
Synthesis of Crosslinked Cellulose/PVA Bioplastic Strengthened with
Chitosan as and Alternative to Conventional Plastics
Eka Yulli Kartika and Endang Saepudin
Department of Chemistry, Faculty of Mathematics and Natural Science, Universitas Indonesia, Indonesia
Keywords: Crosslinked cellulose, Chitosan, Conventional Plastics.
Abstract: Environment problem from plastic waste should be reduced and solved. The development of biodegradable
plastic from cellulose can be a solution to solve it. Cellulose was a natural biopolymer that can be used as
bioplastics. Biodegradable plastics made from cellulose can be used as an alternative to conventional plastics,
because they are environmentally friendly, easy to obtain and easily degraded. However, used of cellulose-
based bioplastic requires physical or chemical modification to improve its physical and mechanical properties.
In this study, improvement of physical and mechanical properties of cellulose-based bioplastics was carried
out by adding polyvinyl alcohol (PVA), glutaraldehyde crosslinker and chitosan filler. Optimization the
synthesis of cellulose / PVA layers was carried out by varying the concentrations of glutaraldehyde and
chitosan from 0-56% and 0-33%.The results of the bioplastic film synthesis were evaluated for thickness,
swelling and solubility, biodegradability and mechanical properties, the optimum concentration of each
variation of glutaraldehyde and chitosan was characterized by FT-IR. The results showed that modification
of the cellulose/PVA-crosslinked glutaraldehyde film and the addition of chitosan fillers improve the physical
and mechanical properties of bioplastics, with the optimum concentration of each varian being 56% (w/w)
and 24% (w/w).
1 INTRODUCTION
Plastic is a product that can function as packaging, so
that it can facilitate human work. The high use of
plastic packaging causes an accumulation of plastic
waste, which causes environmental pollution
(Selvamurugan & Sivakumar, 2019). Plastic
packaging, especially food packaging that is
produced non-biodegradable, leads to environmental
problems because it takes thousands of years to
degrade. Biodegradable means that these materials
can be completely consumed by microorganisms
without leaving harmful pollutants in the
environment. To solve this problem, one solution is
to replace the non-biodegradable plastic base material
into a easily degraded material, namely biodegradable
plastic (Pikon & Czop, 2014). The material used for
the manufacture of plastics plays an important role in
determining the quality of the films produced. In the
manufacture of films can mix natural polymer
materials and synthetic polymer materials (Isroi &
Cifriadi, 2017).
In recent years, the use of natural fibers as
reinforcements in polymers has attracted much
attention due to the environmental concern. Cellulose
fibers exhibit high mechanical properties. Besides,
they also offer a number of other advantages over
conventional reinforcing materials, such as low cost,
worldwide availability, and biodegradability.
Particular attraction is its low density which leads to
high specific mechanical properties (Niu, et al. 2015).
These unique properties have made cellulose an ideal
candidate for high performance biocomposites.
Various biodegradable polymers, suchas chitosan
(Angadi, et al. 2012), starch, polycaprolactone (PCL)
(H.Lonnberg, et al. 2011), poly (3-hydroxybutyrate-
co-3-hydroxyvalerate) (PHBV) (E. Ten, et al. 2010),
polylactic acid (PLA) (L. Suryanegara, et al. 2010),
poly (furfuryl alcohol)(PFA) (H. Dekaa, et al. 2013),
and polyvinyl alcohol (PVA) (Priya, et al. 2014) have
been explored as potential matrices for this kind of
composites. PVA is the most widely produced
watersoluble synthetic polymer in the world. Itis also
a versatile polymer with broad applications due to its
biodegradability, biocompatibility, high tensile
strength, excellent adhesive properties, chemical
resistance and gas barrier properties (Niu, et al.
2015).
Kartika, E. and Saepudin, E.
Synthesis of Crosslinked Cellulose/PVA Bioplastic Strengthened with Chitosan as and Alternative to Conventional Plastics.
DOI: 10.5220/0010786800003317
In Proceedings of the 2nd International Conference on Science, Technology, and Environment (ICoSTE 2020) - Green Technology and Science to Face a New Century, pages 17-24
ISBN: 978-989-758-545-6
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
17

Plasticizers play a role in increasing the flexibility
and permeability of cellulose to water and gas vapor.
Synthetic polymer which is biodegradable as a
mixture of cellulose for the manufacture of
biodegradable plastic films is PVA. PVA is one of
the largest water-soluble, non-toxic and
environmentally friendly polymers. PVA has a polar
hydroxyl group attached to the carbon which makes
it very hydrophilic. In addition, PVA is widely used
in the plastic packaging industry because of its
solubility (Abdhulkani, et al. 2013). Although both
cellulose and PVA have plenty of hydroxyl groups
along their molecular chains, direct incorporation of
pristine cellulose fibers into PVA matrix cannot
produce composites with good mechanical
properties. This is due to the fact that most hydroxyl
groups in cellulose and PVA molecules have already
formed either intra- or inter-molecular hydrogen
bonds. Therefore, it is difficult to form new hydrogen
bonds between the two components by simple mixing
(Niu, et al., 2015).
Chemical crosslinking in composites is an
efficient way to achieve desired compatibility [4].
Chabba et al. crosslinked soy flour/flax yarns
composites using glutaraldehyde (GA) as the
crosslinking agent to improve the tensile and thermal
properties (Chabba, et al., 2015). Li et al. also used
GA to prepare crosslinked chitosan/PVA blend beads
with high mechanical strength (Li, et al., 2007).
Glutaraldehyde is a crosslinking agent that has the
ability to bind hydroxyl groups to polymeric
materials. Glutaraldehyde is also one of the
crosslinking agents which can cause the matrix cross-
linking to be tighter and increase the tensile strength.
In addition, it can improve the mechanical properties
of the mixed film and can improve the characteristics
of water resistance and film thermal stability (Basuki
& Sanjaya, 2009).
To increase the strength and hardness of
bioplastic products, it can be increased by adding
fillers. Chitosan is a polysaccharide that is abundant
in nature after cellulose. The use of chitosan as an
additive in making plastic films serves to improve the
transparency of the plastic film produced. The more
chitosan is used, the better the mechanical properties
and air resistance of the bioplastic products. In
addition, chitosan is non-toxic, easily biodegradable
and is polyelectrolytic (Mollah, et al., 2016).
Therefore, this research will conduct the
development of bioplastic films that are innovative,
environmentally friendly and biodegradable. The
process of making the film was carried out by
preparing the bioplastic film Cellulose / PVA with
added glutaraldehyde as cross-linker with the
addition of chitosan filler as reinforcement.
2 RESEARCH METHODS
2.1 Materials
Commercial cellulose (99%-cellulose). PVA was
purchased from Merck Company (Germany).
glutaraldehyde, chitosan, dimethyl sulfoxide
(DMSO) was purchased from Merk
MERCKMILLIPORE (Germany), CH
3
COOH,
distilled water, filter paper from the Universitas
Indonesia Materials Technology Laboratory.
2.2 Celulose Activation
Cellulose (0.5 g) was immersed in solvent (water:
DMSO :: 1:3) for 24 hours to swell and to activate
reactive sites on the surface (Kohli, et al., 2017).
2.3 Bioplastic Film Synthesis
2.3.1 Cellulose / PVA Crosslinked
Glutaraldehyde Film
The synthesis process begins with the manufacture of
cellulose / PVA. A total of 2 g of PVA was dissolved
in 23 mL of distilled water, heated at 80
o
C for 1 hour
while stirring at high speed. Then add cellulose
which has been activated overnight and stirred again
for 30 minutes at high speed. Then added
glutaraldehyde (0-56%, w/w). Then the bioplastic is
molded and left at room temperature overnight. Then,
oven for 5 hours at a temperature of 60◦C.
2.3.2 Cellulose / PVA Filler Chitosan Film
Solution of 1% chitosan (w/w) was made using 2%
(w/w) acetic acid solution. The chitosan solution (0-
33% w/w) is added to the cellulose / PVA /
glutaraldehyde mixture by the process described in
2.3.1. The mixing is carried out right after the
crosslinking agent is added, before the mixture
thickens and becomes agar. Then the bioplastic is
molded and left at room temperature overnight. Then,
oven for 5 hours at a temperature of 60◦C.
ICoSTE 2020 - the International Conference on Science, Technology, and Environment (ICoSTE)
18

2.4 Film Characterization
2.4.1 Measurement of Film Thickness
The thickness of the samples was determined with a
couplers micrometer. This tool has an accuracy of up
to 0.01 mm. Measurements were repeated in ten
different regions of each sample. Average values
were calculated and used (Abdollahi, et al., 2012).
2.4.2 Swelling and Water Solubility
Measurements
The sample was cut into small pieces (2x2 cm) and
weighed to determine their dry mass. The weighed
samples were placed in closed beakers containing 30
mL of water and stored at temperature room. The
kinetic of swelling was evaluated by periodically
measuring the weight increment of the samples. The
film is taken, cleaned and shaken several times to
remove the liquid that is on the surface of the
bioplastic film and weighed. The weighing was
continued until equilibrium state. The procedure was
repeated three times for each sample to confirm its
repeatability. The water gain of each sample was
calculated as follows:
Swelling %
x 100%
where Ws is the weight of the film sampel after
immersion and W0 is the weight of the initial film
sampel (Abdollahi, et al., 2012).
After that, the film from the swelling test is dried
until the mass of the sample film is constant and the
solubility is calculated using the equation:
WS %
x 100%
Where Wk is weight of the dried layer after
swelling test (Sa’adah, 2020).
2.4.3 Biodegradability in Soil
In this method, samples films 20 mm × 20 mm × 1
mm small pieces were weighted and placed for 120
days into the agricultural soil in a pot. The pot was
covered with a plastic net and exposed to atmospheric
conditions for 120 days. Variations in film
morphology, the time of films disintegrated and
weight loss were recorded. To determine the weight
loss the specimen of each sample was quickly washed
with cold water and dried in an oven at 70 ◦C to
constant weight. The weights of the sample, before
and after washing were recorded (Priya, et al. 2014).
2.4.4 Mechanical Properties
Tensile strength (TS) and elongation at break (E%) of
the film samples were determined according to
ASTM standard method D882–02 with an Strograph
EII Testing Machine. The film samples were cut in
rectangular specimens (40x20 mm). The test is done
by clamping both ends of the sample to a tensile
testing machine. Then the start knob is turned on and
the tool will pull the sample until it breaks. Saved and
recorded the results of tensile strength and%
elongation that are on the tools.
2.4.5 Fourier Transform Infrared
Spectroscopy (FTIR) Analysis
FTIR spectra were studied with a Nicolet 560
spectrometer (Nicolet Co., USA). KBr solids are
smoothed and inserted into the holder for background
measurement. Bioplastic film samples on top of the
holder and measured in the wave number range 500-
4000 cm
-1
(Abdollahi, et al., 2012).
3 RESULTS AND DISCUSSION
3.1 Film Thickness
Bioplastic film thickness measured using a
micrometer. Thickness is one of the parameters used
to determine the quality of a bioplastic characteristic.
The results showed that the thickness of the bioplastic
did not increase significantly with an increase in the
glutaraldehyde crosslinker concentration. However,
at certain concentrations it will increase the thickness
of the printed plastic (Table 3.1). The results showed
that all variations of glutaraldehyde and chitosan had
the same film thickness. The same thickness value
caused because the dissolved material evaporates
when heated.
Table 1 Film Thickness
Treat
ment
Glutaral
dehyde
(%w/w)
Thick
ness
(mm)
Treat
ment
Chitosan
(%w/b)
Thickness
(mm)
PPP
0
0 0,12
PPP
0
0 0,12
PPP
30
30 0,20
PPP
12
12 0,20
PPP
46
46 0,20
PPP
24
24 0,20
PPP
56
56 0,20
PPP
33
33 0,20
Synthesis of Crosslinked Cellulose/PVA Bioplastic Strengthened with Chitosan as and Alternative to Conventional Plastics
19
Bioplastic thickness influenced by the area of the
mold, the volume of the solution and the total amount
of dissolved solids (Sa’adah, 2020). In this study, the
mold area was made the same, so the influencing
factor was the volume of solute glutaraldehyde and
chitosan. So, the addition of glutaraldehyde and
chitosan increases the thickness.
3.2 Swelling and Water Solubility
The swelling test was conducted to determine the
interaction between the bioplastic film and water.
Conventional plastics circulating in the community
are hydrophobic, making it difficult to dissolve in
water. Therefore, the development of bioplastic films
which are difficult to dissolve in water is carried out
(Sa’adah, 2020).
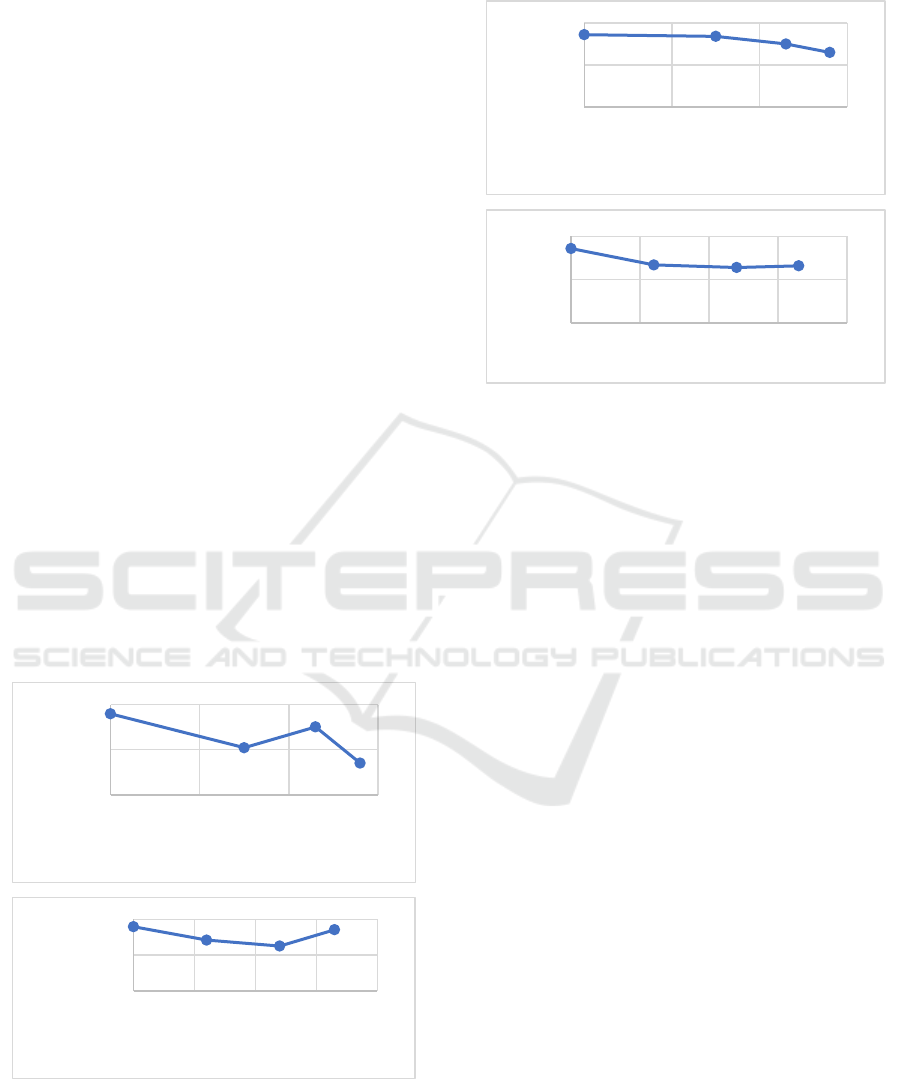
The results of the swelling and solubility test are
shown in Figures 1 & 2. Based on the results of the
study, the higher the concentration of glutaraldehyde
and chitosan added, the lower the% increase in mass
or swelling of bioplastics. This is due to the hydrogen
bonding that occurs between glutaraldehyde and
cellulose as well as chitosan and cellulose in the
bioplastic film, which prevents the formation of holes
that can be traversed by water. In general, the lower
the swelling ability of the bioplastic film, the lower
the film solubility. This is because the lower the
amount of water that enters the cavity, the less the
film component can dissolve (Niu, et al. 2015,
Abdhulkani, et al. 2013, Basuki & Sanjaya, 2009).
Figure. 1 Swelling
Figure 2. Water Solubility
The results showed that in general the swelling
ability of bioplastics decreased with increasing
concentrations of glutaraldehyde and chitosan
(Figure 1). The results showed that the% variation in
the development of glutaraldehyde and the lowest
chitosan in this study was 24% PPP56 and PPP24,
respectively, with a percentage of 141% and 251%.
Meanwhile, the highest% swelling was the PPP0
variation with the proportion of 359%.
Glutaraldehyde acts as a crosslinker and also acts as
a plasticizer. The PPP56 variation has higher
intermolecular interactions between molecules
compared to other variations, so that in other
variations the ability of glutaraldehyde to act as a
plasticizer. The results showed that the higher the
concentration of chitosan was added, the lower the
percentage increase in mass or swelling of
bioplastics. This is due to the hydrogen bonding that
occurs between chitosan and cellulose on the
bioplastic film, so that it prevents the formation of
cavities that can be passed by water (Sa’adah, 2020).
The solubility test results were obtained from the
dried swelling bioplastic film, then the final mass of
the sample was calculated. There is a correlation
between the results of the swelling test and the results
of the solubility test. The higher the swelling ability
of the bioplastic film, the more dissolved bioplastic
film mass will be shown (Figure 2). Addition of
glutaraldehyde and chitosan in Cellulose / PVA
matrix can reduce the solubility of films in water. The
highest yield of bioplastic film solubility at the PPP0
variation was 86%. For the glutaraldehyde variation,
the lowest solubility yield was 65% for PPP56. For
359
209
301
141
0
200
400
0% 20% 40% 60%
Water Uptake (%)
Variations Concentration of …
359
284
251
342
0
200
400
0% 10% 20% 30% 40%
Water Uptake
(%)
Variations Concentration of Chitosan
86 84
75
65
0
50
100
0% 20% 40% 60%
Weight Lost (%)
Variations Concentration of …
86
67
64
66
0
50
100
0% 10% 20% 30% 40%
Weight Lost
(%)
Variations Concentration of Chitosan
ICoSTE 2020 - the International Conference on Science, Technology, and Environment (ICoSTE)
20
chitosan variation, the lowest solubility yield was
64% for PPP24. The results obtained are linear with
the results of the swelling test. This will increase the
interaction between molecules and reduce sensitivity
to water (Basuki & Sanjaya, 2009).
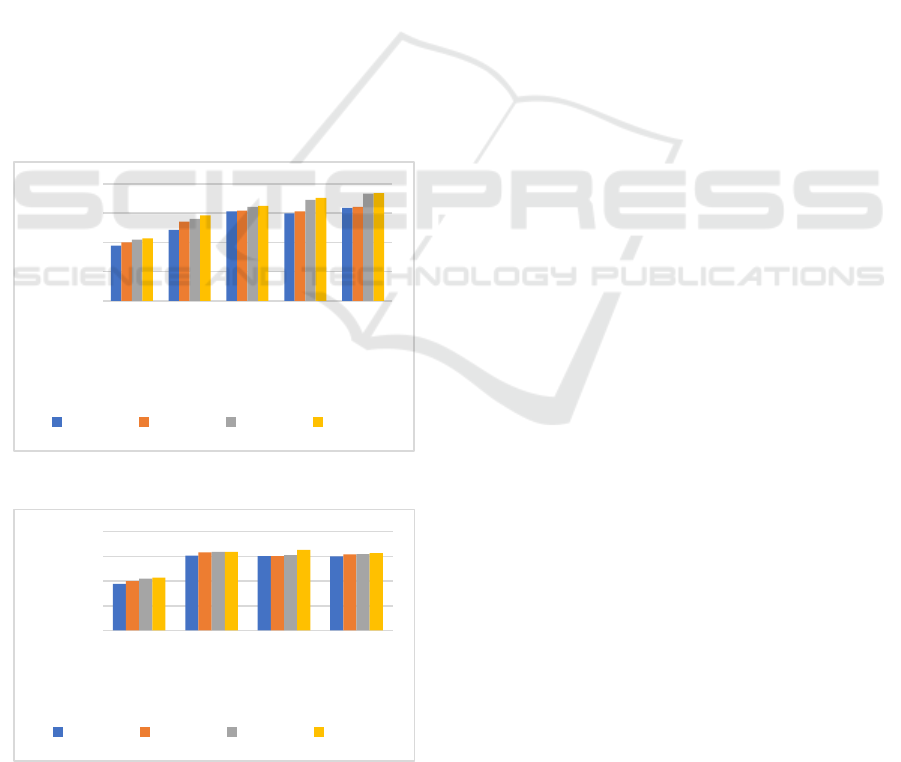
3.3 Biodegradability in Soil
Bioplastic film biodegradation testing was carried out
by means of the burial test method in the soil. The
biodegradation test aims to determine the level of
resistance of the bioplastic film in the soil for a
certain time. The results of the biodegradation
analysis showed that all variations experienced a
decrease in the mass of the bioplastic film, the mass
decrease for each variation did not experience any
difference significant (Figure 3 and 4). The decrease
in the mass of the bioplastic film is an indication that
the bioplastic film is biodegradable. The reduction in
the mass of the bioplastic film was caused by the
attack of microorganisms and the absorption of water
content (swelling) of the bioplastic film which is in
the soil, initiates a hydrolysis reaction so that the
bioplastic polymer can be decomposed into smaller
compounds (Sa’adah, 2020).
Figure. 3 Biodegradability Variation of Glutaraldeyde
Figure. 4 Biodegradability Variation of Chitosan
The results showed that the higher the
concentration of glutaraldehyde and chitosan was
added, the% biodegradability of bioplastics
increased. The results showed that the higher the
concentration of glutaraldehyde added, the%
biodegradability of bioplastics was increased.
Glutaraldehyde acts as a crosslinker and plasticizer,
where the increased glutaraldehyde concentration
will increase its ability as a plasticizer. This can
reduce the interaction between macromolecules, so
that the bonds between macromolecules are easier to
break, so that the bioplastic film will be more easily
degraded by microorganisms. In addition, the
hydrophilic groups present in glutaraldehyde will
make the degradation process easier, thus making
bioplastics more environmentally friendly (Basuki &
Sanjaya, 2009).
The increase in the amount of chitosan in
bioplastics causes the amount of carbon to also
increase. Carbon is one of the elements needed by
microorganisms as a food source, increasing the
amount of chitosan will increase the carbon source
that can be used as food for microorganisms in the
soil. The% increase in biodegradability is due to
increased hydrolysis of random chain cutting which
triggers a reduction in molecular weight. The
reduction in molecular weight will trigger these
molecules to become smaller molecules, so that they
will be easier to degrade by microorganisms
(Abdullah, et al., 2020). There are various kinds of
microorganisms that have been isolated from the soil
using bioplastics as a carbon source. Some of these
microorganisms include Actinobacteria species such
as Amycolatopsis, Thermomactimuces,
Actinomadura, Nanomuraea, Laceyella and
Streptomyces, of which the most common species are
Amycolatopsis and Streptomyces. In addition, there
are also species of Paenibacillus, Pseudomonas,
Bacillus and Bulkholderia. Several types of fungal
species that have been isolated and are responsible
for degrading bioplastics are Aspergilus, Fusarium
and Penicillium, Penicillium is the most common
species (Emadian, et al., 2017).
3.4 Mechanical Properties
The mechanical strength test of bioplastics is carried
out by measuring the tensile strength and strain (%
elongation). Tensile strength (tensile strength) is the
ability of a bioplastic film to withstand a given load
until the bioplastic breaks, while% elongation is the
optimum stretch extension or increase in bioplastic
when it is pulled to break. % elongation is used to
measure the elasticity of a polymer.
0
20
40
60
80
PPPS0 PPPS30 PPPS46 PPPS56 PPPS75
%Biodegradabilitas
Variations Concentration of
Glutaraldehyde
Week1 Week2 Week3 Week4
0
20
40
60
80
PPPS0 PPPS12 PPPS24 PPPS33
%Biodegradabilitas
Variations Concentration of Chitosan
Week1 Week2 Week3 Week4
Synthesis of Crosslinked Cellulose/PVA Bioplastic Strengthened with Chitosan as and Alternative to Conventional Plastics
21
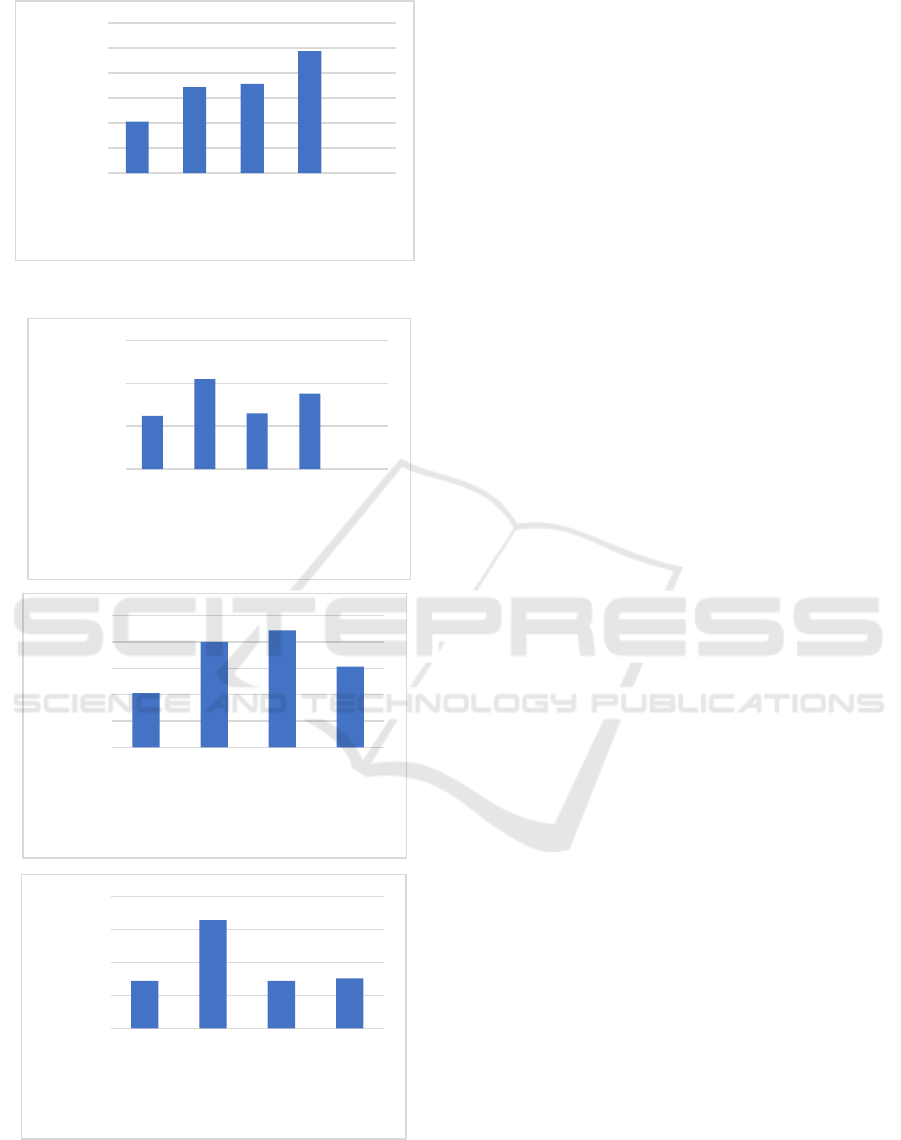
Figure 5. Tensile Strength
Figure 6. Elongation
The presence of cross-linking will also increase
the molecular weight of the bioplastic, where the
tensile strength of the polymer increases with
increasing molecular weight and reaches a saturation
level at a certain molecular weight. At lower
molecular weights, the polymer chains are loosely
bound and the chains are easier to move so that the
strength of the polymer is low (even though it has a
higher crystallinity), whereas polymers with large
molecular weights have large chains and give strength
to the polymer. The results of the bioplastic tensile
strength test with variations in the concentration of
chitosan are shown in Figure 5. The results of the
tensile strength test showed that the higher the
concentration of chitosan added, the tensile strength
of the bioplastic was increased. This is due to the
presence of filler so that it can improve the
mechanical properties of the bioplastic film. The
improved mechanical properties of bioplastics can
also be attributed to the good interface adhesion,
which can form hydrogen bonds between cellulose
and filler chitosan (Sa’adah, 2020).
The effect of glutaraldehyde on% elongation is
shown in Figure 6. The parameter of% elongation is
used to determine the flexibility and tensile strength
of the bioplastic film. An increase in the
glutaraldehyde concentration will increase the%
elongation of the bioplastic film. This is because the
role of glutaraldehyde as a plasticizer can reduce the
interaction of the intermolecular bonds of the
bioplastic polymers and replace them with hydrogen
bonds formed between the plasticizer and the
polymer, thereby reducing stiffness and increasing
the flexibility of the film. The addition of chitosan to
bioplastics will reduce the% elongation (elongation)
of the bioplastic. This is because the addition of
chitosan will increase the stiffness of the bioplastic,
thus reducing the% elongation ability of a bioplastic
film. In addition, increasing the amount of chitosan
filler will increase the hydrogen bonding interaction
between the filler and the cellulose matrix, thereby
reducing the flexibility of the bioplastic film (Mollah,
et al., 2016).
3.5 Fourier Transform Infrared
Spectroscopy (FTIR) Analysis
The analysis of functional groups on the effect of
adding glutaraldehyde and chitosan was carried out
by FTIR, which is shown in Figure 7. In the FTIR
spectra of cellulose / PVA / glutaraldehyde films
showed a widened peak at wave number 3200-3500
cm
-1
indicating the presence of an OH group. The
absorption band at the numbers 1700-1715 cm
-1
4,12
6,87
7,12
9,75
0
2
4
6
8
10
12
0% 30% 46% 56%
Tensile Strength (MPa)
Variations Concentration of …
0
50
100
150
0% 30% 46% 56%
% Elongation
Variations Concentration of
Glutaraldehyde
4,12
8
8,87
6,12
0
2
4
6
8
10
0% 12% 24% 33%
Tensile Strength (MPa)
Variations Concentration of
Chitosan
55
60
65
70
75
0% 12% 24% 33%
% Elongation
Variations Concentration of
Chitosan
ICoSTE 2020 - the International Conference on Science, Technology, and Environment (ICoSTE)
22
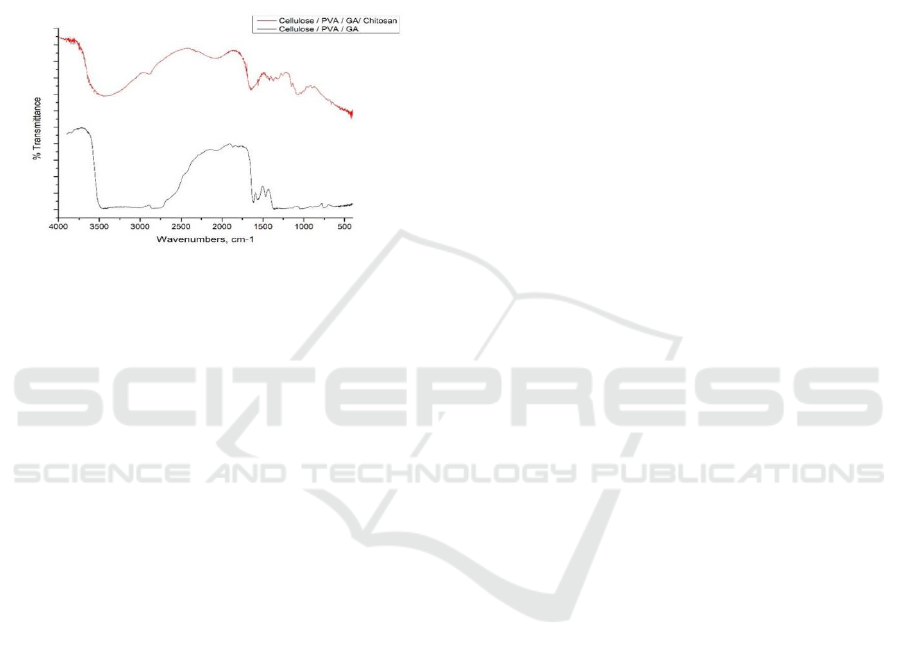
shows the peak area for the aldehyde group. The
FTIR spectra results of the cellulose / PVA /
glutaraldehyde / chitosan film showed absorption at
the wave number 3445.1 cm
-1
which is the –OH
region and water. The absorption band at wave
number 2928 cm
-1
shows the peak area for the C-H
group. The absorption at wave numbers 1659.7 and
1376.1 cm
-1
is the peak area for the N-H group of the
amine group and the C-H group on CH
3
(Basuki &
Sanjaya, 2009).
Figure 7: FTIR analysis
Figure 7. Results of the cellulose/PVA/glutaraldehyde/
chitosan
4 CONCLUSION
Based on the research that has been done, it can be
concluded that the variation in the concentration of
cross-linking agent glutaraldehyde and chitosan filler
has an effect on the physical and mechanical
properties of the bioplastic film layer, with each
optimum concentration to improve the physical and
mechanical properties of the bioplastic is 56% (w / w)
and 24% (w / w). Evidenced by the% swelling and the
lowest solubility and the highest tensile strength
values. Increasing the concentration of
glutaraldehyde and chitosan at a certain concentration
decreased the physical and mechanical properties of
the bioplastic film.
REFERENCES
Abdulkhani, A., Hojati, E., & Ashori, A, 2013, Preparation
of Cellulose / Polyvinyl Alcohol Biocomposite Films
Using 1-n-butyl-3-methylimidazolium Chloride,
International Journal of Biological Macromolecules,
Vol. 62, pp. 379–386.
Abdullah, A. H. D., Putri, O. D., Fikriyyah, A. K., Nissa, R.
C., Intadiana, S., 2020, Effect of Microcrystalline
Cellulose on Characteristics of Cassava Starch-based
Bioplastic, Polymer-Plastics Technology and
Materials, Vol. 59, pp. 1-9.
Abdollahi, M., Rezaei, M., & Farzi, G., 2012, A Novel
Active Bionanocomposite Film Incorporating
Rosemary Essential Oil and Nanoclay into Chitosan,
Journal of Food Engineering, Vol. 111, No. 2, pp.
343–350.
Angadi, S.C., L.S. Manjeshwar., T.M. Aminabhavi, 2012,
Novel Composite Blend Microbeads of Sodium
Alginate Coated with Chitosan for Controlled Release
of Amoxicillin, International Journal of Biological
Macromolecules, pp. 45–55.
Basuki, B. R., Sanjaya, I. G. M., 2009, Sintesis Ikat Silang
Kitosan dengan Glutaraldehid serta Identifikasi
Gugus Fungsi dan Derajat Deasetilasinya Cross-
linked Chitosan Synthesis Using Glutaraldehyde and
Functional Group Identification as well as Its
Deacetylation Degree, Jurnal Ilmu Dasar, Vol. 10,
No.1, pp. 93–101.
Chabba, S., G.F. Matthews., A.N. Netravali, 2005, Green
Composites Using Cross-linked Soy Flour and Flax
Yarns, Green Chemistry, pp. 576–581.
Emadian, S. M., Onay, T. T., Demirel, B, 2017,
Biodegradation of Bioplastics in Natural
Environments, Waste Management, Vol. 59, pp. 526–
536.
Elena Ten, J. Turtle, D. Bahr, L. Jiang, M. Wolcott, 2010,
Thrmal and Mechanical Properties of Poly (3-
Hydroxybutyrate-co-3-hydroxyvalerate) / Cellulose
Nanowhiskers Composites, Polymer, Vol. 51, pp.
2652–2660.
H. Dekaa, M. Misraa, A. Mohanty, 2013, Renewable
Resource Based “All Green Composites” from Kenaf
Biofiber and Poly (Furfuryl Alcohol) Bioresin,
Industrial Crops and Products, Vol. 41, pp. 94–101.
Isroi, I., Cifriadi, A., 2017, Bioplastic Production from
Cellulose of Oil Palm Empty Fruit Bunch, IOP
Conference Series: Earth and Environmental Science
Vol. 65 International Conference on Biomass:
Technology, Application and Sustainable
Development.
Kohli, D., Garg, S., Jana, A. K., Maiti, M., 2017, Synthesis
of Graft Copolymers for Green Composite Films and
Optimization of Reaction Parameters using Taguchi
(L16) Orthogonal Array, Indian Chemical Engineer,
Vol. 59, No. 2, pp. 136–158.
L. Suryanegara, A.N. Nakagaito, H. Yano, 2010, The
Synergetic Effect of Phenylphosponic Acid Zinc and
Microfibrillated Cellulose on the Injection Molding
Cycle Time of PLA Composites, Cellulose, Vol. 17,
pp. 771–778.
Li, M., S. Cheng., H. Yan, 2007, Preparation of
Crosslinked Chitosan/Poly(Vinyl Alcohol) Blend
Beads with High Mechanical Strength, Green
Chemistry, Issue 8, pp. 894–898.
Mollah, M. Z. I., Akter, N., Quader, F. B., Sultana, S., &
Khan, R. A., 2016, Biodegradable Colour Polymeric
Film (Starch-Chitosan) Development :
Characterization for Packaging Materials, Open
Synthesis of Crosslinked Cellulose/PVA Bioplastic Strengthened with Chitosan as and Alternative to Conventional Plastics
23

Journal of Organic Polymer Materials, Vol. 06, No.
01, pp. 10–24.
Niu, Y., Xiaofang, Z., Xu, H., Jiangqi, Z., Wei, Z., &
Canhui, L., 2015, Effective Dispersion and
Crosslinking in PVA/Cellulose Fiber Biocomposites
Via Solid-State Mechanochemistry, International
Journal of Bilogical Macromolecules, pp. 855-861.
Pikoń, K., & Czop, M. (2014). Environmental Impact of
Biodegradable Packaging Waste Utilization, Polish
Journal of Environmental Studies, Vol. 23, No. 3, pp.
969-973.
Priya, B., Gupta, V. K., Pathania, D., Singha, A. S., 2014,
Synthesis, Characterization and Antibacterial Activity
of Biodegradable Starch/PVA Composite Films
Reinforced with Cellulosic Fibre, Carbohydrate
Polymers, Vol. 109, pp. 171–179.
Sa’adah, S.M., 2020, Sintesis Bioplastik Pati-g-PLA/PVA
Termodifikasi Ikat Silang dan Pengisian Selulosa
yang diperkaya dengan Aditif Antioksidan dan
Antimikroba, Tesis, FMIPA Kimia, Universitas
Indonesia.
Selvamurugan. M, P. Sivakumar, 2019, Bioplastics – An
Eco-Friendly Alternative to Petrochemical Plastics,
Current World Enivronment, Vol. 14, No. 1, pp. 49-
59.
ICoSTE 2020 - the International Conference on Science, Technology, and Environment (ICoSTE)
24
