
Single Case Report: Diffuse Cutaneous Mastocytosis with
Generalized Bullae Mimicking Bullous Pemphigoid
Muhammad Ridlo
1*
, Irma D. Roesyanto-Mahadi
1
, Remenda Siregar
2
1
Department of Dermatology & Venereology, University of Sumatera Utara, Faculty of medicine, Universitas Sumatera
Utara Hospital - H.Adam Malik Hospital, Medan
2
Department of Dermatology & Venereology, University of Sumatera Utara, Faculty of medicine, Universitas Sumatera
Utara Hospital - Dr.Pirngadi Medan
Keywords: Diffuse cutaneous mastocytosis, mastocytosis bullous, bullous pemphigoid
Abstract: Introduction:Mastocytosisis a rare disease; it is defined as mast cells infiltration in some organs. Skin is the
most commonly involved organ.Diffuse cutaneous mastocytosisis a form of skin mastocytosiswhich can be
manifested as bullous lesions. Case: A 3-month-old male infant is presentedwith generalized dermatosis,
characterized by multipleblisters and brownish spots all over his bodysince one month ago.On dermatologic
examination, multiple tense bullae of various sizes with brownmacules and plaques are located throughout
the body.Some bullae rupture to form an erosion.Darier sign showserythema/mild urticaria lesions(positive).
Routine blood tests within normal limits. The histopathologic examination reveals sub-epidermal bullae
with aninflammatory cell and the dermis is filled with inflammatory cells and mast cells. Patients were
diagnosed with diffuse cutaneous bullous mastocytosis. The patient was treated with 0.9% NaCl
compresses, 2% mupirocin cream, and 0.1% betamethasone cream. Monitoring therapy is done routinely to
evaluate the prognosis of the disease.Discussion:Diffuse cutaneous bullous mastocytosis is one of the rarest
forms of skin mastocytosis. Cutaneous mastocytosis in children who persist until adolescence develops
systemic mastocytosis in 15-30% cases so early diagnosis of mastocytosis in children is very important to
get a goodprognosis. Conclusion: Diagnosisdiffuse cutaneous mastocytosis is not easy because the
prevalence is difficult to determine and often misdiagnosed. Here,we report this case due to the similarity
clinical manifestation of bullous diseases, especially in a newborn with scattered blisters and erosions.
1 INTRODUCTION
Mastocytosisisa diseasewhich shows a large number
of abnormal proliferation and accumulation of mast
cells in one or more organ systems including the
skin, bone marrow, liver, spleen, lymph nodes and
digestive tract with various variants of clinical
manifestations (Dines et al, 2014). There are two
types of mastocytosis that arecutaneous
mastocytosis (CM) and systemic mastocytosis
(SM). CM is often found in children, whereas SM is
more common in adults (Lange et al, 2015).
According to the 2017 World Health Organization
(WHO) classification, CM encompassesurticaria
pigmentosa or maculopapular cutaneous
mastocytosis (UP), diffuse cutaneous mastocytosis
(DCM), and mastocytoma in the skin.The bullous
eruption is most commonly associated with DCM,
although bullae can occur in all forms of cutaneous
mastocytosis (Hans et al, 2018). In patients with
DCM, bullous eruptions are widespread during the
early stages of life. The blisters present in a variety
of sizes and initially contain clear fluid that may
become hemorrhagic with time and can leave a
hyperpigmented brown macula. Bullous lesions
may occur in linear or grouped fashion and often
develop on the trunk, scalp, and extremities. The
bullous lesions typically resolve by 3-5 years of
age. A small number of patients have been reported
with yellow-orange infiltrated. Over time, the skin
becomes thickened and has a doughy consistency.
Other cutaneous manifestations may include
pruritus, urticaria, a positive Darier's sign and
marked dermographism (Metcalfe et al, 2016; Eui et
al, 2010).
The prevalence of mastocytosis is still
challenging to determine, but it is estimated that the
incidence of mastocytosis is around 5-10 per
1,000,000 inhabitants per year. This disease can
occur at any age.Approximately 50% of the onset of
436
Ridlo, M., Mahadi, I. and Siregar, R.
Single Case Report: Diffuse Cutaneous Mastocytosis with Generalized Bullae Mimicking Bullous Pemphigoid.
DOI: 10.5220/0009991004360440
In Proceedings of the 2nd International Conference on Tropical Medicine and Infectious Disease (ICTROMI 2019), pages 436-440
ISBN: 978-989-758-469-5
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

mastocytosis that occurs in children can appear
during infancy, especially in the neonatal period
until the age of 2 years. There is no racial difference
and sexual dominance in this disease (Metcalte et
al, 2016).
Spontaneous resolution of 50-60% of bullous
lesions can occur in most patients with DCM until
before the age of 5 years. However,reliable
prognostic clues are lacking, especially for
predicting the risk of systemic involvement with
life-threatening manifestations that they should
undergo annual investigations and careful follow-up
(Lange et al, 2015; Magliacanel et al, 2014).
2 CASE
A 3-month-old male infant is checked in a
polyclinic with generalized dermatosis
characterized by multiple blisters and brownish
spots all over his bodysince one month ago.
According to his mother, since the age of 1
month,the blisters appear for the first time in the
abdominal area. One month later, blistersspread
overthe limbs, trunk, and scalp. When the blister
bursts, it will leave behind red which then becomes
brownish spots. History of flushing, complaints of
vomiting, and diarrhea in patients are denied. Due
to the distance of health facilities are far from where
they live, patients have never been taken to a health
facility and have never received treatment. The
patient was the third child with a history of normal
birth, spontaneous crying with a birth weight of
2900 grams. There is no history of the same
complaints in previous births. There is no history of
asthma and allergies in the patient's family.
Physical examination within normal limits, and
there are no abnormalities or congenital disabilities
experienced by the patient. Dermatologic
examination revealed skin was dry and facial skin is
thickened with numerous tense bullous vary in size
over normal skin et regio temporoocipital, oralis,
mentalis, medial antebrachii sinistra, and femoralis
dextra posterior. Multiple erosions vary in size are
seen on regio infraclavicularis, lateral dextra brachii
posterior and vertebralis. Brownish plaques and
macules vary in size circumscribed multiple discrete
et regio abdominals, antebrachii dextra et sinistra,
vertebralis and anterior-posterior femoralis dextra et
sinistra (Figure 1). Darier sign shows
erythemalesions (positive). Examination of the
Nikolsky sign is negative. Based on the anamnesis
and physical examination, we considered diffuse
cutaneous mastocytosis bullous, bullous
pemphigoid, and bullous epidermolysis as a
differential diagnosis. Routine blood tests within
normal limits. Patients were then biopsied on bullae
lesions which are newly formed less than 24 hours
located in the medial region of the left antebrachii.
Histopathologic examination results with staining of
hematoxylin-eosin, showing sub-epidermal blister
with a dome consist of squamous epithelial cells, an
inflammatory cell eosinophils in bullae. Mast cells
densely filling the dermis below the blister
accompanied by vascular congestion and dilatation
(Figure 2). Examination of spinal cord aspiration
was not carried out in these patients because they did
not get the consent of the patient's parents.To assess
the extent and activity of skin lesions, the SCORMA
Index was applied with result 51.8.
The diagnosis of DCM was made based on these
clinical and histopathological findings. Patients were
given 0.9% NaCl compress therapy in erosion
lesions for 15-20 minutes every 6 hours a day, 2%
mupirocin cream and 0.1% betamethasone cream
every 12 hours a day.
3 DISCUSSION
The diagnosis of CM, in this case, is based on
history, clinical manifestations, histopathologically
examination, and the absence of signs of systemic
mastocytosis. Organomegaly examination
(hepatomegaly, splenomegaly, lymphadenopathy) is
needed to look for systemic involvement. A
peripheral blood examination is needed to look for
hematologic abnormalities related to bone marrow
involvement.(Lange et al., 2015;Eui et al., 2010)
The most common variant of CM is UP, that it
manifests as 0,5- 1 cm yellowish-tan to red-brown
macules or slightly raised papules. The affected
areas include the trunk and extremities, while the
face, scalp, palms, and soles tend to be free of
lesions. DCM is an unusual variant of the mast cell
disease characterized by widespread bullae as its
main cutaneous feature. DCM can appear at birth
(congenital and neonatal) or in early infancy.
Widespread involvement of the skin with blistering
and bullae may be the presenting symptoms. The
skin may be leathery and thickened (‘‘peaud'
orange'') due to infiltration with mast cells.
Hyperpigmentation may persist into adulthood, and
dermographism may be prominent.6,7We diagnosed
our case as DCMsince there were multiple bullae all
over his body including face, scalp, trunk and limb
as its main cutaneous feature andwithout systemic
involvement.
Single Case Report: Diffuse Cutaneous Mastocytosis with Generalized Bullae Mimicking Bullous Pemphigoid
437
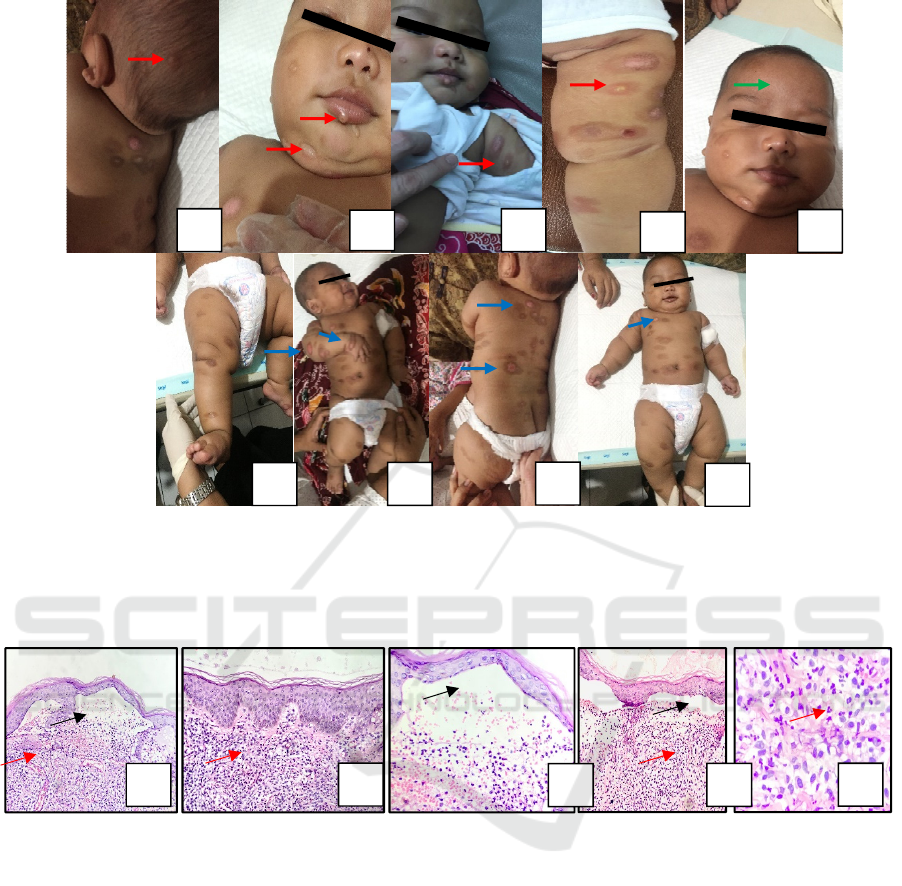
Figure 1. Three-month-old infant with numerous tense bullousvary in size over normal skin et regio temporoocipital, oralis,
mentalis, medial antebrachii sinistra and femoralis dextra posterior (red arrow)(A),(B),(C),(D) and the facial skin is
thickened especially forehead (green arrow) (E). Multiple erosionsvary in size are seen on regio infraclavicularis, lateral
dextra brachii posterior and vertebralis (blue arrow) (G),(H) and (I). Brownish plaques and macules vary in size
circumscribed multiple discrete et regio abdominal, antebrachii dextra et sinistra, vertebralis and anterior posterior femoralis
dextra et sinistra.
Figure 2. Sub-epidermal blister with a dome consists of squamous epithelial cells, an inflammatory cell eosinophils in
bullae (black arrows) (A), (C) and (D).Mast cells densely filling the dermis below the blister accompanied by vascular
congestion and dilatation (red arrows) (A), (B), (D), and (E).
The clinical manifestations of various bullous
diseases in children are almost the same, so clinical
diagnosis is not enough. To confirm mastocytosis in
bone marrow or in blood, the mast cell count should
be more than 20% of the nucleated cells in the bone
marrow or >10% peripheral blood leukocytes.
However, systemic investigations such as bone
marrow aspiration/biopsy or serum total tryptase
level could not be performed because of the
reluctance of the parents.Histopathological and
immunofluorescence exams, particularly DIF (direct
immunofluorescence) are needed for diagnosis.
(Heide et al., 2009).
According to WHO (2017), the
terminology of mastocytosis on the skin can be
established from the results of histopathological
biopsy exams that prove the infiltration of mast cells
in the dermis, and there is no involvement of other
organs or signs of SM. Usually, because no bone
marrow examination was performed and/or clinical
information is lacking.Tran et al. reported that in the
case of CM there was a large amount of infiltration
of eosinophil cells originating from chemotactic
eosinophils a factor secreted by neoplastic mast cells
in the dermis.(Hans-Peter e al., 2018;Arber et al.,
2016)
There are two important biological findings as
markers related to the pathogenesis of
mastocytosisdisease; the presence of somatic
A
B
C
D
E
A
B
C
D
E
F
G
H
ICTROMI 2019 - The 2nd International Conference on Tropical Medicine and Infectious Disease
438

mutations in KIT genes (usually KIT Asp816Val
D816V mutations) and the presence of
immunophenotype deviations associated with CD25
and c-KIT gene expression CD117 which plays a
role in differentiation, maturation, and proliferation
of mast cells.(Arock et al., 2015;Walker et al.,2006)
Mutations from oncogenic KIT D816V are usually
detected in almost 80% of patients with SM.
Mutations KIT D816V is rarely found in CM
patients.As a result of mutations in the c-KIT gene,
this will result in an increase in abnormal
proliferative activity of mast cells.Mast cell
degranulation causes the release of various
mediators such as histamine, the slow-releasing
substance of anaphylaxis (SRSA), eosinophil
chemotactic activating factor (ECAF), heparin and
other mediators that play a role in the Darier sign
mechanism. Darier's sign, which is defined by
wheeling and reddening of lesions upon mechanical
stroking or rubbing, is usually demonstrable. It is not
always positive in adult patients but usually positive
in pediatric patients. The Darier's sign is often not
elicited correctly, resulting in false-negative or false-
positive results.(Tran et al., 2014)
In bullous pemphigoid, IgG autoantibodies are
attached to BP180 antigen (transmembrane
glycoprotein hemidesmosome).The IgG bond and
BP180 antigen will activate complement. The
complement will cause degranulation of mast cells
and withdrawal of neutrophils and eosinophils,
which will release various inflammatory mediators
and proteinases that cause subepidermal domes.
However, there is no histopathological infiltration of
mast cells in the dermis in the bullous pemphigoid,
and the Darier sign does not show erythema/urticaria
lesions (negative).(Wada et al., 2016)
Because there is still no curative treatment for
mastocytosis, the available therapeutic options are
mostly palliative and symptomatic. In the treatment
of CM that occurs in children, it is recommended to
give topical medium-class steroids immediately.
Topical steroid applications in CM that occur in
children have been shown to eliminate local skin
symptoms, and Darier's sign becomes very weak
until it disappears. Treatment with topical steroids is
still better and effective in the case of cutaneous
mastocytosis considering the long time required for
the spontaneous disappearance.(Annalisa et al.,
2015)
Hartmann et al. in a randomized study of
multiple parallel and case-control trials, it was
explained that the topical use of clobetasol for two
weeks in 39 patients with CM had a significant
effect on reducing the size of lesions and the number
of mast cells in the upper dermis.(Hartman et al.,
2010)
In our case patient giving 0.1% betamethasone
creamand 2% mupirocin cream every 12 hours a
dayto prevent secondary infections in open wounds.
In the treatment of mastocytosis in addition to
medical treatment, it is essential to maintain nutrient
intake, which can trigger the release of mast cell
mediators. We can see some food ingredients that
must be watched out for sufferers of mastocytosis
such as; Monosodium Glutamate (MSG), alcohol,
shellfish, artificial food dyes and flavorings, food
preservatives, pineapples, tomatoes & tomato-based
products, and chocolate. .(Annalisa et al., 2015;
Hartman et al., 2010). In our case, we educate the
patient's mother so that after entering the
complementary stage of breastfeeding, it can be
more attentive and careful about food ingredients
that can trigger the release of histamine mediators.
There are still many controversies in defining
and evaluating mastocytosis. One of the aspects that
are missing is a system for clinical evaluation of
mastocytosis of the skin. The clinical use of the
scoring index of mastocytosis (SCORMA). The
scoring of the SCORMA Index was designed in
order to assess the extent and activity of skin lesions.
It is based on a semi-quantitative analysis of the
extent, intensity, and subjective complaints, and it
ranges from 5.2 to 100.(M.Lange et al.,2012)
Despite progress in understanding the
pathogenesis, genetics, and diagnostic criteria of
mastocytosis, reliable prognostic clues are lacking,
especially for predicting the risk of systemic
involvement. According to some, most DCM cases
about 50% of patients tend to improve with time,
whereas others concluded that DCM patients are at
higher risk of developing SM or life-threatening
events such as hypotension or bronchospasm.
Cutaneous mastocytosis in children who persist until
adolescence develops SM in 15-30% cases.
Therefore, evaluation of the prognosis assessment of
cutaneous mastocytosis in children is essential to
determine whether or not an attempt is made to seek
systemic involvement. (Hartman et al., 2010;
M.Lange et al.,2012).
4 CONCLUSION
Due to the similarity between bullous
pemphigoidand CM, we must be considered in the
differential diagnoses of bullous eruptions,
especially in a newborn with scattered blisters and
erosions. It could be concluded that pediatricians
and dermatologists should remain aware of varied
forms of cutaneous mastocytosis because of its rarity
Single Case Report: Diffuse Cutaneous Mastocytosis with Generalized Bullae Mimicking Bullous Pemphigoid
439

and the distinctive management of each individual
case. Our case helps to document the diagnosis of
CM in the pediatric patient population.
REFERENCES
Annalisa P, Michela T. 2015. Topical corticosteroids
versus “wait and see” in the management of solitary
mastocytoma in pediatric patients: a long-term follow-
up. Dermatol therapy. (28):57–61
Arber DA, Orazi A, Hasserjian R, et al. 2016. The 2016
revision to the World Health Organization
classification of myeloid neoplasms and acute
leukemia. Blood. 127(20): 2391–405.
Arock M, Sotlar K, Akin C, et al. 2015. KIT mutation
analysis in mast cell neoplasms: recommendations of
the European Competence Network on Mastocytosis.
Leukemia. 29(6):1223-32.
D. Magliacane1, R. Parente. 2014. Current Concepts on
Diagnosis and Treatment of Mastocytosis.Transl Med
UniSa. 4;(8):65-74.
Dinesh P, Anurag T. 2014. Bullous mastocytosis in a 3-
month-old infant Indian Dermatol Online J. Oct-Dec;
5(4): 497–500.
Eui HL, Mi RK. 2010. Diffuse Cutaneous Mastocytosis
with Generalized Bullae. Ann Dermatol. 22(1):77-80.
Hans-Peter H, Sotlar K, Metzgeroth G, Reiter A, and
Valent P. 2018. Mastocytosis and the Updated WHO
Classification (2016, 2017): What is Really New?.
Ann Hematol Oncol.. 5(2): 1194.
Hartmann K, Siebenhaar F, Belloni B, et al. 2010. Effects
of topical treatment with the raft modulator
miltefosine and clobetasol in cutaneous mastocytosis:
a randomized, double-blind, placebo-controlled trial.
Br J Dermatol 162: 185–190
Heide R, Zuidema E, Beishuizen A, et al. 2009. Clinical
aspects of diffuse cutaneous mastocytosis in children:
two variants. Dermatology. 219: 309–315.
Lange M, Niedosytko M, Renke J, Glen J, Nedoszyko B.
2015. Clinical aspects of pediatric mastocytosis: a
review of 101 cases. JEADV. 27: 97-102.
M. Lange, M. Niedoszytko. 2012. Diffuse cutaneous
mastocytosis: analysis of 10 cases and a brief review
of the literature. J Eur Acad Dermatol Venereol.
Dec;26(12):1565-71
Metcalfe DD, Akin C, Valent P. 2016. Cutaneous
manifestations in patients with mastocytosis:
Consensus report of the European Competence
Network on Mastocytosis; the American Academy of
Allergy, Asthma & Immunology; and the European
Academy of Allergology and Clinical Immunology. J.
Allergy Clin. Immunol. Jan;137(1):35-45.
Tran DT, Jokinen CH, Argenyi ZB, Mitsuaki I, Muneo I.
2014. Cutaneous mastocytosis with abundant
eosinophilic infiltration: a case report with review of
the literature. Int J Clin Exp Pathol. 7(5): 2695–7
Wada M, Nishie W, Ujiie H, Izumi K, Iwata H, Natsuga
K, Nakamura H, Kitagawa Y, Shimizu H. 2016.
Epitope-dependent pathogenicity of Abs targeting a
major bullous pemphigoid autoAg collagen
XVII/BP180, Journal of Investigative Dermatol. 36(5):
938-946
Walker T, von Komorowski G, Scheurlen W, Dorn-
Beineke A, Back W, Bayerl C. 2006. Neonatal
mastocytosis with pachydermic bullous skin without c-
Kit 816 mutation. Dermatology. 212:70–72
ICTROMI 2019 - The 2nd International Conference on Tropical Medicine and Infectious Disease
440
