
Opportunities and Challenges in Fall Risk Management using EHRs
and Artificial Intelligence: A Systematic Review
Henrique D. P. dos Santos
1 a
, Juliana O. Damasio
1 b
, Ana Helena D. P. S. Ulbrich
2 c
and Renata Vieira
3 d
1
School of Technology, PUCRS, Porto Alegre, Brazil
2
Nossa Senhora da Conceic¸
˜
ao Hospital, Porto Alegre, Brazil
3
CIDEHUS, University of
´
Evora, Portugal
Keywords:
Electronic Health Records, Artificial Intelligence, Fall Detection, Fall Risk Prediction.
Abstract:
Electronic Health Records (EHRs) have led to valuable improvements to hospital practices by integrating
patient information. In fact, this data can be used to develop clinical risk prediction tools. We performed
a systematic literature review with the objective of analyzing current studies that use artificial intelligence
techniques in EHRs data to identify in-hospital falls. We searched several digital libraries for articles that
reported on the use of EHRs and artificial intelligence techniques to identify in-hospital falls. Articles were
selected by three authors of this work. We compiled information on study design, use of EHR data types,
and methods. We identified 21 articles, 11 about fall risk prediction and 10 covering fall detection. EHR data
shows opportunities and challenges for fall risk prediction and in-hospital fall detection. There is room for
improvement in developing such studies.
1 INTRODUCTION
Electronic Health Records (EHRs) have played an im-
portant role in hospital environments providing many
benefits in terms of patient safety and health care
quality (Buntin et al., 2011). EHRs are a rich source
of information to build risk prediction models. Cur-
rent machine learning and natural language process-
ing (NLP) techniques can help in the efforts to prevent
or identify several outcomes: readmission, fracture,
diabetes, mortality, and length of stay, among others.
A previous systematic review, presented in (Gold-
stein et al., 2017), described work on risk prediction
models yet it did not consider falls. To the best of our
knowledge, there are no previous studies addressing a
systematic review for the use of machine learning and
NLP methods over EHR data to identify in-hospital
falls. Within hospitals and nursing homes, falls con-
stitute the largest category of adverse event reports.
Approximately 30% of in-patient falls result in in-
jury, with 4% to 6% resulting in serious injury (Hitcho
a
https://orcid.org/0000-0002-2410-3536
b
https://orcid.org/0000-0001-8915-285X
c
https://orcid.org/0000-0001-6910-8210
d
https://orcid.org/0000-0003-2449-5477
et al., 2004).
Traditional fall risk models (Morse et al., 1989)
and fall detection tools (Resar et al., 2006) were de-
veloped for hospital environments without EHR sys-
tems. These tools are useful but time-consuming
and do not consider cultural changes for a variety of
hospitals and countries (De Souza Urbanetto et al.,
2013). A previous review on the matter focused
on non-automated models, listing articles about fall
risk prediction models, predicting falls among inpa-
tients and recording falls in the community (Walsh
et al., 2016). Another fall-related review focused on
sensor information, but used machine learning algo-
rithms in wearable, ambience, and vision-based de-
vices (Mubashir et al., 2013) not EHR.
Both fall detection and fall risk prediction play a
crucial role for hospital risk management and preven-
tion (Swift and Iliffe, 2014). Fall detection enables
the nurse team to map the most common reasons for
fall incidents and to create policies to avoid new in-
cidents. Fall risk prediction is one of these strategies
to prevent inpatient falls: it predicts the patient’s risk
and defines the level of care for this patient.
Thus, the main purpose of this systematic litera-
ture review (SLR) is to understand how EHRs data
626
Santos, H., Damasio, J., Ulbrich, A. and Vieira, R.
Opportunities and Challenges in Fall Risk Management using EHRs and Artificial Intelligence: A Systematic Review.
DOI: 10.5220/0010424306260633
In Proceedings of the 23rd International Conference on Enterprise Information Systems (ICEIS 2021) - Volume 1, pages 626-633
ISBN: 978-989-758-509-8
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
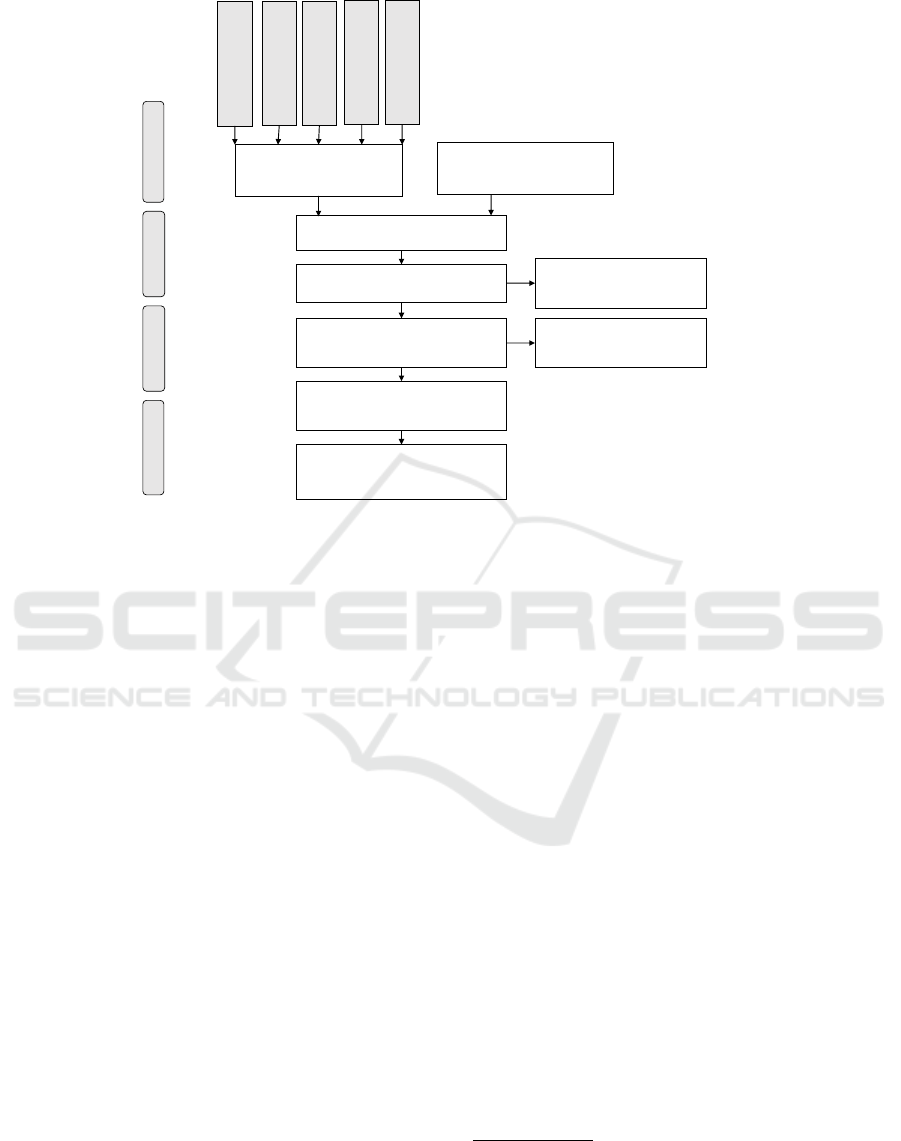
Studies included in quantitative
synthesis (meta-analysis)
(n = 19)
Studies included in qualitative
synthesis
(n = 19)
Full-text articles assessed for
eligibility
(n = 19)
Records screened
(n = 123)
Records after duplicates removed
(n = 123)
Full-text articles excluded,
with reasons
(n = 0)
Full-text articles excluded,
with reasons
(n = 104)
Records identified through
database searching
(n = 155)
Additional records identified
through other sources
(n = 0)
Included
Eligibility
Screening
Identification
ACM (n=1)
IEEE (n=4)
Scopus (n=112)
Pubmed (n=38)
ScienceDirected
(n=0)
Figure 1: PRISMA flow diagram.
has been used to develop and validate automatically
built models for identifying in-hospital fall risks. We
focus on EHRs data because of a large amount of use-
ful information is generated during the patient stay.
We detailed the studies considering research design,
data types, outcomes and evaluation, to summarize
what is relevant and useful for these models. The goal
is to analyse and discuss how the area has been devel-
oped and point to future promissing directions.
The paper is structured as follows. Section 2
shows the protocol used in the SLR. Section 3.1
presents the results obtained with the application of
the protocol. Section 4 presents a discussion about
the results. Finally, in Section 5 the conclusions are
presented.
2 MATERIALS AND METHODS
We used the SLR protocol proposed by (Kitchen-
ham et al., 2009). The main goal of this study is
to identify artificial intelligence techniques (machine
learning, natural language processing, and neural net-
works) to analyze adverse events (falls) in electronic
health records (EHRs).
2.1 Research Questions
Based on the previously defined objective, the follow-
ing research questions were identified:
Research Design
RQ1: What is the purpose of the investigation?
RQ2: What types of data and features are used?
Model Development and Evaluation
RQ3: Which AI techniques are used?
RQ4: How are the algorithms evaluated?
RQ5: What is the performance of the algorithms?
Limitations
RQ6: What are the limitations indicated?
Final discussion
RQ7: What are the challenges and opportunities?
2.2 Search Process
We selected five relevant digital libraries in Comput-
ing Science and Health: ACM Digital Library
1
, Sci-
enceDirect
2
, IEEExplore
3
, Scopus
4
, and Pubmed
5
.
Scopus is a general database that indexes several
other databases, covering approximately 19,500 ti-
tles from more than 5,000 international publishers,
1
https://dl.acm.org/
2
https://www.sciencedirect.com/
3
https://ieeexplore.ieee.org/Xplore/home.jsp
4
https://www.scopus.com/
5
https://www.ncbi.nlm.nih.gov/pubmed/
Opportunities and Challenges in Fall Risk Management using EHRs and Artificial Intelligence: A Systematic Review
627

including coverage of 16,500 peer-reviewed journals
in the scientific, technical, and medical and social
sciences. Afterwards, keywords related to the re-
search topic were identified, such as “fall”, “elec-
tronic health records”, and “artificial intelligence
techniques”. These terms were combined to create
the search expressions. The search expressions were
adapted according to the mechanism of each digital
library, so as not to alter their logical sense. The
searches were performed in the abstract, title, and
keywords fields.
For “fall” concept were used the search expres-
sion (fall OR falls) AND; For “electronic health
records” were used (electronic health records
OR EHR OR electronic medical records OR
EMR OR narratives OR free-text records
OR clinical notes) AND; For “artificial intelli-
gence techniques” were used (machine learning
OR data mining OR text mining OR neural
networks OR natural language processing
OR information extraction OR decision
trees OR prediction).
2.3 Selection Criteria
We included all papers (including conference pro-
ceedings) published in English and regardless of year
of publication. We followed some selection criteria
for the inclusion and exclusion of publications:
Exclusion. In the case of similar or duplicate publi-
cations, only the most recent were considered; Results
that did not use data from medical records or did not
use computational methods or did not focus on fall
adverse events; Books, PhD or Masters theses, and
abstracts from conference presentations.
Inclusion. The results must bear some relation with
the research topic of this work in the title, in the key-
words, or in the abstract.
2.4 Quality Assessment, Data
Collection, Data Analysis
We applied the search expression to each digital li-
brary in May 2019. We used the StArt
6
tool to help
organize the SLR. The results extracted from each
digital library were imported into StArt, with all rel-
evant information about the publication, such as title,
abstract, year, authors, and keywords. To check the
quality of the publications we used the Kappa Method
6
http://lapes.dc.ufscar.br/tools/start tool
for Measurement of Interrater reliability (McHugh,
2012).
For that purpose, first one author applied the selec-
tion criteria in the publications; subsequently, two re-
searchers individually reviewed the accepted and ex-
cluded publications. This was important to reduce
bias. To better evaluate the articles, we consulted the
TRIPOD (Moons et al., 2015) guidelines that state
how to report risk prediction studies. Finally, we per-
formed an analysis of the publications.
We first identified a total of 157 publications.
After applying the selection criteria, 21 studies re-
mained. Figure 1 shows the PRISMA flow diagram
(Liberati et al., 2009) with the number of papers ac-
cepted each step of the research. Publications from
the years 2009 to 2020 were found in our results.
These publications were related to two tasks: fall
risk prediction and fall incident detection. Fall risk
prediction is used to label a patient in her admis-
sion, to inform and aware the healthcare professionals
about her condition. The fall detection is a Risk Man-
agement department task to identify adverse events
in the hospital environment and create preventive risk
protocols and safety policies.
We analysed the number of publications by coun-
try. The highest concentration of papers was the
United States of America (13 papers), followed by
Japan (n = 3), Korean (n = 2), and Brazil (n = 2). The
number of papers per country was collected according
to the country of the first author’s institutional affilia-
tion. In regards to the distribution of publications by
year, the year 2015 showed the largest amount of pa-
pers (n = 5), followed by 2019 (n = 4), 2016 (n = 3),
2018 (n = 3), 2012 (n = 2), and 2017 (n = 2).
We present a summary of the studies in Tables 1
and 2. The analysis of these works are presented in
detail in the next section, answering questions pre-
sented in 2.1.
3 RESULTS
3.1 Design of EHR Prediction/Detection
Studies
Referring to question RQ1, we see that the studies
main purpose are divided in detection and prediction
of falls. For papers on detection of in-hospital fall
events (n = 8) we present Table 1. For papers re-
lated to the prediction of the patients’ risk of falling
(n = 11) we present Table 2. Most studies used a
cohort design from a single EHR system and a sin-
gle hospital (n = 12) (Tremblay et al., 2009; Toyabe,
ICEIS 2021 - 23rd International Conference on Enterprise Information Systems
628

Table 1: Characteristics of Fall Incident Detection Studies Included in the Review.
Author Year Patients Data Points Source Algorithms Eval Ss Sp F1
Tremblay 2009 NR 2,157 notes K-Means, LR T/T 0.83 0.93
Toyabe 2012 NR 4,821 notes, inc-rep Syntactic Rules DV 0.87 0.98 0.84
McCart 2013 2,241 26,010 notes LR, SVM T/T 0.93 0.94 0.85
Rochefort 2015 NR NR inc-rep NR NR 0.83 1.00
Luther 2015 1,652 26,010 notes SVM T/T 0.94 0.83 0.90
Bates 2016 NR 8,288 radiology reports SVM CV 0.94 0.93
Shiner 2016 NR 2,730 notes MaxEnt, CRF T/T 0.97 0.44
Topaz 2019 NR 750 notes Random Forest T/T 0.90 0.89
dos Santos2019 367 1,078 notes LSTM CV NR NR 0.90
Santos 2020 NR 3,468 notes BiLSTM-CRF CV NR NR 0.81
NR = Not Reported; notes = Clinical Notes; inc-rep = Incident Reports; LR = Logistic Regression; Eval =
Evaluation Method; T/T = Split dataset in train and test sets once; DV = Direct Validation; CV = Cross
Validation; Ss = Sensitivity; Sp = Specificity; F1 = F-Measure; Every Fall Detection study used textual
information as the source of detection.
Table 2: Characteristics of Fall Risk Prediction Studies Included in the Review.
Author Year Patients Data Points Source Algorithms Eval Ss Sp AUC
Marier 2016 5,129 133,781 pat, adm, med LR T/T NR
Weed-Pfaff 2016 1,080 NR adm, med Syntactic Rules DV NR
Lee 2016 8,390 NR pat, adm LR T/T 0.95
Yokota 2016 46,241 1,230,604 pat, adm LR T/T 0.71 0.66
Yokota 2017 45,257 1,223,687 pat, adm SVM T/T 0.64 0.69
Zhu 2017 114 1,558 notes Query DV NR
Bjarnadottir2018 36,583 1,046,053 notes Query NR NR
Choi 2018 75,036 220,904 pat, adm Syntactic Rules DV 0.30 0.70
Lucero 2018 814 NR pat, adm, med LR CV 0.73 0.85 0.88
Cho 2019 35,479 NR adm, med Bayes Network CV 0.96
Oshiro 2019 57,678 NR adm, pat, med LR T/T 0.73 0.70 0.72
NR = Not Reported; notes = Clinical Notes; adm = Administrative Data; med = Medication Orders; pat =
Patient Data; LR = Logistic Regression; DV = Direct Validation; T/T = Split dataset in train and test sets once;
CV = Cross Validation; Ss = Sensitivity; Sp = Specificity; AUC = Area Under the ROC Curve;
2012; Rochefort et al., 2015; Lee et al., 2016; Bates
et al., 2016; Yokota and Ohe, 2016; Yokota et al.,
2017; Zhu et al., 2017; Bjarnadottir and Lucero, 2018;
Lucero et al., 2019; Topaz et al., 2019; Oshiro et al.,
2019; dos Santos et al., 2019; Santos et al., 2020), the
other studies used data from multiple hospitals (with
the same EHR system)(n = 7) (McCart et al., 2013;
Luther et al., 2015; Shiner et al., 2016; Marier et al.,
2016; Weed-Pfaff et al., 2016; Choi et al., 2018; Cho
et al., 2019).
Considering question RQ2, fall detection stud-
ies based their predictive models on textual informa-
tion. Fall events reported in EHRs are usually pre-
sented as non-structured data (text). Clinical notes
were the most common sources used (n = 6) (Trem-
blay et al., 2009; Toyabe, 2012; McCart et al., 2013;
Luther et al., 2015; Shiner et al., 2016; Topaz et al.,
2019; dos Santos et al., 2019; Santos et al., 2020),
other sources included were radiology reports (n = 1)
(Bates et al., 2016) and incident reports (n = 2) (Toy-
abe, 2012; Rochefort et al., 2015).
For fall risk detection models, most studies used
structured data, as follows:
Administrative Data (n = 9) (Marier et al., 2016;
Weed-Pfaff et al., 2016; Lee et al., 2016; Yokota
and Ohe, 2016; Yokota et al., 2017; Choi et al.,
2018; Lucero et al., 2019; Cho et al., 2019; Os-
hiro et al., 2019): the average number of daily
tests, blood transfusions, number of nurses, Charl-
son score, Morse Fall Scale score, other nursing
scores, International Classification of Diseases
(ICD), type of room, length of hospital stay, med-
ical department, ward, unit, day of the week, nurs-
ing processes.
Patient Data (n = 7) (Marier et al., 2016; Lee et al.,
2016; Yokota and Ohe, 2016; Yokota et al., 2017;
Choi et al., 2018; Lucero et al., 2019; Oshiro
Opportunities and Challenges in Fall Risk Management using EHRs and Artificial Intelligence: A Systematic Review
629

et al., 2019): gender, age, blood pressure, gait
abnormality, other mental disorders, walking is-
sues, Parkinson’s disease, urinary incontinence,
depression, maximum pulse rate, registration as
a severely ill patient, activity and hyponatremia,
osteoarthritis, osteoporosis, other symptoms.
Medication Data (n = 5) (Marier et al., 2016; Weed-
Pfaff et al., 2016; Lucero et al., 2019; Cho et al.,
2019; Oshiro et al., 2019): medication period of
nervous and circulation medicines, psychotropics,
antipsychotic medication, anticonvulsant medica-
tions, in some cases all medications.
Each feature provides the model with a hypothesis
for the possible risk of falls. Other studies did not
use structured data, but only textual information from
clinical notes (n = 2) (Zhu et al., 2017; Bjarnadottir
and Lucero, 2018). In general, studies found a deficit
in the Morse Fall Scale to represent all variables for
risk factors. Some studies regarding fall risk models
described the most relevant features: imbalance and
gait are the main reason to determine a high fall risk.
3.2 Model Development and Evaluation
Regarding question RQ3, Logistic Regression mod-
els were the most common algorithms used (n = 7)
(Tremblay et al., 2009; McCart et al., 2013; Marier
et al., 2016; Lee et al., 2016; Yokota et al., 2017;
Lucero et al., 2019; Oshiro et al., 2019) to develop the
prediction model. Other approaches included syntac-
tic rules or queries (n = 5) (Toyabe, 2012; Weed-Pfaff
et al., 2016; Choi et al., 2018; Zhu et al., 2017; Bjar-
nadottir and Lucero, 2018), Support Vector Machine
methods (n = 4) (McCart et al., 2013; Luther et al.,
2015; Bates et al., 2016; Yokota et al., 2017), Recur-
rent Neural Network (n = 2) (dos Santos et al., 2019;
Santos et al., 2020), Random Forests (n = 1) (Topaz
et al., 2019), Bayes Network (n = 1) (Cho et al.,
2019), and Conditional Random Field (n = 1) (Shiner
et al., 2016). Most fall risk prediction studies incorpo-
rated some form of variable selection (n = 9) (Marier
et al., 2016; Weed-Pfaff et al., 2016; Lee et al., 2016;
Yokota and Ohe, 2016; Yokota et al., 2017; Choi
et al., 2018; Lucero et al., 2019; Cho et al., 2019; Os-
hiro et al., 2019), most often via stepwise approaches.
Most of these works select from patient, admin-
istrative and medication data structured variables as
features (n = 9) (Marier et al., 2016; Weed-Pfaff et al.,
2016; Lee et al., 2016; Yokota and Ohe, 2016; Yokota
et al., 2017; Choi et al., 2018; Lucero et al., 2019;
Cho et al., 2019; Oshiro et al., 2019). On the other
hand, only two papers used textual information (Zhu
et al., 2017; Bjarnadottir and Lucero, 2018).
Considering fall incident detection, all studies
used textual information. A variety of strategies were
used to detect fall in texts as showed in Table 1.
Most work used machine learning algorithms (n =
6) (Tremblay et al., 2009; McCart et al., 2013; Luther
et al., 2015; Bates et al., 2016; Shiner et al., 2016;
Topaz et al., 2019; dos Santos et al., 2019; Santos
et al., 2020) and one used syntactic rules (Toyabe,
2012).
Answering question RQ4, all but two studies used
some form of validation of the model. The most com-
mon form was split sample in train and test (n = 10)
(McCart et al., 2013; Tremblay et al., 2009; Luther
et al., 2015; Bates et al., 2016; Shiner et al., 2016;
Topaz et al., 2019; Yokota et al., 2017; Yokota and
Ohe, 2016; Lee et al., 2016; Marier et al., 2016), fol-
lowed by cross-validation (n = 5) (Lucero et al., 2019;
Cho et al., 2019; Bates et al., 2016; dos Santos et al.,
2019; Santos et al., 2020) and direct validation (n =
4) (Toyabe, 2012; Zhu et al., 2017; Weed-Pfaff et al.,
2016; Choi et al., 2018), with some studies using mul-
tiple forms of validation. Direct validation was used
when the authors developed syntactic rules or queries.
These rules are based on data information, author hy-
pothesis, or/and statistical correlation with the out-
come.
Splitting the sample in train and test is a com-
mon approach used in health science research groups.
Computer science studies usually apply the cross-
validation method. The split proportion varied in the
studies in this review. The authors (Tremblay et al.,
2009) and (Topaz et al., 2019) chose to split each
dataset into a training dataset (80% of the data) and a
validation dataset (20% of the data). In (McCart et al.,
2013) and (Luther et al., 2015) models were trained
with a stratified sample of 70% of documents from
one location (dataset train) and then applied to the re-
maining unseen documents (dataset test). Oshiro et
al. (Oshiro et al., 2019) used a similar split: 72% to
train the model and 22% to test it. While (Marier
et al., 2016) and (Yokota et al., 2017) performed a
split by 50%. In (Shiner et al., 2016) and (Yokota and
Ohe, 2016) did not explicit the split proportion they
used.
For RQ5, we found that the most common metric
to measure the algorithms’ performance was Sensi-
tivity (n = 14) (Tremblay et al., 2009; Toyabe, 2012;
McCart et al., 2013; Rochefort et al., 2015; Luther
et al., 2015; Bates et al., 2016; Shiner et al., 2016;
Topaz et al., 2019; Lee et al., 2016; Yokota and Ohe,
2016; Yokota et al., 2017; Choi et al., 2018; Lucero
et al., 2019; Oshiro et al., 2019), which measures the
proportion of actual positives that are correctly iden-
tified. Specificity was also used in most studies (n
ICEIS 2021 - 23rd International Conference on Enterprise Information Systems
630

= 10) (Tremblay et al., 2009; Toyabe, 2012; McCart
et al., 2013; Rochefort et al., 2015; Luther et al., 2015;
Shiner et al., 2016; Yokota and Ohe, 2016; Yokota
et al., 2017; Lucero et al., 2019; Oshiro et al., 2019);
it measures the proportion of actual negatives that are
correctly identified. Another common metric in ma-
chine learning studies was F-Measure (n = 5) (Toy-
abe, 2012; McCart et al., 2013; Luther et al., 2015;
Bates et al., 2016; Topaz et al., 2019; dos Santos
et al., 2019; Santos et al., 2020), the harmonic mean
of precision and sensitivity. Precision is the fraction
of correctly identified instances among all positives
identified. Three other studies used the Area Under
the Receiver Operating Characteristic (ROC) Curve
(relation between sensitivity and specificity). Aver-
age Sensitivity for in-hospital fall detection was 0.90
(worst: 0.83, best: 0.97) and for fall risk prediction
was 0.67 (worst: 0.30, best: 0.95). In Tables 1 and 2,
we show the individual results for each study.
3.3 Limitations
Besides the contribution of the works, we also anal-
ysed what some authors listed as the limitations
of their studies (RQ6). The most reported limita-
tions were the data sample and data selection (n =
11) (Tremblay et al., 2009; McCart et al., 2013;
Shiner et al., 2016; Yokota and Ohe, 2016; Weed-
Pfaff et al., 2016; Marier et al., 2016; Zhu et al., 2017;
Yokota et al., 2017; Choi et al., 2018; Oshiro et al.,
2019; Topaz et al., 2019; Lucero et al., 2019; dos San-
tos et al., 2019). The subsequent related issue was the
generalization problem of the model (n = 8) (Shiner
et al., 2016; Marier et al., 2016; Zhu et al., 2017;
Bjarnadottir and Lucero, 2018; Choi et al., 2018; Os-
hiro et al., 2019; Topaz et al., 2019; Lucero et al.,
2019). Some studies did not discuss their limitations
(n = 7) (Toyabe, 2012; Luther et al., 2015; Rochefort
et al., 2015; Bates et al., 2016; Lee et al., 2016; Cho
et al., 2019)
Regarding fall incident detection, Tremblay et
al. (Tremblay et al., 2009) showed examples where
the trained model misclassified fall-related adverse
events. The examples featured words such as hip,
pain, and knee, which are commonly found in fall in-
cidents; however, that is not always the case. McCart
et al. (McCart et al., 2013) discussed the pitfalls in the
gathered dataset. A shortfall in the reported incidents
added bias to the trained model.
The authors (Shiner et al., 2016) warned about
their small and random sample to identify falls. Their
study design reduced the possible variation in the way
falls are described. They stated that further work
should test multiple methods for fall identification, in-
cluding incident reports, manual records reviews, and
patient self-reports.
The reported limitations in fall risk prediction also
referred to dataset issues. Marier et al. (Marier et al.,
2016) pointed to limitations in the model generaliza-
tion related to the selection procedure. Their sample
was restricted to nursing homes that disproportion-
ately represent higher-quality institutions. Further-
more, there was missing data on selected risk fac-
tors for some residents. Weed-Pfaff et al. (Weed-
Pfaff et al., 2016) noted that errors of omission or in-
accurately recorded data could have affected results.
In (Yokota and Ohe, 2016) also mentioned human
errors and estimated that fall incidents occurred 1.3
times more often than the reported falls. They stated
that the process of choosing variables was a time-
consuming task. In their following work (Yokota
et al., 2017), Yakota and colleagues noted that the
representation of a single day did not evaluate the pa-
tients’ status changes over the course of the day. In
this study, the constructed model was a black box. It
did not specify which feature contributes more with
the outcome.
Regarding dataset size and sample selection, Zhu
et al. (2017) also noted the limited size of fall inci-
dents. The authors (Bjarnadottir and Lucero, 2018)
highlighted the lack of generalization when a model is
trained over a single-site observation. Moreover, the
lack of labeled data only allowed them to conclude
that their model might contain risk factors that have
been theoretically and empirically linked to fall risk.
Choi et al. (2018) remembered the importance of eval-
uating the model generality again. They observed that
their model was built upon discrete diagnosis infor-
mation that are not usually present in EHR systems.
Lucero et al. (2019) were also concerned about fea-
ture selection. Their findings presented limited gen-
eralization because the data derives from only one ter-
tiary teaching hospital. Oshiro, Cho et al.(2019) de-
bated that further models should improve the identifi-
cation of injurious falls and the detection of falls over-
all.
The drawbacks listed above provide inputs on op-
portunities and challenges. The next section discusses
the possible room for improvement in the develop-
ment of fall incident detection and fall risk prediction
models.
4 DISCUSSION
Over the last decade, many studies on the develop-
ment of models for fall detection and risk prediction
using EHR data have been published. In this section,
Opportunities and Challenges in Fall Risk Management using EHRs and Artificial Intelligence: A Systematic Review
631

we discuss the challenges and opportunities for arti-
ficial intelligence in fall risk management, answering
question RQ7.
The EHR data has the advantage of providing a
large number of patients for cohort studies. On top
of that, it is also able to provide a large number of
features: potential predictors. However, we verified
that many studies did not fully use every piece of in-
formation about the patients available in their medical
records as predictor variables.
The validation process adopted by some studies
did not assure the applicability of the proposed model
in other scenarios. A multivariable model for fall risk
prediction should be validated with an independent
sample and should evaluate its impact in real scenar-
ios before being used as a clinical decision-support
system. Only four studies used multiple sites, but
none validated the model across these sites. This
shows a lost opportunity to validate the prediction al-
gorithm in external data. Even if the scores perform
worse externally, it is important to answer how well
the models will fit another site.
Four insights for improvements in development
of fall incident detection and fall risk prediction:
Dataset. Combining all EHR data: laboratory, med-
ication, patient data, and clinical notes; Algorithms.
Evaluating Machine and Deep Learning approaches
over all features; Model. Training site-specific mod-
els since each hospital has different patient profiles
and specific environments; Validation. Consider mul-
ticenter studies and evaluations on real scenarios;
5 CONCLUSIONS
Our results indicate that many models for fall detec-
tion (to map the occurrence of incidents) and fall risk
prediction (to avoid new incidents) have been devel-
oped, in the last ten years, based on information from
Electronic Health Records. Both tasks play a crucial
role in fall prevention for hospital risk management.
Most studies in risk prediction used structured data
related to administrative, medication, and patient in-
formation. The most commonly used algorithms were
Generalized linear models. Thus, the most common
metric to measure the algorithms’ performance was
Sensitivity. We believe that one of the challenges for
the next few years is to use all available EHR data
to build both predictive and detection models. In ad-
dition, further work should focus on improving and
validating existing models, considering the TRIPOD
guidelines to provide quality reporting. Also, it is im-
portant to create automated methods that focus on pa-
tient safety avoiding spending the time of health care
professionals with questionnaires, annotations, proto-
col assessments and notifications.
ACKNOWLEDGMENTS
This work was partially funded by CAPES
(Coordenac¸
˜
ao de Aperfeic¸oamento de Pessoal
de N
´
ıvel Superior) Foundation (Brazil), UFRGS
(Federal University of Rio Grande do Sul), Google
Latin America Research Awards, and the Foundation
for Science and Technology of Portugal, under the
projects UIDB/00057/2020, CEECIND/01997/2017
and by CAPES - Finance Code 001.
REFERENCES
Bates, J., Fodeh, S., Brandt, C., and Womack, J. (2016).
Classification of radiology reports for falls in an hiv
study cohort. Journal of the American Medical Infor-
matics Association, 23(e1):e113–e117.
Bjarnadottir, R. I. and Lucero, R. J. (2018). What can we
learn about fall risk factors from ehr nursing notes? a
text mining study. eGEMs, 6(1):1–8.
Buntin, M. B., Burke, M. F., Hoaglin, M. C., and Blu-
menthal, D. (2011). The benefits of health infor-
mation technology: a review of the recent literature
shows predominantly positive results. Health affairs,
30(3):464–471.
Cho, I., Boo, E.-H., Chung, E., Bates, D., and Dykes,
P. (2019). Novel approach to inpatient fall risk
prediction and its cross-site validation using time-
variant data. Journal of medical Internet research,
21(2):e11505.
Choi, Y., Staley, B., Henriksen, C., Xu, D., Lipori, G.,
Brumback, B., and Winterstein, A. (2018). A dynamic
risk model for inpatient falls. American Journal of
Health-System Pharmacy, 75(17):1293–1303.
De Souza Urbanetto, J., Creutzberg, M., Franz, F., Ojeda,
B., da Silva Gustavo, A., Bittencourt, H., Steinmetz,
Q., and Farina, V. (2013). Morse fall scale: Trans-
lation and transcultural adaptation for the portuguese
language [morse fall scale: Traduc¸
˜
ao e adaptac¸
˜
ao tran-
scultural para a l
´
ıngua portuguesa]. Revista da Escola
de Enfermagem, 47(3):569–575.
dos Santos, H. D. P., Silva, A. P., Maciel, M. C. O., Burin,
H. M. V., Urbanetto, J. S., and Vieira, R. (2019).
Fall detection in ehr using word embeddings and deep
learning. In 2019 IEEE 19th International Conference
on Bioinformatics and Bioengineering (BIBE), pages
265–268.
Goldstein, B., Navar, A., Pencina, M., and Ioannidis, J.
(2017). Opportunities and challenges in developing
risk prediction models with electronic health records
data: A systematic review. Journal of the American
Medical Informatics Association, 24(1):198–208.
Hitcho, E. B., Krauss, M. J., Birge, S., Claiborne Dunagan,
ICEIS 2021 - 23rd International Conference on Enterprise Information Systems
632

W., Fischer, I., Johnson, S., Nast, P. A., Costantinou,
E., and Fraser, V. J. (2004). Characteristics and cir-
cumstances of falls in a hospital setting: a prospec-
tive analysis. Journal of general internal medicine,
19(7):732–739.
Kitchenham, B., Brereton, O. P., Budgen, D., Turner, M.,
Bailey, J., and Linkman, S. (2009). Systematic litera-
ture reviews in software engineering–a systematic lit-
erature review. Information and software technology,
51(1):7–15.
Lee, J., Jin, Y., Piao, J., and Lee, S.-M. (2016). Devel-
opment and evaluation of an automated fall risk as-
sessment system. International Journal for Quality in
Health Care, 28(2):175–182.
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C.,
Gøtzsche, P. C., Ioannidis, J. P., Clarke, M., Dev-
ereaux, P. J., Kleijnen, J., and Moher, D. (2009).
The prisma statement for reporting systematic reviews
and meta-analyses of studies that evaluate health care
interventions: explanation and elaboration. PLoS
medicine, 6(7):e1–e34.
Lucero, R., Lindberg, D., Fehlberg, E., Bjarnadottir, R.,
Li, Y., Cimiotti, J., Crane, M., and Prosperi, M.
(2019). A data-driven and practice-based approach to
identify risk factors associated with hospital-acquired
falls: Applying manual and semi- and fully-automated
methods. International Journal of Medical Informat-
ics, 122:63–69.
Luther, S., McCart, J., Berndt, D., Hahm, B., Finch, D.,
Jarman, J., Foulis, P., Lapcevic, W., Campbell, R.,
Shorr, R., Valencia, K., and Powell-Cope, G. (2015).
Improving identification of fall-related injuries in am-
bulatory care using statistical text mining. American
Journal of Public Health, 105(6):1168–1173.
Marier, A., Olsho, L., Rhodes, W., and Spector, W. (2016).
Improving prediction of fall risk among nursing home
residents using electronic medical records. Jour-
nal of the American Medical Informatics Association,
23(2):276–282.
McCart, J., Berndt, D., Jarman, J., Finch, D., and Luther,
S. (2013). Finding falls in ambulatory care clini-
cal documents using statistical text mining. Jour-
nal of the American Medical Informatics Association,
20(5):906–914.
McHugh, M. L. (2012). Interrater reliability: the kappa
statistic. Biochemia medica: Biochemia medica,
22(3):276–282.
Moons, K. G., Altman, D. G., Reitsma, J. B., Ioannidis,
J. P., Macaskill, P., Steyerberg, E. W., Vickers, A. J.,
Ransohoff, D. F., and Collins, G. S. (2015). Trans-
parent reporting of a multivariable prediction model
for individual prognosis or diagnosis (tripod): expla-
nation and elaboration. Annals of internal medicine,
162(1):W1–W73.
Morse, J., Morse, R., and Tylko, S. (1989). Development
of a scale to identify the fall-prone patient. Canadian
Journal on Aging / La Revue canadienne du vieillisse-
ment, 8(4):366–377.
Mubashir, M., Shao, L., and Seed, L. (2013). A survey on
fall detection: Principles and approaches. Neurocom-
puting, 100:144 – 152. Special issue: Behaviours in
video.
Oshiro, C., Frankland, T., Rosales, A., Perrin, N., Bell, C.,
Lo, S., and Trinacty, C. (2019). Fall ascertainment and
development of a risk prediction model using elec-
tronic medical records. Journal of the American Geri-
atrics Society.
Resar, R., Rozich, J., Simmonds, T., and Haraden, C.
(2006). A trigger tool to identify adverse events in
the intensive care unit. Joint Commission Journal on
Quality and Patient Safety, 32(10):585–590.
Rochefort, C., Buckeridge, D., and Abrahamowicz, M.
(2015). Improving patient safety by optimizing the
use of nursing human resources. Implementation Sci-
ence, 10(1).
Santos, J., dos Santos, H. D. P., and Vieira, R. (2020).
Fall detection in clinical notes using language mod-
els and token classifier. In 2020 IEEE 33rd Interna-
tional Symposium on Computer-Based Medical Sys-
tems (CBMS), pages 283–288.
Shiner, B., Neily, J., Mills, P., and Watts, B. (2016). Iden-
tification of inpatient falls using automated review of
text-based medical records. Journal of Patient Safety.
Swift, C. G. and Iliffe, S. (2014). Assessment and preven-
tion of falls in older people–concise guidance. Clini-
cal medicine, 14(6):658.
Topaz, M., Murga, L., Gaddis, K., McDonald, M., Bar-
Bachar, O., Goldberg, Y., and Bowles, K. (2019).
Mining fall-related information in clinical notes:
Comparison of rule-based and novel word embedding-
based machine learning approaches. Journal of
Biomedical Informatics, 90.
Toyabe, S.-I. (2012). Detecting inpatient falls by using natu-
ral language processing of electronic medical records.
BMC Health Services Research, 12(1).
Tremblay, M., Berndt, D., Luther, S., Foulis, P., and French,
D. (2009). Identifying fall-related injuries: Text min-
ing the electronic medical record. Information Tech-
nology and Management, 10(4):253–265.
Walsh, M., Frances Horgan, N., Walsh, C., and Galvin, R.
(2016). Systematic review of risk prediction models
for falls after stroke. Journal of Epidemiology and
Community Health, 70(5):513–519.
Weed-Pfaff, S., Nutter, B., Bena, J., Forney, J., Field, R.,
Szoka, L., Karius, D., Akins, P., Colvin, C., and Al-
bert, N. (2016). Validation of predictors of fall events
in hospitalized patients with cancer. Clinical Journal
of Oncology Nursing, 20(5):E126–E131.
Yokota, S., Endo, M., and Ohe, K. (2017). Establishing
a classification system for high fall-risk among inpa-
tients using support vector machines. CIN - Comput-
ers Informatics Nursing, 35(8):408–416.
Yokota, S. and Ohe, K. (2016). Construction and evalua-
tion of find, a fall risk prediction model of inpatients
from nursing data. Japan Journal of Nursing Science,
13(2):247–255.
Zhu, V. J., Walker, T. D., Warren, R. W., Jenny, P. B.,
Meystre, S., and Lenert, L. A. (2017). Identifying
falls risk screenings not documented with administra-
tive codes using natural language processing. In AMIA
annual symposium proceedings, volume 2017, page
1923. American Medical Informatics Association.
Opportunities and Challenges in Fall Risk Management using EHRs and Artificial Intelligence: A Systematic Review
633
