, UI actions, or HTTP calls.
Design patterns can fit into an Enterprise
architecture (Zachman, 2019) as well as with the
architecture of FHIR (FHIR Arch, 2021) as part of an
overall information architecture. In addition to
resources organized in the mentioned layers, FHIR
also includes a Resource Contextualization
component containing FHIR profiles and graphs.
Within this context, this paper will propose a meta
resource, which is a set of resources created using
containment and relationships and which is geared
towards solving specific problems in the design of
healthcare applications. We believe that meta
resources can fit into the FHIR’s resource
contextualization layer, thereby extending its
architecture with additional relationship types,
attributes, and constraints.
The intent is to have an identifiable construct that
can meaningfully relate multiple FHIR resources in
an ordered manner and that allows sharing and
exchange to happen at a higher granularity level akin
to a design pattern. To facilitate this process, we
define the meta resource as a named construct that
associates multiple resources so that they can then be
utilized in multiple contexts.
For this purpose, we leverage the extension
concepts of FHIR profiles along with the grouping
operation of Bundle resources to integrate seamlessly
into the FHIR standard. This provides a design
pattern-like capability that can augment and extend
the existing functionality of FHIR by higher-level
conceptual named constructs that, for particular
workflows, clearly define the involved resources and
their interactions into one unified artifact.
To illustrate the potential of the concepts of this
paper, we will leverage our work on the development
of a mobile health application and framework
(Agresta et al., 2020) for medication reconciliation
that integrates information from multiple EHRs. We
show how the FHIR resources used in our solution
can be better conceptualized into a meta resource,
which also could be effectively utilized and deployed
in different settings for purposes related to medication
reconciliation. For this, we combine the resources
(Patient, Medication, MedicationStatement, Detected
Issues, etc.) into a MedicationReconciliation meta
resource which supports the actions of the
application, including: retrieving medications from
multiple EHRs, personal health records, and other
HITs; combining and reconciling medications into a
best medication list that identifies potential conflicts
between the same or different medications; and,
supporting an adaptive multi-use algorithm for
medication reconciliation.
The remainder of this paper has four sections.
Section 2 provides background information on the
critical FHIR concepts and our medication
reconciliation work which will be used for examples
throughout the paper. Section 3 presents a model that
extends FHIR with meta resources. Section 4
explores the algorithmic process for integrating meta
resources based on FHIR bundles. Section 5 contains
concluding remarks and outlines ongoing work.
2 BACKGROUND
This section provides background material in three
areas. Section 2.1 explores medication reconciliation
and its importance for healthcare. Section 2.2 presents
the concepts of the FHIR relevant for the paper.
Section 2.3 discusses our medication reconciliation
architecture and app.
ICSOFT 2021 - 16th International Conference on Software Technologies
168

2.1 Medication Reconciliation
Since a patient’s medication regimen is the basis for
treatment decisions, medication lists must be accurate
to maximize therapeutic impact and prevent
potentially life-threatening events. Discrepancies
between the medication lists in HITs where patients
receive care can potentially harm patients. This
challenge is significant enough that in Connecticut
(CT), the CT General Assembly passed Special Act
18-6: An Act Requiring the HIT Officer to Establish
a Work Group to Evaluate Issues Concerning
Polypharmacy and Medication Reconciliation
(CTACT, 2018) which produced a number of
recommendations to the legislature which includes
the development of technology to support the ability
to generate the Best Possible Medication History via
an automated electronic means (OHS, 2019).
Medication Reconciliation is defined as: “the
process of comparing a patient’s medication orders to
all of the medications that the patient has been taking.
This reconciliation is done to avoid medication errors
such as omissions, duplications, dosing errors, or
drug interactions.” (JC, 2006) Transitions of care
(hospital to ambulatory care, nursing facility to home)
can be particularly dangerous without an accurate
medication list. Different EHRs at these locations can
lead to medication errors that contribute to this harm.
Medication errors account for over 1M ED visits,
3.5M office visits, and 125k hospital admissions
annually (ADE, 2020). Medication reconciliation can
address these issues by compiling a list of
medications from various EHRs into a single source-
of-truth resource for seamless access by users for
medication management.
In regards to prior work, we highlight a number
of related efforts, the first (Coons, et al., 2019) is the
development of a mobile application for a medication
list leveraging FHIR. The next related effort
(Pandolfe, et al. 2016) provides a framework that
improves accuracy of the medication reconciliation
process focusing on an outpatient medication list
through a centralized architecture. Another study
(Schnipper, et al., 2012) concluded that concordance
between documented and patient-reported medication
regimens and reduction in potentially harmful
medication discrepancies can be improved with a
PHR medication review tool linked to the provider’s
medical record. Finally, (Yang et al., 2018) discussed
the importance of improving standardization of the
process and technology used for Medication
Reconciliation by exposing some of the challenges
and potential harms when a clinician attempts to
discontinue a medication electronically using an
approved HL7 messaging standard called CancelRx
that their system has only partially implemented. It
left medications that should no longer be taken on
different EHR and Pharmacy HIT system lists.
2.2 The FHIR Standard
FHIR is structured around the concept of resources,
which are comprehensive data elements that hold the
information that can be expressed in FHIR. Formally,
a FHIR resource R is defined as an entity with the
properties set P = (Identity, Type, (Data Item*),
Version). The Identity property is used to address a
given resource entity within a FHIR system
consisting of one or more FHIR servers. The type
property specifies one of the resource types that are
provided by the FHIR specification. The data items
property is a set of structured data elements, which
holds the resource's actual data content as specified
by its definition. The identified version property
implements a version counter which tracks changes
that occur to the contents of a resource through its
lifetime. The record version automatically changes
each time the resource changes, allowing a full audit
trail. The business version changes each time the
content in the resource changes. The FHIR standard
defines representation formats in XML, JSON and
Turtle. Figure 1 contains an abbreviated portion of the
patient resource XML schema and Figure 2 for the
Medication resource. Note that for these two
examples and all other examples we have omitted
some of the details as it impacts the single column
display; see (FHIR Res, 2021) for complete versions.
<Patient xmlns="http://hl7.org/fhir">
<identifier><!-- 0..* Identifier -->
</identifier>
<active value="[boolean]"/><!-- 0..1 -->
<name><!-- 0..* HumanName --></name>
<telecom><!-- 0..* ContactPoint -->
</telecom>
<gender value="[code]"/><!-- 0..1 -->
<birthDate value="[date]"/><!-- 0..1 -->
<deceased[x]><!-- 0..1 boolean|dateTime -->
</deceased[x]>
<address><!-- 0..* Address --></address>
<maritalStatus>
<!-- 0..1 CodeableConcept -->
</maritalStatus>
<!--omitted material due to length -->
<other>
<!-- 1..1 Reference(
Patient|RelatedPerson) -->
</other>
<type value="[code]"/><!-- 1..1 -->
</link>
</Patient>
Figure 1: Patient XML schema.
Extending the Fast Healthcare Interoperability Resources (FHIR) with Meta Resources
169
<Medication xmlns="http://hl7.org/fhir">
<identifier><!-- 0..* Identifier -->
</identifier>
<code><!-- 0..1 CodeableConcept --></code>
<status value="[code]"/><!-- 0..1 -->
<manufacturer>
<!-- 0..1 Reference(Organization) -->
</manufacturer>
<form><!-- 0..1 CodeableConcept --></form>
<amount><!-- 0..1 Ratio --></amount>
<ingredient>
<!-- 0..* Active/inactive ingredient -->
<item[x]>
<!-- 1..1 CodeableConcept|
Reference(Substance|Medication) -->
</item[x]>
<isActive value="[boolean]"/><!-- 0..1 -->
<strength><!-- 0..1 Ratio --></strength>
</ingredient>
<batch>
<!-- 0..1 Packaged medications details -->
<lotNumber value="[string]"/><!-- 0..1 -->
<expirationDate value of="[dateTime]"/>
<!-- 0..1 -->
</batch>
</Medication>
Figure 2: Medication XML schema.
The FHIR standard provides an explicit extension
mechanisms called profile to satisfy the need for
adjustments to specific organizational needs. This is
integrated already at the core specification level and
allows to define extensions, constraints, and additional
APIs for FHIR implementations that need to provide
functionality in addition to the built-in capabilities.
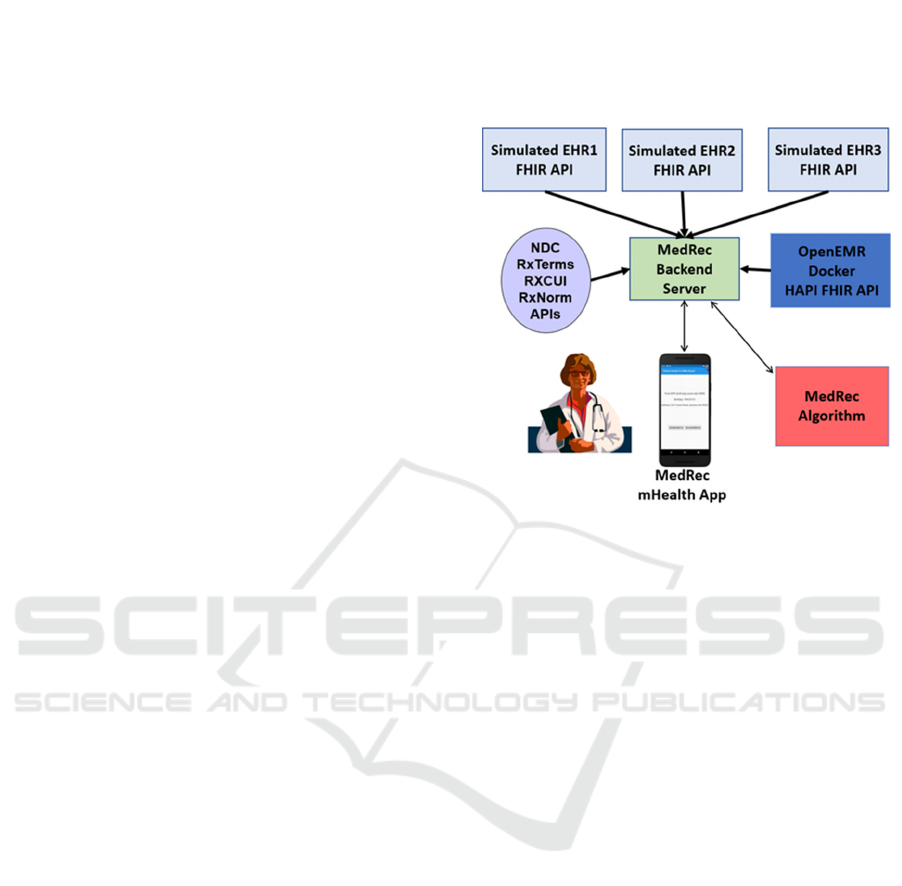
2.3 MedRec Architecture and App
Following a hackathon in spring 2019 (OHS, 2019),
we developed an architecture for our MedRec
application, as shown in Figure 3. To establish our test
environment, we set up four separate HITs: one as a
gold standard to have the exact correct medications for
every patient without any duplications or problems
and three HITs with perturbed versions of that gold
standard with missing medications, different
medications, errors in dosage, old medications etc.
This scenario models what happens in practice.
A HAPI FHIR client iterates over a list of other
FHIR sources, parses the response, and merges them
into a single Bundle. Reconciliation is then performed
on the Bundle by making requests to the RxNorm API
(RxNorm, 2021) for similar medications on a per-
medication basis, then attempting to find duplicates
within the Bundle. Duplicates are removed from the
queue for RxNorm requests for efficiency, since they
have already been matched. When a duplicate is
found, a DetectedIssue resource (FHIR, 2021) is
created and added to the bundle. The returned
medication list is intact as returned from the multiple
FHIR sources, and the duplicates must be displayed in
the app through processing the DetectedIssue
resources, so that final authority as to which
medications are duplicates rests with the user.
Figure 3: MedRec Architecture.
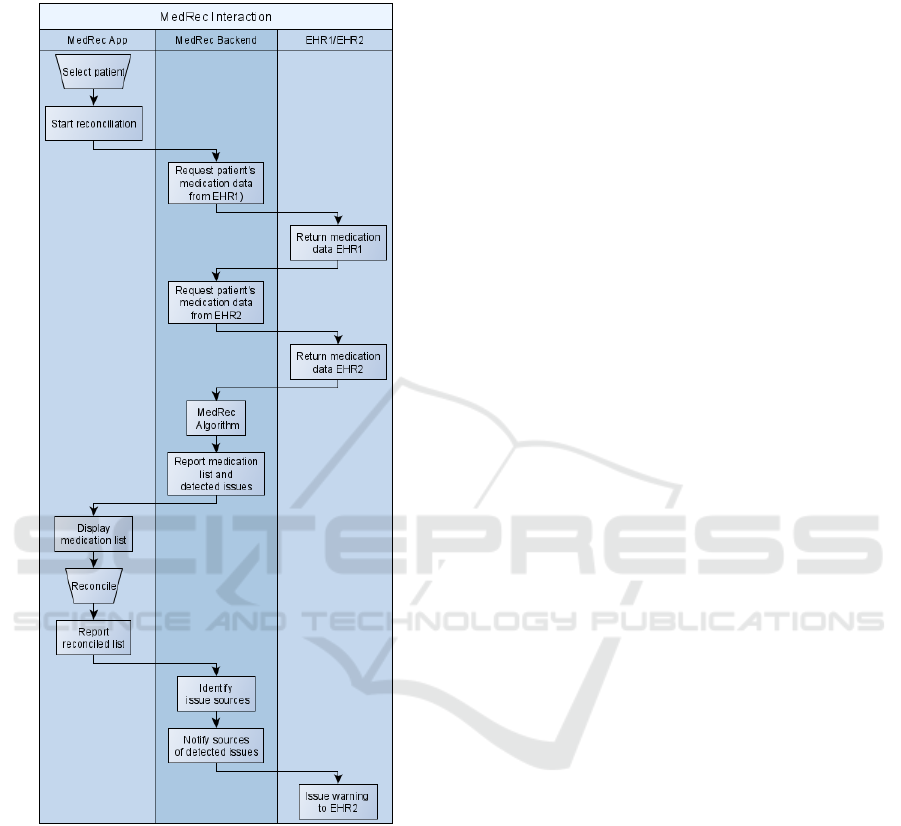
To explain medication reconciliation, Figure 4
presents a simplified process: a User selects a patient;
App initiates the reconciliation and triggers the
backend; Backend requests medications for the patient
from both EHRs; MedRec algorithm processes
medication lists and detects issues; App displays
medication list with highlighted issues; User
reconciles conflicts; Reconciled list is returned to the
backend; Backend identifies which sources were
contributing the data causing identified issues;
Affected sources are notified (in this example a
warning is sent to EHR2); and, Sources may issue a
warning to their users.
3 META RESOURCE MODEL
This section introduces a formal model for the meta
resource extension of FHIR. Section 3.1 presents a set
of core definitions for resources from the FHIR
standard used as the foundation for the meta resource
definitions in Section 3.2.
3.1 Core Definitions
The Fast Healthcare Interoperability Resources
(FHIR) standard is built around data elements called
resources and application programming interfaces for
exchanging them. Definitions Defn. 1 to 5 describe
ICSOFT 2021 - 16th International Conference on Software Technologies
170
the core capabilities of FHIR resources from a general
perspective in order to support the rest of the meta
resource model to be presented in Section 3.2.
Figure 4: Medication Reconciliation Process.
Defn. 1. The resource identity (𝑅
) is given by
an URL that allows to identify and address a
resource uniformly.
Defn. 2. Let 𝓡𝓣
𝑡
,𝑡
,…,𝑡
be the
list of different resources that are defined in the
resource list of the FHIR specification (FHIR
Res, 2021). Then the resource type is defined as
𝑅
𝑡
∈ 𝓡𝓣
.
Defn. 3. The resource data ( 𝑅
) is the
specific collection of data fields that a resource of
type 𝑅
receives in the FHIR specification.
Defn. 4. The resource version (𝑅
) is a
sequence number that increases each time the
resource changes.
Definitions Defn. 1 to 4 support the following two
definitions.
Defn. 5. An FHIR resource is described by the
four-tuple 𝑅 𝑅
,𝑅
,𝑅
,𝑅
Defn. 6. Let 𝓡𝑅
,𝑅
,…𝑅
be the set of
all FHIR resources.
Example 1 illustrates a Patient (FHIR Res, 2021)
instance according to the schema in Figure 1 using the
model concepts introduced in Defn.1 to 6.
Example 1. An FHIR Patient resource instance
for patient John Doe after five changes is
represented by
𝑅
𝑅
,𝑡
,𝑅
,𝑥
where
𝑅
ℎ𝑡𝑡𝑝://𝑡𝑒𝑠𝑡. 𝑓ℎ𝑖𝑟. 𝑜𝑟𝑔/𝑟𝑒𝑠𝑡/𝑃𝑎𝑡𝑖𝑒𝑛𝑡/123
and
𝑡
𝑃𝑎𝑡𝑖𝑒𝑛𝑡
and
𝑅
"𝑖𝑑𝑒𝑛𝑡𝑖𝑓𝑖𝑒𝑟" ∶ "ea44426f",
"active": "true",
"name": "John Doe",
"telecom": "555-370-8047",
"gender": "male",
"𝑏𝑖𝑟𝑡ℎ𝐷𝑎𝑡𝑒" ∶ "1970 12 12", . . .
and
𝑥 5
3.2 Meta-Resource Definitions
We are introducing the concept of meta resources to
leverage the design pattern idea and define higher-
level design constructs that can represent multiple
resources needed to implement a particular
application. This transcends the resource-centric view
of FHIR on clinical data. Meta resources provide
reusable workflow-centric patterns that allow a
conceptual view for implementing functionalities in
an already FHIR enabled system. Sample workflows
include medication reconciliation, patient admission,
or vaccination support.
The definition of a specific meta resource
determines the properties, components, relationships,
and requirements that the given meta resource has to
an implementing system. When facing implementing
a particular workflow, a meta resource from a library
of previously implemented solutions can be used as a
pattern for the high-level design of applications and
as a schema against which actual implementations are
checked for full functionality.
In detail, a meta resource is described by a
selection of descriptive properties, a set of resources
Extending the Fast Healthcare Interoperability Resources (FHIR) with Meta Resources
171

organized using composition and relationships, and
API extensions for interaction. The related model is
given in Defn. 7 to Defn. 12. First, to support the
classification and management of meta resources,
they require identifying properties.
Defn 7. The meta resource identifier (𝑀𝑅
) is
a unique identifier for the meta resource.
Defn 8. The meta resource name (𝑀𝑅
) is
a human-readable name for identifying the meta
resource.
The overall objective of a meta resource is the
larger granular organization of resources for a
specific health-related workflow, described via
human-readable description.
Defn 9. The meta resource description
(𝑀𝑅
) is a textual description of the medical
workflow that a meta resource enables.
Combining definitions Defn 7 through Defn 9, we
define a meta resource.
Defn. 10 (v1). A meta resource (intermediate) is
described by the three-tuple 𝑀𝑅
𝑀𝑅
,𝑀𝑅
,𝑀𝑅
.
Defn 11. Let 𝓜𝓡 𝑀𝑅
,𝑀𝑅
,…,𝑀𝑅
be
the set of all meta resources.
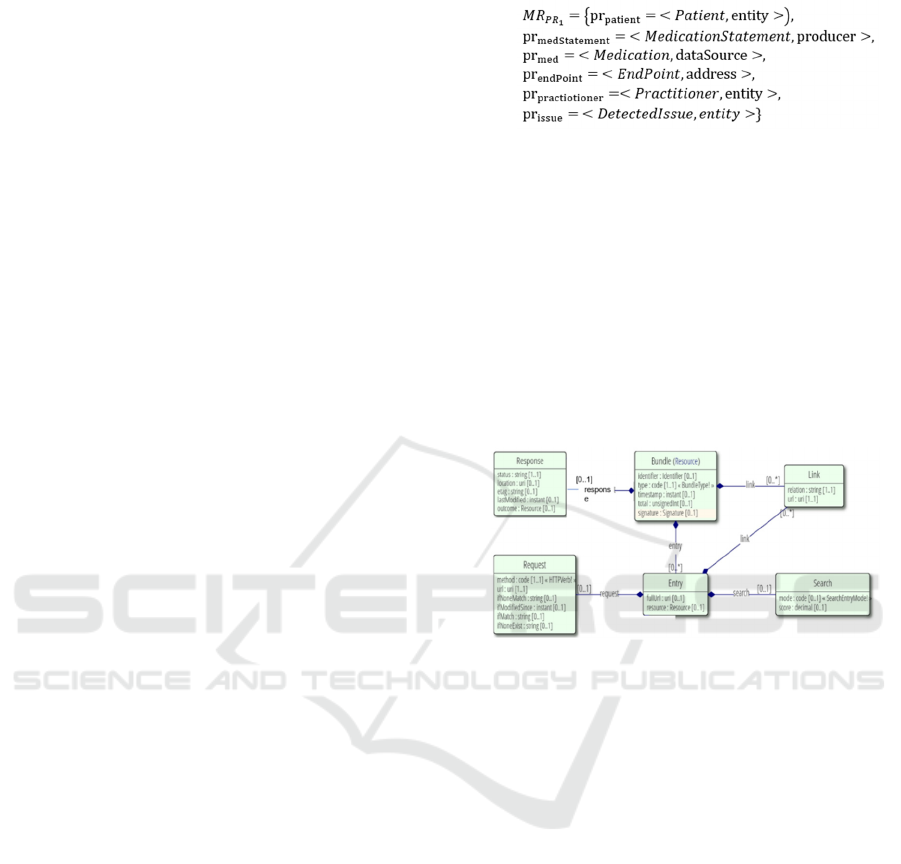
Example 2 shows an instance of a
MedicationReconciliation meta resource for the
medication reconciliation app in Section 2.1 and
implementing the structure shown in Figure 5, which
gives an overview of the meta resource: the Patient
resource holds demographic information of the
affected patient. It references one or more
MedicationStatement resources. The
MedicationStatement identifies that a patient is or
was taking a medication. It contains a Medication
resource identifying the actual medication. It also
references one Endpoint resource to indicate from
where the statement was retrieved. Finally, it also
references one Practitioner resource that identifies
that individual that should be notified about issues
regarding this statement. A DetectedIssue resource
references two or more medication statements,
indicating a potential problem between those
statements, which must be resolved during
reconciliation. An Endpoint records information on
which system needs to be notified regarding a
discovered issue. It references a practitioner as
designated contact in the scope of this endpoint. An
endpoint represents a health information technology
system such as an electronic health record that could
be at a hospital, a rehab facility, a clinician’s office,
etc., that must be notified whenever a change is made.
A Practitioner resource identifies the practitioner that
needs to be contacted regarding issues detected for a
given medication statement during reconciliation.
Example 2. The MedicationReconciliation meta
resource is represented by
𝑀𝑅
𝑀𝑅
,MR
,𝑀𝑅
where
𝑀𝑅
ℎ𝑡𝑡𝑝://𝑡𝑒𝑠𝑡. 𝑓ℎ𝑖𝑟. 𝑜𝑟𝑔/𝑟𝑒𝑠𝑡/𝑚𝑒𝑡𝑎
/𝑀𝑒𝑑𝑖𝑐𝑎𝑡𝑖𝑜𝑛𝑅𝑒𝑐𝑜𝑛𝑐𝑜𝑛𝑐𝑖𝑙𝑖𝑎𝑡𝑖𝑜𝑛/123
and
𝑀𝑅
𝑀𝑒𝑑𝑖𝑐𝑎𝑡𝑖𝑜𝑛𝑅𝑒𝑐𝑜𝑛𝑐𝑖𝑙𝑖𝑎𝑡𝑖𝑜𝑛
and
𝑀𝑅
”Medication reconciliation is the
process of comparing a patient’s medication orders
to all the medications that the patient has been taking
and eliminating potential errors, resulting in a new
up to date list of medications.”
Patient
MedicationStatement
1
1..*
DetectedIssue
Endpoint
2..*
0..*
Medication
1..*
1
Practicioner
1..*
1..*
Figure 5: Medication Reconciliation Meta Resource.
Each of the resources in a meta resource is
classified according to the service it provides, such as
consumer, producer, data source, or data generator.
For example, in Figure 5, the Medication resource
will serve as data sources for performing the
reconciliation. In contrast, the FHIR
MedicationStatement can serve as a producer of
further medication resources.
Defn 12. Let 𝓡𝓒
𝑐
,𝑐
…,𝑐
be
the set of all recognized classification categories
for a resource within the scope of a meta resource.
Example 3 shows the definition of a category.
Example 3. The MedicationReconciliation meta
resource utilizes categories 𝓡𝓒
𝑐
,𝑐
,
𝑐
,𝑐
⊂
𝓡𝓒
.
Fundamentally, a meta-resource is a composition
of standard FHIR resources enriched with meta-
information to define the structure and interactions of
ICSOFT 2021 - 16th International Conference on Software Technologies
172
FHIR resources on the design level. A meta-resource
definition contains a specification of FHIR resources
that must be available to instantiate the meta resource,
known as the participating resources.
Defn. 13. The participating resources of a meta
resource are defined as the set 𝑀𝑅
𝑝𝑟
,
𝑝𝑟
,
…𝑝𝑟
,
, where a participating resource
𝑝𝑟
𝑟
,𝑐
is a tuple in which 𝑟
is
identifying either an FHIR standard resource or
recursively a meta resource, therefore 𝑟
∈𝓡 ∪
𝓜𝓡. The category of the participating resource
is given by 𝑐
∈ 𝓡𝓒.
For supporting a meta-resource, the application
being developed needs to provide a matching API:
Defn. 14. The meta resource API extension
(𝑀𝑅
) is defined as an FHIR profile (FHIR Pro,
2021) providing the API extensions needed for
interacting with the meta resource.
Participating resources relate to one another by
reference or composition, leading to:
Defn 15. The meta resource reference structure
is defined as the set 𝑀𝑅
𝑟𝑒𝑓
,𝑟𝑒𝑓
,…𝑟𝑒𝑓
, where 𝑟𝑒𝑓
𝑝𝑟
,𝑝𝑟
with 𝑝𝑟
,𝑝𝑟
∈𝑀𝑅
and 𝑖 𝑗 is a tuple of
distinct participating resources and 𝑝𝑟
references
𝑝𝑟
.
Defn 16. The meta resource composition
structure is defined as the set 𝑀𝑅
𝑐𝑜𝑚
,𝑐𝑜𝑚
,…𝑐𝑜𝑚
, where 𝑐𝑜𝑚
𝑝𝑟
,𝑝𝑟
with 𝑝𝑟
,𝑝𝑟
∈𝑀𝑅
and 𝑖 𝑗 is a
tuple of distinct participating resources and 𝑝𝑟
is
contained in 𝑝𝑟
.
Definitions Defn. 11 to 16 require a revision of
Defn.10:
Defn. 10 (v2). A meta resource is described by
the seven-tuple
𝑀𝑅 𝑀𝑅
,𝑀𝑅
,𝑀𝑅
,
𝑀𝑅
,𝑀𝑅
,𝑀𝑅
,𝑀𝑅
>.
Example 4 illustrates the revised Defn.10.
Example 4. An instance of the meta resource
MedicationReconciliation can be represented by
𝑀𝑅
𝑀𝑅
,𝑀𝑅
,𝑀𝑅
,𝑀𝑅
,𝑀𝑅
,
𝑀𝑅
,𝑀𝑅
where
𝑀𝑅
http://test.fhir.org/rest/meta/
MedicationReconciliation/123
and
𝑀𝑅
𝑀𝑒𝑑𝑖𝑐𝑎𝑡𝑖𝑜𝑛𝑅𝑒𝑐𝑜𝑛𝑐𝑖𝑙𝑖𝑎𝑡𝑖𝑜𝑛
and
𝑀𝑅
𝑠𝑡𝑟𝑖𝑛𝑔
𝑠𝑒𝑒 𝐸𝑥𝑎𝑚𝑝𝑙𝑒 2
and
and
𝑀𝑅
𝑝𝑟𝑜𝑓𝑖𝑙𝑒
and
𝑀𝑅
𝑝𝑟
,𝑝𝑟
,
𝑝𝑟
,𝑝𝑟
,
𝑝𝑟
,𝑝𝑟
,
𝑝𝑟
,𝑝𝑟
and
𝑀𝑅
𝑝𝑟
,𝑝𝑟
4 FROM META RESOURCES TO
FHIR BUNDLES
Figure 6: FHIR Bundle in UML Representation.
This section presents the process and an associated
algorithm that can automatically transition a meta
resource definition into a destination FHIR bundle at
the schema level. FHIR bundles are container
resources built explicitly into the standard to group
other references, for example, in the context of search
results or for the exchange of messages. FHIR
bundles allow both references and containment and
are therefore capable of reflecting the relationships
shown in the meta resource in Figure 5. In Figure 6,
the UML representation of the FHIR Bundle (FHIR
Res, 2021) is shown, where we focus on the Bundle,
Link, and Entry. The corresponding XML schema for
the FHIR bundle is found in Figure 7. As one
transitions from design to the development of the
healthcare application, the FHIR bundle can facilitate
the exchange with another system. This approach
aims to ensure baseline compatibility with systems
that are not aware of meta resources while
simultaneously leveraging existing communication
APIs for interacting with other meta resource enabled
systems.
Extending the Fast Healthcare Interoperability Resources (FHIR) with Meta Resources
173

To illustrate the process, a meta resource as
presented in Defn. 10v2 in Section 3.2 needs to be
transitioned to a suitable FHIR artifact to construct a
representation that is amenable to realization within a
particular healthcare application. Figure 6 has the
UML representation of an FHIR bundle that gives an
overview of its components and interdependencies.
Conceptually, a bundle consists of meta-information
about the bundle itself (such as an identifier), entries
encapsulating FHIR resources, and links between
those entries (and potentially further outside
resources). Additionally, the request, response, and
search components can be used to make the bundle
suitable for workflows such as search queries to a
system. To transition from a meta resource to a
bundle, we utilize only the Bundle, Link, and Entry
components shown in Figure 6.
Figure 7: FHIR Bundle XML Schema.
Our algorithmic process takes the meta resource
as defined in Defn. 10v2 in Section 3.2 and, using the
XML schema of Figure 7, creates a meta resource
bundle that captures all components and
relationships. For the meta resource presented in
Figure 5, this would use all the defined resources in
the figure and the indicated relationships and
components to generate the medication reconciliation
bundle XML as shown in Figure 10. Note that adding
any Search, Request, or Response components of
Figure 6 is left to the responsibility of the developer
of the healthcare application where required.
Figure 8 gives an overview of the processing steps
that our approach takes to generate the bundle.
Specifically, for every medication reconciliation meta
resource, the initial generation is of the top-level
bundle element and a single patient element. Then the
participating resources, as given in Figure 5, are
processed into matching bundle entries. The
comments in the pseudo code relate which parts of the
example in Figure 10 are generated by a given step.
Figure 8: Pseudo-code Algorithm for Bundle Generation
(comments indicate lines generated in Figure 10.)
In summary, Figure 10 contains an excerpt of the
bundle schema (shortened for readability) that was
generated for a MedicationReconciliation meta
resource (see Figure 5) for a patient John Doe. The
resource recorded a drug interaction between two
drugs while reconciling the patient’s medication
statements from an openEHR instance and another
HIE system. Lines 1-4 define the bundle, assign an id
and a collection type, which defines a simple
collection without further constraints. Lines 5-8
contain an entry for the participating Patient resource.
Note that for every entry, a fullUrl property defines
the referenceable identity for the serialized resource
of the entry. We use this URL to reference
participating resources within the bundle itself.
Entries for the participating Practitioner (lines 9-
13) and Endpoint resources (lines 14-18) provide the
location and the person necessary for issuing an alert
about the drug interaction. Lines 19-32 contain the
participating MedicationStatement resource for a
medication which the patient is taking. The structural
references to the related Patient, Practitioner, and
Endpoint resources (as introduced in Figure 5) are
explicitly serialized to the link elements in lines 21-
23. While links handle the references introduced by
the meta resource concept, aggregations are serialized
to FHIR built-ins, i.e., the contained element of
MedicationStatement, as shown in line 27. Note that a
reference is always serialized to a link for consistency
within the meta resource model representation. This is
the case even if a potential built-in exists (e.g., the
Patient resource in the subject entry in line 29).
ICSOFT 2021 - 16th International Conference on Software Technologies
174
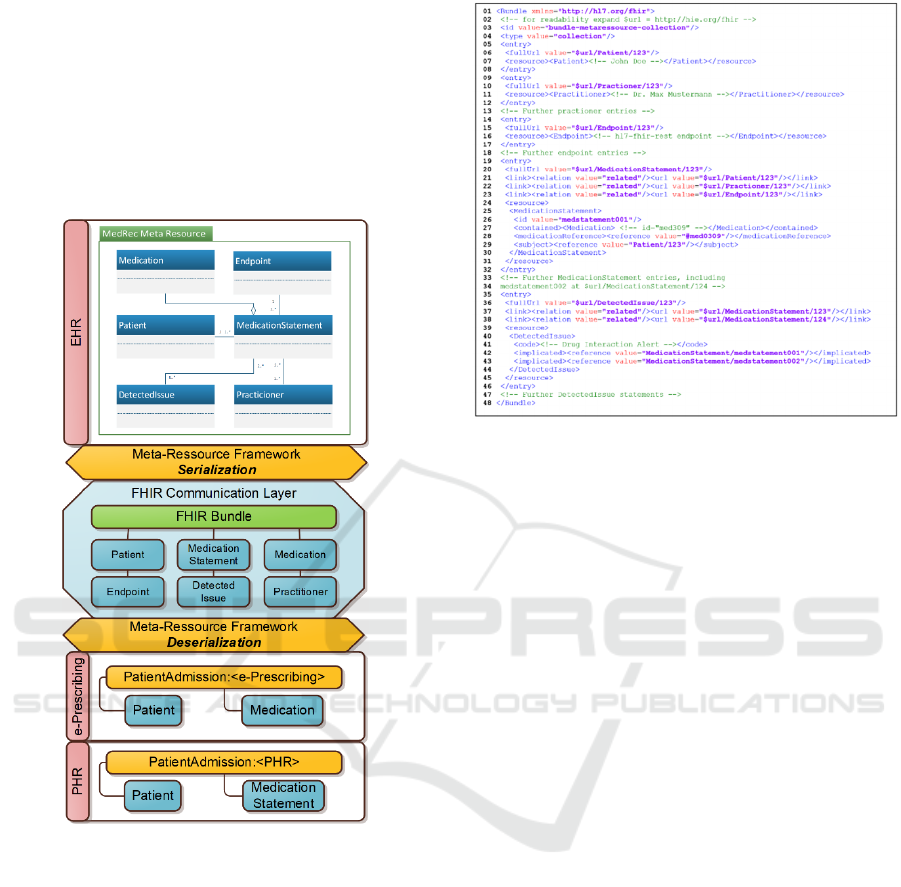
Further medication resources are omitted in this
example (lines 33-34). The drug interaction risk is
recorded in lines 35-46, where a participating
DetectedIssue resource flags two medication
statements. References from the meta resource are
expressed as links for a possible meta resource
deserialization (lines 37-38). Additionally, the built-in
FHIR relation in the implicated elements (lines 42-43)
is preserved for usage as regular FHIR resources.
Figure 9: Meta Resource Serialization to Bundle.
The generic high-level concept of our
transformation approach and its related information
flow between systems is illustrated in Figure 9. The
EHR at the top of the figure uses the previously
introduced medication reconciliation meta resource
for enabling a reconciliation workflow. However, the
information encapsulated within the meta resource’s
participating resources can be read by different
involved HITs. For this, the meta resources are
serialized to an FHIR bundle by iterating over the
meta resource’s participating resource and adding
them to the generated bundle one by one. The
resulting bundle conforms to the FHIR standard and
reflects the contents of the meta resource in a flat
structure (depicted in the middle of Figure 9). In the
serialization
process,
the
relationships
introduced
in
Figure 10: Generated Bundle.
the bundle by the meta resource are preserved in three
cases: by using FHIR built-ins (for cases such as the
relationship between a MedicationStatement and a
Medication resource); by setting appropriate link
elements in the bundle; or by adding them to
extensions via the StructureDefinition mechanism
(for cases such as the relation between a Practitioner
and an Endpoint resource). The first case can be
understood by systems without meta resource
knowledge, while the two later cases must support the
meta resource extension themselves for deserializing.
Other healthcare applications can use the
generated bundles by processing the participating
resources as direct information or reconstructing the
bundle’s meta resource. This operation is depicted at
the bottom of Figure 9, where an e-Prescribing system
extracts just the Patient and Medication resources
from the bundle while a PHR system processes only
on the Patient and MedicationStatement resources.
5 CONCLUSION AND ONGOING
WORK
This paper has presented the extension of the FHIR
standard with the concept of a meta resource which
allows for a design pattern level capability to support
reusable components that can be automatically
generated and easily exchanged among healthcare
applications that need to share the similar kind of
data. The meta resource extension can be
incorporated into the information architecture of
FHIR in the resource contextualization layer as
Extending the Fast Healthcare Interoperability Resources (FHIR) with Meta Resources
175

discussed in the introduction. To present our work,
we reviewed background on FHIR and the medication
reconciliation application that we use for examples in
Section 2. Next we presented a formal model to
represent meta resources in Section 3, which in turn
was used as the basis to present an algorithm and
associated process that automatically translates a
meta resource to a FHIR XML bundle schema in
Section 4. The generated bundle schema can be used
as a basis for the developer to create an instance of
the schema as the healthcare application is
transitioned from a design level to an implementation.
The resulting healthcare application would have
the bundled component that would be more easily
exchanged and transferred among different HITs. Our
work could improve healthcare interoperability by
having meta resources for recurrent parts of clinical
workflow such as for medication reconciliation.
Ongoing work is focusing on several different
areas. We intend to work with the team of the
medication reconciliation application to assist in
reformulating their usage of resources into the
medication reconciliation bundle as given in Section
4. This will allow us to fully understand the way to
transition from the bundle schema to the actual
exchangeable bundle instance. Another area is to
formally define the appropriate notation so that the
meta resource can fully fit into the defined FHIR
standard by using only predefined FHIR conventions.
REFERENCES
ADE, 2020. Office of Disease Prevention and Health
Promotion, “Adverse Drug Events,” February 2020.
https://health.gov/our-work/health-care-quality/adver
se-drug-events.
Agresta, T., Demurjian, S., Sanzi, E., DeStefano, J., Ward-
Charlerie, S., Rusnak, R., Tran, R., 2020. A Mobile
Health Application for Medication Reconciliation
Using RxNorm and FHIR. Proc. of the Fifth Intl. Conf.
on Informatics and Assistive Technologies for Health-
Care, Medical Support and Wellbeing, HEALTHINFO.
Coons, J. C., Patel, R., Coley, K. C., & Empey, P. E. (2019).
Design and testing of Medivate, a mobile app to achieve
medication list portability via Fast Healthcare
Interoperability Resources. Journal of the American
Pharmacists Association
CTACT, 2018. CT State Legislature, "Connecticut
SB00217 | 2018 | General Assembly," LegiScan,.
https://legiscan.com/CT/bill/SB00217/2018
De Pietro C, Francetic, I., 2018. E-health in Switzerland:
The laborious adoption of the federal law on electronic
health records (EHR) and health information exchange
(HIE) networks. Health Policy, 122(2), 69-74.
FHIR, 2021. HL7 International, “FHIR Overview,”
https://www.hl7.org/fhir/overview.html.
FHIR Arch, 2021. HL7 International, “FHIR Architecture,”
https://www.hl7.org/fhir/overview-arch.html
FHIR Pro, 2021. HL7 International, “FHIR Profiling,”
https://www.hl7.org/fhir/profiling.html
FHIR Res, 2021. HL7 International, “FHIR Resource List,”
https://www.hl7.org/fhir/resourcelist.html
Gamma, E., 1995, Design patterns: elements of reusable
object-oriented software Addison-Wesley.
Heath, S., 2016. Outpatient EHR Adoption Reaches 92%,
Nears Market Saturation. EHR Intelligence.
https://ehrintelligence.com/news/outpatient-ehr-
adoption-reaches-92-nears-market-saturation
JC, 2006. Medication reconciliation sentinel event alert.
The Joint Commission. https://www.jointcommission.
org/resources/patient-safety-topics/sentinel-event/senti
nel-event-alert-newsletters/sentinel-event-alert-issue-3
5-using-medication-reconciliation-to-prevent-errors/
OHS, 2019. OHS Connecticut, “Work Group – Final
Report,” Please see pages 25-76 of https://portal.ct.gov/-
/media/OHS/Health-IT-Advisory-Council/MRP/MRP_
WG_FINAL_Recommendations.pdf
ONC, 2O21. Office of the National Coordinator for Health
Information Technology, “ONC's Cures Act Final
Rule,". https://www.healthit.gov/curesrule/
Pandolfe, F., Crotty, B. H., & Safran, C. (2016). Medication
Harmony: A Framework to Save Time, Improve
Accuracy and Increase Patient Activation. AMIA
Annual Symposium Proceedings.
RxNorm, 2021. National Library of Medicine, “RxNorm,”
https://www.nlm.nih.gov/research/umls/rxnorm/
Schnipper, J., et al. 2012. “Effects of an online personal
health record on medication accuracy and safety: a
cluster-randomized trial. Journal of the American
Medical Informatics Association, 19, 728-734.
Yang, U., et al. 2018. Analysis of Medication Therapy
Discontinuation Orders in New Electronic Prescriptions
and Opportunities for Implementing CancelRx. Journal
of the American Medical Informatics Association, 25,
1516-1523.
Smart, 2021. Smart on FHIR. https://smarthealthit.org/
Zachman, 2021. Enterprise architecture.
https://www.zachman.com/resources/zblog/item/enter
prise-architecture-defined-architecture-abstractions
Ziminski, T., Demurjian, S. A., Sanzi, E., Agresta, T.,
(2015). “Toward Integrating Healthcare Data and
Systems: A Study of Architectural Alternatives.
Chapter 16 in Maximizing Healthcare Delivery and
Management through Technology Integration, Iyamu,
T., Tatnall, D. eds.), pp 270-304.
Ziminski, T. B., Demurjian, S. A., Sanzi, E., Baihan, M.,
Agresta, T., 2020. Chapter 14: An Architectural
Solution for Health Information Exchange. Virtual and
Mobile Healthcare: Breakthroughs in Research and
Practice, Information Resources Management
Association, pp. 283-327, IGI.
ICSOFT 2021 - 16th International Conference on Software Technologies
176
