
Development of a Framework for a Functional-Structural Seagrass
Transplantation Simulation using GAMA Platform
Therese Anne Rollan
a
, Ariel Blanco
b
and Edgardo Macatulad
c
Department of Geodetic Engineering, University of the Philippines, Diliman, Quezon City, Philippines
Keywords: Agent-based Modelling, Geographic Information System, Seagrass Growth Simulation, Seagrass
Transplantation, Plant Growth Modelling, Functional-Structural Approach, GAMA Platform.
Abstract: A massive decrease in seagrass coverage in the Philippines has been observed in the past several years due to
coastal eutrophication and typhoons. It is key to observe the changes and probable damage in seagrass habitat
and develop a way to scientifically back up recovery strategies such as transplantation to increase the
probability of rehabilitation success. This study describes the framework development of a transplantation
scenario evaluation tool that performs Thalassia hemprichii growth simulation within an uproot site in
Palawan as a case study. The growth parameters used include shoot leaf area, spacer length, plastochrone
interval, and life expectancy, and horizontal apex density. Base scenario and three scenarios with varying
combinations of transplantation density and distribution were applied to the three 4 x 4 grid plots with 24 x
24 cm cell size from classified drone imagery. Results show that transplantation distribution has a greater
weight than density with respect to the percent cover responses. Based on the mean and standard deviation of
percent cover responses, scenario 1 having 4 transplants with 24 cm intervals is the most suitable for plots 1
and 2, while scenario 2 having 8 transplants (2 per cell) with 24 cm intervals for plot 3.
1 INTRODUCTION
Seagrasses are clonal flowering plants that share the
same architecture (Marba, Duarte, Alexandria, &
Cabaco, 2004), submerged in shallow marine waters
(Florida Fish and Wildlife Conservation
Commission, n.d.), usually located on semi-enclosed
lagoons and along coastlines, and co-existing with
intertidal mangroves and corals (Fortes M. D., A
Review: Biodiversity, Distribution and Conservation
of Philippine Seagrasses, 2013). They play an
important role in providing food and shelter for
various marine species (Duarte & Chiscano, 1999;
Heiss, Smith, & Probert, 2000), stabilizing the sea
bottom (Borowitzka, Lavery, & van Keulen, 2006),
maintaining water quality, supporting the livelihood
of local economies and holds around 12% of the total
ocean carbon stock (UNEP, 2004). Among the
thriving species of seagrass in the Philippines,
Thalassia hemprichii (T. hemprichii) is one of the
dominant ones that exhibits horizontal expansion
a
https://orcid.org/0000-0002-8436-8165
b
https://orcid.org/0000-0002-0489-9979
c
https://orcid.org/0000-0001-7977-2932
typically in a span of approximately 5 to 11 days
(Lopez, Unpublished; Vermaat, et al., 1995).
A massive decrease in seagrass coverage in the
Philippines has been observed in the past several
years. It is key to monitor the changes and probable
damage and develop a way to scientifically justify
recovery strategies such as transplantation to increase
the probability of rehabilitation success. This study
aims to develop a framework for simulating seagrass
recovery in order to increase the certainty of
transplantation spatial strategy success in order to
help a seagrass meadow recover from a typhoon
damage. It will assist local communities, government
authorities, and researchers to formulate effective
strategies not only for seagrass recovery but also for
its rehabilitation and conservation.
The main question is how can a simulation be
applied to possibly increase the certainty of
transplantation spatial strategy success and help a
seagrass meadow recover from a typhoon damage.
The framework aims to answer the following:
248
Rollan, T., Blanco, A. and Macatulad, E.
Development of a Framework for a Functional-Structural Seagrass Transplantation Simulation using GAMA Platform.
DOI: 10.5220/0010520502480255
In Proceedings of the 11th International Conference on Simulation and Modeling Methodologies, Technologies and Applications (SIMULTECH 2021), pages 248-255
ISBN: 978-989-758-528-9
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

What are the primary datasets needed?
What steps must be done to run a seagrass
transplantation simulation?
From the results of the transplantation simulation
scenarios, the following are the questions aimed to be
answered:
What are the corresponding seagrass percent
cover tracks?
What metrics can be used to describe the
behavior of these tracks?
From the scenario factors considered, which of
them are statistically significant? Which have
greater bearing on the metrics considered?
2 LITERATURE REVIEW
2.1 Seagrasses
2.1.1 Overview
Seagrasses belong to plants producing flowers known
as angiosperms, evolved to thrive in marine waters
and typically have ribbon-like, grassy leaves
(McKenzie, 2008) with its general structure divided
into above-ground including leaf, blade and stem and
below-ground consisting of rhizome (horizontal) and
root (El Shaffai, 2016). They follow a clonal
mechanism called vegetation proliferation sharing
similar architecture but with varying plant size and
growth rate across species (Marba, Duarte,
Alexandria, & Cabaco, 2004).
2.1.2 Disturbances, Rehabilitation,
Transplantation and Recovery
Since some are located in deep areas, light becomes
crucial and exposure to disturbances becomes higher
(Greve & Binzer). These disturbances are caused by
anthropogenic activities and natural phenomena
(Short & Wyllie-Echeverria, Natural and human-
induced disturbances of seagrass, 1996). Oil spill
incidents were reported in the Philippines in 1987
(Fortes M. D., 1991), Puerto Rico in 1962 and
California in 1969 (Zieman, Orth, Phillips, Thayer, &
Thorhaug, 1984). In the U.S., a continuous decrease
in percent cover was observed from 2003 to 2008
including a dramatic decline by 2006 due to a storm
wave (Buchanan, 2009). In Banate Bay, Philippines,
seagrasses were uprooted by typhoon Haiyan (The
Philippine Star, 2016). Although, their response to
environmental and population changes are species-
specific (Marba, Duarte, Alexandria, & Cabaco,
2004), as observed as well by Duarte et al. (1987).
Their recovery relies on vegetative growth, regrowth
from fragments of transported plants, and recovery
from seeds (Vanderklift, et al., 2016). Rollon et al.
(1998) showed that the projected duration of post-
disturbance recovery ranges from 2 to 10 years in full
recovery both for artificially created gaps of 0.25
sq.m. Another study observed gradual recovery
within 2 to 6 years after a cyclone and consequent
flood (Campbell & McKenzie, 2004).
2.1.3 Thalassia Hemprichii
Genus Thalassia consists of two species, T.
testudinum and T. hemprichii, a.k.a. “twin species”
because they can only be distinguished through the
counts and dimensions of the styles and stamens of
their flowers appearance-wise (van Tussenbroek, et
al., 2006). Both grow in highly organized and rigid
pattern which primarily depends on the active tip of
the horizontal (h.) rhizomes called apical meristem or
apex for expansion (Tomlinson, 1974). To survive,
vertical rhizomes utilize surrounding resources,
deploying leaves and roots at the same (Hemminga &
Duarte, 2000). T. hemprichii is a commonly
widespread species and is considered to be stable
despite threats and disturbances (Short, et al., 2010).
In the Philippines, it commonly thrives on mud-coral-
sand or coarse coral-sand substrates and grows up to
6 meters deep (Menez, Phillips, & Calumpong, 1983).
2.2 Agent-based Modeling
2.2.1 Overview
Agent-Based Modeling (ABM) utilizes objects called
agents possessing attributes and behaviors, and
playing specified roles in the model through specified
rules and constraints. Its advantages include capturing
of emergent phenomena, provision of a natural
environment for the study of certain systems, and
flexibility (de Smith, Goodchild, & Longley, 2018).
Moreover, it is capable of handling high
heterogeneity in characteristics, interactions between
agents and environments, and their dynamics,
feedbacks and adaptation (Auchincloss & Garcia,
2015). In the past decades, this method has already
been widely used in different fields. However, due to
lack of awareness in the significant importance of
seagrass, studies on seagrass growth simulation
employing this method is still relatively sparse.
Development of a Framework for a Functional-Structural Seagrass Transplantation Simulation using GAMA Platform
249
2.2.2 Gama Platform
GIS Agent-based Modeling Architecture Platform
(GAMA) is an open-source environment that
combines agent-based simulations with spatial
applications (Grignard, et al., 2013). It uses its own
programming language GAML (GAMA Modeling
Language) coded in Java which makes them similar
in syntax and structure (GAML, 2018). In
conjunction with the visualization, instantaneous
statistics of the agents and the simulation can be
displayed using graphs.
2.2.3 Functional-Structural Plant Model
One approach is Cellular Automata which treats a
seagrass plot as a grid having each grid cell a value
representing percent cover or biomass (Marsili-
Libelli & Giusti, 2004). However, this can be quite a
generalized approach if the target is to visualize and
analyze the components in detail. To achieve these,
ABM must be employed wherein Functional-
Structural Plant Model (FSPM) can be applied. It is
suitable in simulating seagrass growth because the
plant is modelled in a much finer detail (Godin &
Sinoquet, 2005) such as its roots, leaves and branches
to simulate the higher-level outcomes (Dejong, Da
Silva, Vos, & Escobar-Gutiérrez, 2011). Related
studies include the works of Sintes et al. (2005),
Renton et al. (2011), and Whitehead et al. (2018).
3 MATERIALS AND METHODS
This research is divided into three main procedures
namely 1) Pre-processing, 2) Data Processing and
Visualization, and 3) Analysis and Validation.
Figure 1: This is the research workflow for the development
of the seagrass transplantation simulation framework.
3.1 Pre-processing
This stage involves literature review, gathering of
growth parameters (Table 1), gathering of site
datasets shown in Figure 2, seagrass percent cover
extraction using Mixed-Tuned Matched Filtering
(MTMF) method in ENVI software, and
identification of three random plots within the
blowout scenarios that will represent three plots or the
number of repetitions of the transplantation
simulation runs.
Table 1: T. hemprichii parameters used in the developed
transplantation simulation are summarized below as
adapted and derived from the work of Vermaat, et al.,
(1995), and from Ms. Rose Lopez and Dr. Rene Rollon.
Parameter Value Standard
deviation
Shoot leaf area (sq.cm, single-sided) 26.56 0.02
Shoot spacing along rhizome
(spacer length, cm)
6.77 2.90
Shoot
p
lastochrone interval (PI, days) 4.03 0.34
Shoot life expectancy (days) 229 17
Rhizome apex density per sq. m 58 -
Rhizome life ex
p
ectanc
y
(years) 4 -
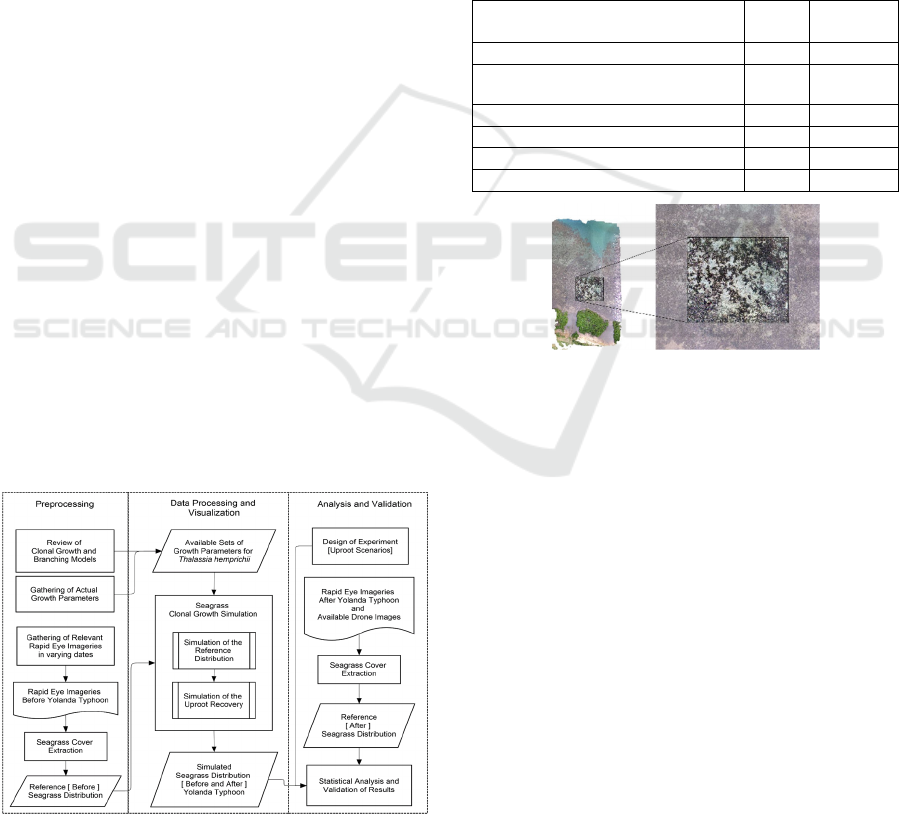
Figure 2: This is a drone imagery captured by a project team
under the IAMBlueCECAM Program on September 2017
on a seagrass blowout site in Palawan, Philippines.
In the seagrass extraction, two drone image spatial
resolutions were used: the original resolution 6 cm and
the resampled 24 cm. To obtain the grids, ArcMap
Fishnet tool was used to generate a 24 x 24-cm grid
resolution. To determine the three random plots within
the blowout that will represent three transplantation
simulation repetitions, ArcMap Create Random Points
tool was used. The extent for the previously generated
grid served as a coverage constraint in order to ensure
that the points will not fall outside the blowout site.
Minimum allowable distance from each of the points
was set to 20 meters to avoid them from being too close
to each other. The grid cell where these points fell into
are the upper left corner of the 4 x 4 grid, having cells
with dimensions 24 x 24 cm. In Figure 3, the
preparation for the input grids is demonstrated. Values
in percent are converted to their decimal format and
used in the transplantation simulation as comma-
separated (csv) files.
SIMULTECH 2021 - 11th International Conference on Simulation and Modeling Methodologies, Technologies and Applications
250

Figure 3: Obtaining the input percent cover grids from the
MTMF classification output and the randomly generated
locations: Illustrations above represent a single random plot
from a random location shown as yellow marker at the
upper leftmost cell of the 4 x 4 grid.
3.2 Data Processing and Visualization
In this stage, parameters in Table 1 and the extracted
seagrass percent cover for each plot are the input for
the seagrass transplantation simulation. Prior to the
simulation proper, the input csv files for each scenario
of the three random plots must be prepared.
Following Table 2, four scenarios of varying level
combinations for low and high of factors a) planting
distribution and b) planting density. Low level (L) for
the planting distribution means wide intervals
between plants and high level (H) corresponds to
closer intervals. On the other hand, L for planting
density denotes 1 plant per grid cell of 24 x 24 cm and
H indicates 2 plants per cell. There will be five
percent cover responses: Sum, Mean, Standard
Deviation, Minimum, and Number of Extreme Drops.
To represent these scenarios as input files for the
transplantation simulation, corresponding template
for each were created by computing their density (per
grid cell of size 24 x 24 cm) contributions as shown
below by the equations 2 and 3. These templates are
grids with values having the dimensions with the
plots. The shoot leaf area from Table 1 cannot be
directly used since it assumes that the leaf is
completely horizontal, facing the drone upon imagery
capture. Due to water depth and current, seagrass
leaves are angled, if not upright. Thus, we use a
reduction factor which in this case is 1/3 according to
our consultation with Dr. Rollon as demonstrated in
equation 1.
Table 2: This table illustrates the Design of Experiments
(DOE) for the four seagrass transplantation scenarios with
varying level combinations for low and high of factors a)
planting distribution and b) planting density.
Scenario
Planting
Distribution
Planting
Densit
y
Percent
Cover
Response
LHL H
1× × R1
2× × R2
3×× R3
4× × R4
Derived shoot leaf area per plant (sq.cm.) =
(1/3)26.56 = 8.85
(1)
Derived shoot leaf area density for Planting
Density (L) = (1 plant x 8.85)/(24 x 24) = 0.015
(2)
Derived shoot leaf area density for Planting
Density (H) = (2 plants x 8.85)/(24 x 24) =
0.031
(3)
In Figure 4, L stands for low factor level and H
the high factor level. The first letter denotes the level
of factor a) planting distribution while the second
letter for b) planting density such that (L)(L) stands
for transplantation scenario #1 from Table 2. These
scenario grids are added to each of the three random
plots creating five transplantation simulation runs for
each namely: i) scenario 0 - base scenario or the actual
percent cover based on the drone-obtained imagery,
and ii) scenario 1 to 4. They are then converted to csv
files as inputs for the reference meadow with percent
cover values.
Figure 4: Seagrass transplantation scenario templates with
the computed values of the percent cover contribution of the
seagrasses to be planted.
Figure 5 describes the flow of the simulation
starting from the initial pairs of h. rhizome and shoot.
Due to the lack of firm literary basis for the initial
seagrass plants per unit area that may populate and
turn into a meadow, the chosen ratio between the
number of initial pairs to the percent cover is 1:10.
Development of a Framework for a Functional-Structural Seagrass Transplantation Simulation using GAMA Platform
251
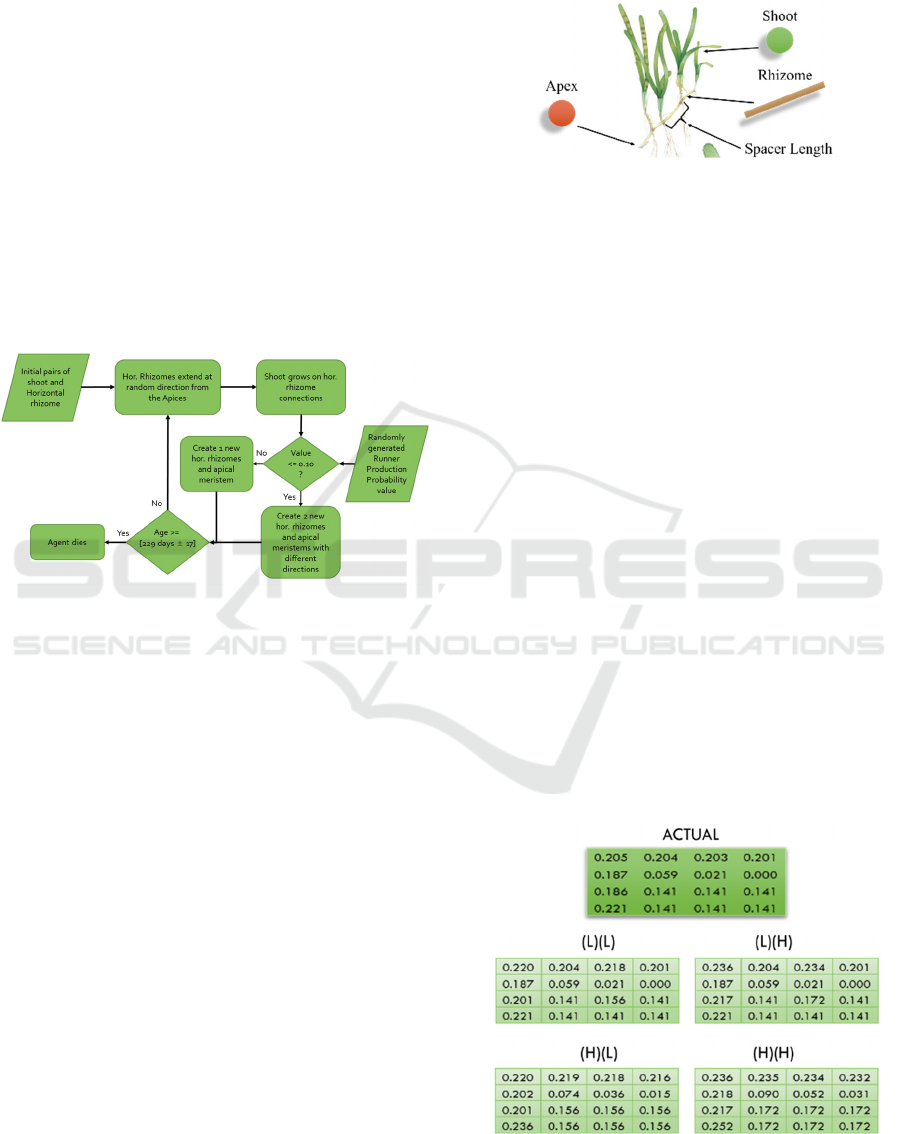
Though this is a part of the assumptions, this ratio is
still reasonable since it partially and relatively
describes the reference meadow.
Every transplantation simulation cycle, new h.
rhizomes will emerge from the locations of the apices
in a random direction. Randomized runner production
probability determines whether there will be one or
two emerging rhizomes. If the probability is ≤ 10%, a
runner is produced and two new h. rhizomes will be
created. From the current apex agents, shoot agents
will grow on the next cycle. In addition, old apex
agents will die (disappear in the transplantation
simulation) and new apex agents will grow from the
tips of the new h. rhizomes. Previously created shoot
and h. rhizome agents will remain until they reach the
maximum age imposed. Every cycle, agent count and
percent cover value are logged and graphed.
Figure 5: Seagrass Transplantation Simulation
Implementation Workflow.
The geo-simulation uses agents to represent the
main components of seagrass growth, namely the
apical meristem or apex, the h. rhizome and the shoot
as illustrated in Figure 6. Apex (apical meristem) is
represented as red circle, shoot as green circle and h.
rhizome as brown line. The growing tip or meristem
of a shoot is represented by the Apex which can
produce new rhizomes and apices. Shoots grow at the
nodes of h. rhizomes. Since the simulation is limited
to two-dimensional top view visualization, shoots are
simplified and represented as green circles.
The transplantation simulation parameters used
include apex density, plastochrone interval (denoted
by P.I.; number of days within which h. rhizome is
produced), horizontal elongation rate, branching rate,
h. rhizomes between shoots, shoot spacing along
rhizome and median maximum age of shoot and
rhizome. The time step used is 4.03 ± 0.34 days which
represents the duration of rhizome growth and a
threshold of 58 apices denoting the maximum number
in an almost 1 sqm. plot. Simulation run starts from
randomly distributed pairs of apex and rhizome over
a specified relatively small plot and grow into
meadows covering a spatial distribution with respect
to a corresponding reference seagrass percent cover.
Figure 6: A simplified top view representations of seagrass
agents T.hemprichii with its photo adapted from
(SeagrassWatch.org).
3.3 Analysis
The final stage of the methodology is the analysis in
which the graphs for each of the plot’s scenario result
was observed and the trends of the values are
discussed and explained. Using Design Expert
software, the analysis of variance (ANOVA) and
interaction among factor levels were examined. This
will show if the factors and their levels are significant.
4 RESULTS AND DISCUSSION
4.1 Reference Seagrass Meadows
Figure 7 summarizes the actual or the reference
percent cover plots (scenario 0) and their four
scenarios obtained by taking into account the
combinations of the levels of the two factors in the
DOE table (Table 2). Each row of the said figure
represents a repetition (or the plots as discussed in
Section 3.1). All of these are formatted as csv files
and were used as inputs in the transplantation
simulation.
Figure 7: Percent cover grid per plot for the actual values
and the scenario factor levels.
SIMULTECH 2021 - 11th International Conference on Simulation and Modeling Methodologies, Technologies and Applications
252

4.2 Seagrass Transplantation
Simulation Results
In the transplantation simulation, four quantities were
kept track over time (1 transplantation simulation
cycle = 1 P.I. from Table 1) for a total span of 5 years
namely percent cover, and shoot, horizontal rhizome
and apical meristem counts. For each plot scenario,
the maximum potential seagrass percent cover is
66%. This is due to the agent constraints which are
imposed based on the maximum number of apical
meristems per plot and the agents’ respective
lifespans according to Vermaat, et. al. (1995) and Dr.
Rollon. Since percent cover is calculated from an
orthogonal viewpoint wherein only the shoots are
visible, the percent cover progression can be derived
from the trend of the shoots. Abrupt percent cover
drops result from a number of shoot agents that occur
simultaneously which in turn dies simultaneously.
Hence, extreme percent cover drops do not
necessarily mean that the seagrass meadow will
continuously thin.
In choosing the best planting scenario, standard
deviation and mean were considered. Standard
deviation accounts for the fluctuations of the percent
cover values. Hence, the best planting scenario per
study plot is characterized by low standard deviation
and high mean. Figure 8 illustrates the comparison
between two scenarios. Apparently, scenario 1 is
better than scenario 4 in this case (Figure 9).
Figure 8: This figure compares two scenarios (1 and 4) in
order to choose the better planting scenario. Note that the
comparisons are among the four scenarios per plot.
Figure 9: This shows the transplantation simulation results
of Plot 1 with Scenario 1 as the best scenario.
4.3 DOE Results
The importance of the factors with respect to the
responses by observing their relative significance
through their corresponding weights summarized in
Table 3. Sum and Mean are the most and least
significant percent cover response, respectively, both
to the planting distribution and planting density.
Furthermore, the factors were found to be
independent of each other a factor can be examined
separately without considering the other.
Table 3: This table contains the weight of each percent
cover responses shown.
Percent Cover
Res
p
onse
Planting
Distribution
Planting
Densit
y
Sum 0.993 0.548
Mean 0.002 0.001
Standar
d
Dev. 0.005 0.003
Minimum 0.009 0.012
No. of Extreme Dro
p
s 0.750 0.375
The DOE table as shown in Table 2 was
completed with five percent cover responses sum,
mean, standard deviation, minimum, and number of
extreme percent cover drops. It was observed that the
Development of a Framework for a Functional-Structural Seagrass Transplantation Simulation using GAMA Platform
253

model factors planting distribution and planting
density, and levels in this case study are not
significant using 95% confidence level with respect
to the previously enumerated responses. However,
based on how seagrass transplantation are practically
planned and carried out, these factors are still viewed
as worthy of research attention. It is just unfortunate
that this result may be due to a number of study
limitations brought about by the short duration and
lack of fieldwork budget of the project under which
this study was undertaken. These limitations include
the lack of field-obtained datasets such as drone
images in varying dates which can facilitate a
formulation of a sophisticated calibration and
validation procedure. Another is the lack of powerful
computers to simulate larger seagrass plots in longer
period. Nonetheless, these can serve as areas of
improvement for future researchers.
5 CONCLUSIONS AND
RECOMMENDATIONS
The study was able to develop and demonstrate a
framework for seagrass transplantation simulation.
The two factors planting distribution and planting
density appeared to be insignificant in the setup of
this study due to the presented limitations. However,
the importance (weight) of the factors with respect to
the responses can be observed based on the
coefficients derive from the DOE analysis. Majority
of the responses show that planting distribution has a
greater weight than planting density. Mean and
standard deviation were used to determine which
scenario will fit given the initial percent cover of a
plot -- Scenario 1 having 4 plants with 24 cm intervals
for Plots 1 and 2, while Scenario 2 having 8 plants (2
plants per grid cell) with 24 cm intervals for Plot 3.
Visualization techniques such 3D view of
seagrass agents closer to their real appearances can be
used in order to make non-technical persons
understand more easily the simulation outcomes.
Furthermore, a stand-alone software with more user-
friendly interface can be developed for government
and academic purposes. These future programs must
be optimized for usage efficiency to account for
machine capability limitations.
For the validation, it is highly encouraged to use
imageries of the same resolution as the reference
imagery. One method is to extract and compare
percent covers from the “after the simulation date”
imageries and compare them to the simulation percent
cover outcome.
ACKNOWLEDGEMENTS
This work is funded by the Department of Science
and Technology, Philippine Council for Industry,
Energy and Engineering Technology Research and
Development with Project No. 04041, 2018.
REFERENCES
Auchincloss, A., & Garcia, L. (2015, November). Brief
introductory guide to agent-based modeling and an
illustration from urban health research. Cad Saude
Publication, 65–78.
Borowitzka, M., Lavery, P., & van Keulen, M. (2006).
Epiphytes of seagrasses. In A. Larkum, R. Orth, & C.
Duarte, Seagrasses: biology, ecology and conservation
(pp. 441-461). the Netherlands: Springer.
Buchanan, S. W. (2009). Cape Cod National Shore
Resource Brief: Seagrass Monitoring. U.S. Department
of the Interior - National Park Service.
Campbell, S., & McKenzie, L. (2004). Flood related loss
and recovery of intertidal seagrass meadows in southern
Queensland, Australia. Estuar. Coast. Shelf Sci. 60,
477-490. Retrieved from http://refhub.elsevier.com/
S0025-326X(17)30741-5/rf0060
de Smith, M., Goodchild, M., & Longley, P. (2018).
Geospatial Analysis: A Comprehensive Guide to
Principles Techniques and Software Tools 6th Edition.
UK: The Winchelsea Press.
Dejong, T., Da Silva, D., Vos, J., & Escobar-Gutiérrez, A.
(2011). Using functional–structural plant models to
study, understand and integrate plant development and
ecophysiology. Annals of Botany 108, 987-989.
Duarte , C., & Chiscano, C. (1999). Seagrass biomass and
production: a reassessment. Aquatic Botany 65, 159-
174.
Duarte, C. M., & Kalff, J. (1987). Weight-density
relationships in submerged aquatic plants: the
importance of light and plant geometry. Oecologia,
72(4), 612-617.
El Shaffai, A. (2016). Field Guide to Seagrasses of the Red
Sea.
Florida Fish and Wildlife Conservation Commission. (n.d.).
Importance of Seagrass. Retrieved from Florida Fish
and Wildlife Conservation Commission Web site.
Fortes, M. D. (1991). Seagrass-mangrove ecosystems
management: a key to marine coastal conservation in
the ASEAN region. Marine Pollution Bulletin 22, 113-
116.
Fortes, M. D. (2013). A Review: Biodiversity, Distribution
and Conservation of Philippine Seagrasses. Philippine
Journal of Science 142, 95-111.
Fortes, M. D., Go, G. A., Bolisay, K., Nakaoka, M., Uy, W.
H., Lopez, M., Edralin, M. (2012). Seagrass response to
mariculture-induced physico-chemical gradients in
Bolinao, northwestern Philippines. 12th International
Coral Reef Symposium. Cairns, Australia.
SIMULTECH 2021 - 11th International Conference on Simulation and Modeling Methodologies, Technologies and Applications
254

GAML. (2018, September 8). Retrieved from GAMA
Platform Github Web site: https://gama-
platform.github.io/wiki/GamlLanguage
Godin, C., & Sinoquet, H. (2005). Functional-structural
plant modelling. New Phytologist 166, 705-708.
Greve, T. M., & Binzer, T. (n.d.). Chapter 4: Which factors
regulate seagrass growth and distribution?
Grignard, A., Taillandier, P., Gaudou, B., Vo, D., Huynh,
N., & Drogoul, A. (2013). GAMA 1.6: Advancing the
Art of Complex Agent-Based Modeling and
Simulation. In G. Boella, E. Elkind, B. Savarimuthu, F.
Dignum, & M. Purvis, PRIMA 2013: Principles and
Practice of Multi-Agent Systems. PRIMA 2013. Lecture
Notes in Computer Science, vol 8291 (pp. 117-131).
Berlin, Heidelberg: Springer. Retrieved from GAMA
Platform Homepage: https://gama-platform.github.io/
Heiss, W., Smith, A., & Probert, P. (2000). Influence of the
small intertidal seagrass Zostera novazelandica on
linear water flow and sediment texture. New Zealand J
Mar Fresh 34, 689-694.
Hemminga, M., & Duarte, C. (2000). Seagrass Ecology.
Cambridge, UK: Cambridge University Press.
Jackson, E. L., Rowden, A. A., Attrill, M. J., Bossey, S. J.,
& Jones, M. B. (2001). The importance of seagrass beds
as a habitat for fishery species. Oceanography and
Marine Biology: An Annual Review, 269-303.
Retrieved from ResearchGate: https://www.research
gate.net/publication/236683422
Lopez, R. (Unpublished). Master Thesis (University of the
Philippines Marine Science Institute).
Marba, N., Duarte, C., Alexandria, A., & Cabaco, S. (2004).
Chapter 3: How do seagrass grow and spread?
Marsili-Libelli, S., & Giusti, E. (2004). Cellular Automata
Modelling of Seagrass in the Orbetello Lagoon. 9th
International Congress on Environmental Modelling
and Software. Provo, Utah: BYU ScholarsArchive.
McKenzie, L. (2008, Feb). Seagrass-Watch: Seagrass
Educators Handbook.
Menez, E., Phillips, R., & Calumpong, H. (1983).
Seagrasses in the Philippines. Washington:
Smithsonian Institution Press.
Renton, M., Airey, M., Cambridge, M., & Kendrick, G.
(2011). Modelling seagrass growth and development to
evaluate transplanting strategies for restoration. Annals
of Botany 108, 1213–1223.
Rollon, R., De Ruyter Van Steveninck, E., Van Vierssen,
W., & Fortes, M. (1998). Contrasting Recolonization
Strategies in Multi-Species Seagrass Meadows. Marine
Pollution Bulletin Vol. 37, Nos. 8-12, 450-459.
SeagrassWatch.org. (n.d.). ID_Seagrass Images. Retrieved
from SeagrassWatch.org: http://www.seagrass
watch.org/ID_Seagrass/images
Short, F., & Wyllie-Echeverria, S. (1996). Natural and
human-induced disturbances of seagrass.
Environmental Conservation, 17-27.
Short, F., Carruthers, T., Waycott, M., Kendrick, G.,
Fourqurean, J., Callabine, A., Dennison, W. (2010).
The IUCN Red List of Threatened Species. Retrieved
from Thalassia hemprichii: http://www.iucnredlist.org/
details/173364/0
Sintes, T., Marba, N., Duarte, C., & Kendrick, G. (2005).
Nonlinear processes in seagrass colonisation explained
by simple clonal growth rules. OIKOS 108, 165-175.
The Philippine Star. (2016, April 3). Pfizer supports young
scientists to save environment. PhilStar Global.
Tomlinson, P. (1974). Vegetative morphology and
meristem dependence—the foundation of productivity
in seagrasses. Aquaculture 4, 107-130.
UNEP. (2004). Seagrass in the South China Sea. Bangkok:
UNEP.
van Tussenbroek, B., Vonk, J., Stapel, J., Erftemeijer, P.,
Middelburg, J., & Zieman, J. (2006). The Biology of
Thalassia: Paradigms and Recent Advances in Research
. In A. W. al., Seagrasses: Biology, Ecology and
Conservation (pp. 409-439). Springer.
Vanderklift, M., Bearham, D., Haywood, M., McCallum,
R., McLaughlin, J., McMahon, K., Lavery, P. (2016).
Recovery mechanisms: understanding mechanisms of
seagrass recovery following disturbance. Report of
Theme 5 – Project 5.4 prepared for the Dredging
Science Node, Western Australian Marine Science
Institution. Perth, Western Australia: Western
Australian Marine Science Institution.
Vermaat, J. E., Agawin, N. S., Duarte, C. M., Fortes, M. D.,
Marba, N., & Uri, J. S. (1995). Meadow maintenance,
growth and productivity of a mixed Philippine seagrass
bed. Marine Ecology Progress Series, 215-225.
Whitehead, S., Cambridge, M., & Renton, M. (2018). A
functional–structural model of ephemeral seagrass
growth influenced by environment. Annals of Botany
121, 897–908.
Zieman, J., Orth, R., Phillips, R., Thayer, G., & Thorhaug,
A. (1984). The effects of oil on seagass ecosystems. In
J. Cairns, & A. Buikema, Recovery and restoration of
marine ecosystems (pp. 37-64). Stoneham,
Massachusetts, USA: Butterworth Publications.
Development of a Framework for a Functional-Structural Seagrass Transplantation Simulation using GAMA Platform
255
