
Histopathology of Rat Kidney Organ Due to Ethanol Extract of
Phaleria Macrocarpa Treatment Induced by Isoniazid
Ali Napiah Nasution* and Sri Lestari R. Nasution
Faculty of Medical, Universitas Prima Indonesia, Indonesia
Keywords: Phaleria macrocarpa, INH, Histopatology of the kidney.
Abstract: The use of anti-tuberculosis drugs has side effects on human organs, especially the kidneys. Phaleria
macrocarpa is now widely used as traditional medicine. The content of phaleria macrocarpa contains high
antioxidants and this fruit is spread in North Sumatra. This study aims to determine the histopathological
picture of induced kidney organ isoniazid (INH) by giving ethanol extract of phaleria macrocarpa. Animals
test used in this study were 9 wistar rats which were divided into 3 treatment and experiments groups for 10
days. The results showed that the histopathology of kidney organ caused by INH were highly damage.
However, giving Ethanol Extract of Phaleria macrocarpa at a dose of 3.4 g / 200 g was able to protect the
kidneys (nephroprotector) from the toxicity of isoniazid drugs with minimal damage or almost normal. The
administration of phaleria macrocarpa extract together with the anti-tuberculosis drug INH shows the
protective effect of the kidneys against the INH drug.
1 INTRODUCTION
In 2030 the World Health Organization (WHO)
devised a strategy to end tuberculosis (WHO, 2019).
Indonesia also aims to become a tuberculosis-free
country by 2050 (MOH RI, 2018). An adherence to
taking anti-tuberculosis drugs plays an important role
in the success of the treatment of tuberculosis
(Sitorus, Fatmawati and Rahmaniah, 2017).
Development of rapid industrialization generated
many negative consequence for environmental
pollution affecting human health. The previous
research of Nasution et all., (2015) explained that
P.macrocarpa contained hydrogen stretching due to
inter and intramolecular interaction of alcohol, phenol
and carboxylic acid. This functional group will
contribute to preventing metal ion from destroying
organs.
The kidney is the second most common organ
after the livertarget destroyer by xenobiotics. This
isdue to many chemical substancesexcreted in the
urine. One of the most common parts of the
kidneydamage caused by chemicals is the proximal
tubule. Some compounds that can be xenobiotic
include alcohol / ethanol andgentamicin (Panjaitan,
2003).Microscopic changes onkidney including
changes in the structure of the glomerulus, swelling
or enlargementkidneys and increased numbers of fat,
protein and water cells. This effect willchange the
ability of the kidneys to function normally (Booggan,
2003).
The most common side effect in patients taking
first-line OAT is nephrotoxicity (Pratiwi, 2018).
Histologically the administration of isoniazid causes
mild to moderate kidney damage (Muzika et al.,
2016).
Phaleria macrocarpa fruit is one of the ingredients
of traditional medicine that oftenly used to treat
various diseases and has antioxidant, antimicrobial,
and anti-inflammatory activity (Hendra et al., 2011).
Ramadhan Research, 2019 also stated that the
phaleria pacrocarpa fruit has an effect as a
nephroprotector in mice which induced by
paracetamol.
The results of Parapaga, Durry and Lintong, 2018
stated that antioxidant activity can inhibit and prevent
oxidative damage. Antioxidants can also reduce the
production of free radicals and protect body cells
from oxidative stress (Octaria, 2019).
According to Yatman (2012), the antioxidant
process throughoxidation and reduction reactions that
form oxidizing free radicalswith reactive oxygen.
Because of its reactivity, free radicals willoxidizes
beneficial substances for the body causing a
numberdamaged body tissue. Hence it is easily
Nasution, A. and R. Nasution, S.
Histopathology of Rat Kidney Organ Due to Ethanol Extract of Phaleria Macrocarpa Treatment Induced by Isoniazid.
DOI: 10.5220/0010296202430247
In Proceedings of the International Conference on Health Informatics, Medical, Biological Engineering, and Pharmaceutical (HIMBEP 2020), pages 243-247
ISBN: 978-989-758-500-5
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
243

oxidized, free radicals, for that matterperoxyl radical
(ROO) will oxidize xanthone rapidly so that it is
radicalthe peroxyl will turn into R-H. This change
occurs because of the oxygen moleculereduced by
garsinone B as a xanthone derivative, the reaction is
inhibitoryfree radicals of various types.Free radicals
can interfere with cellular function by
performinglipid peroxidation resulting in damage to
cell membranes. This damage can becauses changes
in electric charge in cells, changes in osmotic
pressure,causes cell swelling and ends in cell death.
Nakatni et al., (2004) conducted a study on the in
vitro anti-inflammatory activity of γ-mangostin
against the synthesis of PGE2 and cyclooxygenase
(COX) in glioma cells mouse C6. These two
compounds and enzymes are the most important
mediators in the inflammatory reaction. From this
research results can be made:mangostindirect PGE2
production in the inflammatory process.
In order to develop research on extracts of the
crown of the godsas a herbal medicine, this research
was conducted with the aim to find outthe effect of
giving the extract of the Dewa's Crown on the
histopathological picture of the kidney of rats which
was induced by isoniazid and the optimal dose of the
extract had an effect on the liver and kidneys. The
results obtained are expectedused as a source of
information about the benefits of extract of the crown
of gods especiallyon the kidneys and serve as the
basis for further researchdevelopment of mangosteen
peel extract as a standardized herbal medicine.
This study aims to determine the
histopathological picture of kidney organ induced by
INH (Isoniazid) by giving ethanol extract of Phaleria
macrocarpa fruit.
Nasution et al., (2019) Cd(II) ion is a heavy metal
that has atoxic abilty in the human body. phaleria
macrocarpa has been used as anticancer, Diabetes
Mellitus and antimicrobe because its consists of
flavonoid, steroid and tannin. The result of
exsperimental rats exposed witg Cd(II) ion, there are
significant decreasing of all the observed parameters
including MAD, SGOT and SGPT with percentage
71,5 %, 72,1 % and 93, 6 % respectively. The rats
given with the antidote of phaleria macrocarpa flesh
fruit were able to protect the liver from damage due
to exposure to Cd(II) as seen from the decrease in
liver function enzyme parameters namely SGOT and
SGPT
2 METHOD
This research (conducted at the Pharmacy
Laboratory, University of North Sumatra in
December 2019) used an experimental design with a
post-test only control group design pattern.
Experimental samples were 9 male Wistar white rats
(Rattus Novergicus), weight 150-200 grams were
used. Rats were in accordance with the code of
conduct issued by the Ethics Committee of the
Faculty of Medicine, University of Prima Indonesia.
The tools used were laboratory glass, analytical
balance, blender, incubator, oven, rotary evaporator,
water bath, animal scales, animal house, injection
equipment, surgical instruments, microtome, object
glass, cover glass, light microscope. The ingredients
used were phaleria macrocarpa, aquadest, isoniazid,
CMC-Na 0.5%, 10% formalin buffer, 96% ethanol,
preparations dyes.
The phaleria macrocarpa fruit was separated from
the skin and seeds and washed with running water.
The phaleria macrocarpa meat was cut into small
pieces and then dried and blended until smooth into
powder, then put into a container that can be tightly
closed.
The 500 mg simplicia powder was macerated with
96% ethanol, then evaporated with a rotary
evaporator at ≤70oC. Re-evaporated with waterbath
(water bath) until the extract results become thick
(Lestari, 2019; Ramadan, 2019).
Nephroprotector Effectiveness Test Samples were
divided into 3 groups, so that each group amounted to
3 rats. The treatment of each group was as follows:
Control group (P1) without treatment (only aquades).
Treatment Group 2 (P2) isoniazid 300 mg / 70 kg BW
induced. Treatment group 3 (P3) was given ethanol
extract of Crown Fruit of a dose of 3.4 g / 200 g BW
with isoniazid induction dose of 300 mg / 70 kg BW.
The treatments were orally contucted once per day for
10 days. At the end of the experiment, the rats were
anesthetized with chloroform before being dissected,
kidney organs were taken and then put into containers
containing 10% formalin buffer that had been labeled.
Preparations made with a thickness of 4-6 mm,
stained with HE and viewed under a microscope to
see histopathological changes.
3 RESULTS
3.1 Histopathology of the Kidney
Organs
The results of histopathology of the renal organs
examined in the control group are given in Figure 1.
In the control rat the glomerulus and renal tubules
were seen in normal condition, the nucleus was
clearly visible and the renal capsule was also clearly
HIMBEP 2020 - International Conference on Health Informatics, Medical, Biological Engineering, and Pharmaceutical
244
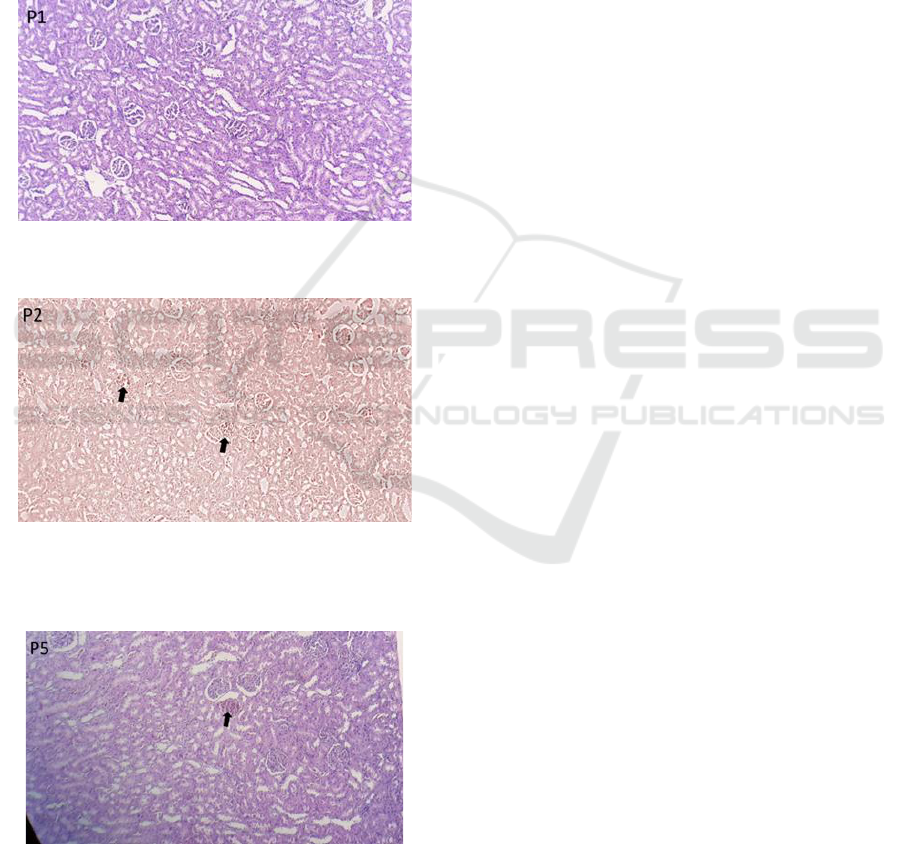
visible. Mice induced with isoniazid 300 mg / 70 kg
bw (KP2) were found with moderate glomerular
atrophy with necrosis. We found damage in the
kidneys as shown in Figure 2.
Mice given Ethanol Extract of Phaleria
Macrocarpa (EEPM) with isoniazid induction (KP3)
were seen with mild glomerular atrophy with
necrosis. In contrast to those who were only given
INH only in group 2, there was a lot of damage and
necrosis in the kidney organs. The administration of
the extract phaleria macrocarpa fruit can proverly
protect the kidneys from isoniazid induction.
Figure 1. Histopathology of rat kidney organ controls (P1).
10 x 10 magnification
Figure 2. Histopathology of rats kidney organ (P2) = INH
300 mg / 70 kg bw.
Description: glomerular atrophy (black arrow).
Figure 3. Histopathology of rats kidney organ (P3) =
EEPM + INH. Description: glomerular atrophy (black
arrow).
According to Zakhari et al., (2006), the results of
alcohol metabolism that occur inliver, namely the formation
of acetate, acetyldehyde, and increased reactive
oxygenspecies (ROS) enter the systemic blood circulation
which can damagethe structure of the cells of the extra
hepatic tissue namely the brain, lung, heart and
tissuekidney. ROS which are highly reactive can cause the
breakdown of molecular complexescellular (Wu and
Caderbaum, 2005). After ethanol intoxication,the balance
between prooxidants and antioxidants is disturbed
thuscauses oxidative stress from biomolecules, such as fat,
protein, or DNA,and ultimately cause cell damage (Das and
Vasudeven, 2007).
According to Yatman (2012), the process of
antioxidants through oxidation reactions andreduction
which forms free radicals which are oxidizing agents
withoxygenreactive. Because of their reactivity, these free
radicals willoxidize substances that arebeneficial to
thebody, causing a number of body tissues to be damaged.
Bybecause it is easily oxidized, free radicals, in this case
theperoxyl radical (ROO) willoxidizes xantones quickly, so
that the peroxyl radical will changeto be R-H. This
changeoccurs because the oxygen molecule is reduced by
garsinon Bas a xanthone derivative, its reaction can inhibit
free radicals of varioustype.
The molecular structure of each of the cell and tissue
flavonoid groupsthe body is constantly exposed to the
damaging effects caused by free radicalsand reactive
oxygen species (ROS) free radicalsnormally formed during
oxygen metabolism or induced by damageexogenous. Free
radicals can interfere with cellular function by
performinglipid peroxidation resulting in damage to cell
membranes. This damage can becauses changes in electric
charge in cells, changes in osmotic pressure,causes cell
swelling and ends in cell death. Flavonoidscan interfere
with more than 3 different free radical generating systems,
andcan also enhance endogenous antioxidant function.
Antioxidant activityhere is the antioxidant mechanism of
flavonoids, namely binding radicalsdirectly (direct radical
scanning venging), through nitric oxide, xanthinoxidase,
immobilization of leukocytes, interactions with other
enzyme systems (Nijveldtet al., 2001).
4 RESULTS
This research was conducted to determine the
effectiveness of etal extract of Phaleria macrocarpa
fruit on nephroprotectors in isoniazid-induced white
rats. Treated the rats with the EEPM at a dose of 3.4
g / 200 g bw can neutralize kidney damage due to
isoniazid with kidney histology indicators in mice.
Based on the results of research in the negative
control group (mice without treatment that was only
given aquadest) had a slight inflammation.
Supposedly in the negative control group there was
no damage to hepatocyte cells, this might be caused
by external variables that could not be controlled,
such as the psychological condition of the rat and the
Histopathology of Rat Kidney Organ Due to Ethanol Extract of Phaleria Macrocarpa Treatment Induced by Isoniazid
245

initial condition of the liver and kidney of the rat
before treatment. Changes in the environment of mice
also affect the behavior patterns of mice (Suharyadi,
Sukohar and Muhartono, 2014).
The histopathological picture assessed in the
kidney organ in the form of glomerular atrophy is
characterized by a decrease in the size of the
glomerular tissue due to the reduced number of cells.
The widening of the tubular lumen was due to the
presence of a protein cast or protein deposition on the
tubules.
According to Anggriani(2008), microscopic
picture of epithelial cells of the proximal tubuleswell
with granular cytoplasm due to shifting of
waterextracellular into cells. This shift occurs
because the toxin causeschanges in the surface
electric charge of tubular epithelial cells, active
transport of ions and acidsorganic, and the ultimate
concentrating ability of the kidneycauses the tubule to
be damaged, the flow decreases. Picture of swelling
of these cellscalled albuminous degeneration or
parenchymal or cloudy degenerationswelling (cloudy
swelling). This may cause the tubular lumenproximal
narrowing to close. According to researchconducted
by Liu et al., (2002) who stated that giving
ethanol50% with a dose of 10 ml / kg of rats can cause
kidney damage.
The treatments with the Phaleria macrocarpa
extract for 10 days did not cause any toxicity, because
there were no deaths and poisoning in white rats
during the study. Based on the histopathology that has
been studied, the EEPM is able to protect the kidneys
due to isoniazid induction because it has high
antioxidants.
The antioxidant content of flavonoids as
inhibitors of CYP3A activity and acts as a free radical
scavenger (Hassanin, Abd El-Kawi and Hashem,
2013). Flavonoid compounds contained in the
phaleria macrocarpa fruit have an influence in
protecting the kidneys.
5 CONCLUSIONS
The results showed that an effect after giving an
EEPM at a dose of 3.4 g / 200 g bw on the kidney
tissue damage were highly reduced as evidenced by
histopathological examination. Preventive use of the
EEPM can act as a protective kidney when
consuming OAT INH together.
6 SUGGESTION
The next researcher is suggested to design experiment
using the phaleria macrocarpa fruit in the different
dosage of concentration with a longer trial period.
REFERENCES
Boggan B. 2003. Alcohol, Chemistry and You. Effect of
Ethyl Alcohol on Organ Function.
Depkes RI (2018)
‘WaspadaiPeningkatanPenyakitMenular’, p. 1.
Hassanin, K. M. A., Abd El-Kawi, S. H. and Hashem, K. S.
(2013) ‘The prospective protective effect of selenium
nanoparticles against chromium-induced oxidative and
cellular damage in rat thyroid’, International Journal of
Nanomedicine, 8, pp. 1713–1720. doi:
10.2147/IJN.S42736.
Hendra, R. et al. (2011) ‘Flavonoid analyses and
antimicrobial activity of various parts of Phaleria
macrocarpa (Scheff.) Boerl fruit’, International Journal
of Molecular Sciences, 12(6), pp. 3422–3431. doi:
10.3390/ijms12063422.
Lestari, T. (2019) ‘Uji EfektivitasEkstrakBuahKurma
(Phoenix dactylifera) Dan EkstrakBuahMahkota Dewa
(Phaleria macrocarpa) Dari Pemeriksaan SGOT dan
SGPT TerhadapTikus Yang Di Induksi Paracetamol’,
1(1), pp. 8–15.
Liu CF, Lin MH, Lin CC, Chang HW, Lin SC. 2002.
Protective effect of tetramethylpyrazine on absolute
ethanol-induced renal toxicity in mice. J Biomed
Science. 9(4) : 299-302.
Nasution AN, Aziz H, Tjong DH, Zein R, Effect of Phaleria
macrocarpa flesh fruits extract on MDA level, SGOT
and SGPT activity in serum of experimental rats
contaminated by Cd(II). Macedonian journal of medical
sciences, 2019 Dec 15; 7(23):3950-3954
Nasution An, Amrina Y, Zein R, Aziz H, Munaf E,
Biosorption characteristics of Cd(II) ion using herbal
plant of mahkota dewa (phaleria macrocarpa). Journal
of chemical and pharmaceutical Research. 2015:7(7):
189-96
Nakatani K, Atsumi M, Arakawa T, Oosawa K, Shimura S,
Nakahata N, Ohizumi Y. 2002. Inhibition of histamine
release and prostaglandin e2 synthesis by mangostin in
-: Chaverri JP, Rodriguez NC, Ibarra MO, Rojas JMP.
pp. 34: 344−47
Nijveldt RJ, van HDEC, Bgoelens PG, van NK, van LPAM
2001. Flavonoids: A review of probable mechanisms of
action and potential applications. Am. J. Clin. Nutr. hlm
418-425.
Muzika, V. et al. (2016) ‘Histological study of isoniazid-
rifampicin related nephrotoxicity in Wistar rats’, Folia
Med. Fac. Med. Univ. Saraeviensis, 51(1), pp. 4–9.
Oktaria, R. (2019) ‘EfekProtectif Thymoquinone Terhadap
Gambaran HistopatologiGinjalTikusPutih (Rattus
novergicus) Galur Sprague dawley yang
HIMBEP 2020 - International Conference on Health Informatics, Medical, Biological Engineering, and Pharmaceutical
246

DiinduksiRifampsin’, Journal of Chemical Information
and Modeling, 53(9), pp. 1689–1699. doi:
10.1017/CBO9781107415324.004.
Parapaga, V. F. S., Durry, M. F. and Lintong, P. M. (2018)
‘EfekPemberianEkstrakDaunSirsak (Annona muricata
L.) terhadap Gambaran HistopatologikHatiTikus
Wistar (Rattus Norvegicus) yang
DiinduksiRifampisin’, Jurnal e-Biomedik, 6(2), pp.
195–199. doi: 10.35790/ebm.6.2.2018.22173.
Pratiwi, E. P. (2018) ‘EfekSampingObat Anti
TuberkulosisKategori I dan II
PasienTuberkolosisParuDewasa di RumahSakit Hasan
Sadikin’, Indonesian Journal of Clinical Pharmacy,
7(4), p. 252. doi: 10.15416/ijcp.2018.7.4.252.
Ramadhan, A. (2019) ‘Uji EfektivitasEkstrakBuahKurma
(Phoenix dactylifera) Dan EkstrakBuahMahkota Dewa
(Phaleria macrocarpa)
SebagaiNefroprotektorTerhadapTikus Yang Di Induksi
Paracetamol’, 1(1), pp. 8–15.
Sitorus, B., Fatmawati and Rahmaniah, S. E. (2017) ‘Peran
PengawasMenelanObat (PMO)
TerhadapPengobatanPenderitaTuberkulosa Di Wilayah
Kerja Unit PengobatanPenyakitParu-Paru (UP4)
Pontianak’, JurnalIlmiahIlmu Sosial dan IlmuPolitik
Universitas Tanjungpura, p. 16. Available at:
http://jurnal.untan.ac.id/index.php/jpmis/article/view/2
0108/pdf.
Suharyadi, A., Sukohar, A. and Muhartono (2014)
‘PengaruhPemberianEkstrakEtanolDaunSirsakterhada
p Gambaran HistopatologiGinjalTikus yang
DiinduksiDmba’, Majority, 3(4), pp. 28–34. Available
at:
http://juke.kedokteran.unila.ac.id/index.php/majority/a
rticle/view/240.
WHO (2019) ‘The End TB Strategy’, pp. 1–25.
Yatman E. 2012. Kulitbuahmanggismengandungxanton
yang berkhasiattinggi. wawasan: Universitas
Borobudur. Tahun 29 No. 324. hlm. 2−9.
Zakhari S. 2006. Overview: How is Alkohol Metabolized
by the Body. National Institute on Alcohol Abuse and
Alcoholism (NIAAA) 5635, Fisher Lane. MSC 9304
Bethesda. hlm 62-88.
Histopathology of Rat Kidney Organ Due to Ethanol Extract of Phaleria Macrocarpa Treatment Induced by Isoniazid
247
