
Fixed Drug Eruption Due to Ambroxol
Puteri Wulandari
1*
, Kristo A. Nababan
1
1
Department of Dermatology & Venereology, Universitas Sumatera Utara,
Faculty of medicine, Universitas Sumatera Utara Hospital
Adam Malik Hospital, Medan.
Keyword: Fixed drug reaction, ambroxol
Abstract: Introduction: Fixed drug eruption (FDE) is a common cutaneous drug eruption characterized by the
development of one or more annular, oval, erythematous and hyperpigmented patches as a result of systemic
exposure to a drug. The lesion may recur at the same site and/or at the new sites with re-exposure to the
offending drug(s). More than 100 drugs have been implicated in causing FDEs including ibuprofen,
sulfonamides, naproxen and tetracyclines. There was only one case report fixed drug eruption due to
Ambroxol in Japan. This is a second case report fixed drug eruption due to ambroxol. Case: A 37-years old
Male came to Adam Malik Hospital Medan with the chief complaint an itchy, two similar
violaceousmacular lesions on the left back of the hand anderythematosus macular lesion in his genital part.
Three days ago, he was taking ambroxolthat he buy over-the-counter for treating his sore throats. Then the
cutaneous lesion in the left back of the hand appeared about one day later followed by a cutaneous lesion in
his genital part. He recalled a history of 2 similar episodes in the same location 1 year and 6 months ago due
to the same medication (ambroxol) that resolved about 7-10 days without any treatment, leaving post-
inflammatory hyperpigmentation. This patient was given an education to avoid the offending drug
(ambroxol) because this has been the third times he was experienced. Then he was given cetirizine 10 mg
once daily and topical desoximetasone cream applied on the lesion twice daily. Conclusion: There is an
increased risk of cutaneous drug reactions with expectorants containing ambroxol. We must increase
awarenessof fixed drug reaction cases due to the medications that are often used freely especially an over-
the-counter medication.
1 INTRODUCTION
Fixed drug eruption (FDE) is a common cutaneous
drug eruption characterized by the development of
one or more annular, oval, erythematous and
hyperpigmented patches as a result of systemic
exposure to a drugand account for 16% of all
cutaneous drug eruption (Breathnach, 2004).
The
lesion may recur at the same site and/or at the new
sites with re-exposure to the offending drug(s)
(Ayanlowo, 2015). The reaction may be
erythematous, eczematous, urticarial, bullous and
pigmented with a necrotic center sometimes
mimicking the target lesions of erythema
multiforme. The lesion resolve with
postinflammatory hyperpigmentation (Ayanlowo,
2015; Butler et al, 2015).
The number of diagnosed FDE cases is
increasing steadily, due in part to increased
awareness by physicians as well as increased
requests by patients to identify the precise cause of
repeated eruptions and pigmentation (Lee, 2000).
Fixed drug eruption is the second most common
adverse cutaneous eruption reported in both in- and
outpatients units, occurring at all ages and in all
races (Ayanlowo, 2015). The list of drugs keeps
growing with the introduction of new medications
(Ayanlowo, 2015). Barbiturates, antibiotics
(sulfonamides, tetracyclines, penicillin, and
erythromycin) and non-steroidal anti-inflammatory
drugs are common and well-known causative agents
(Lee, 2000).
Mucoactive substances such as ambroxol,
available in several countries as an over-the-counter
medicines that used for the treatment of acute and
chronic bronchitis.
6
There was an old publication in
2006 about safety and usage pattern of an over-the-
counter ambroxol cough syrup. This study confirms
that ambroxol is used according to the advice given
in the patient’s leaflet and supports the already
established safety and efficacy of this product in
416
Wulandari, P. and Nababan, K.
Fixed Drug Eruption Due to Ambroxol.
DOI: 10.5220/0009990504160419
In Proceedings of the 2nd International Conference on Tropical Medicine and Infectious Disease (ICTROMI 2019), pages 416-419
ISBN: 978-989-758-469-5
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
acute bronchitis (Schulz et al., 2006). But in 2015,
there was an emerge to update ambroxol safety
because accumulating evidence from case reports
and literature demonstrating that ambroxol is
potentially responsible for severe cutaneous adverse
reactions (SCARs) (Health Product Regulatory
Authority, 2015).
2 CASE REPORT
A 37-years old Male came to Adam Malik Hospital
Medan with the chief complaint an itchy, two similar
violaceous macular lesions on the left back of the
hand and erythematosus macular lesion in his genital
part. Three days ago, he was taking ambroxol that he
buy over-the-counter for treating his sore throats.
Then the cutaneous lesion in the left back of the
hand appeared about one day later. He recalled a
history of 2 similar episodes in the same location 1
years and 6 months ago due to the same medication
(ambroxol) that resolved about 7-10 days without
any treatment, leaving post-inflammatory
hyperpigmentation.
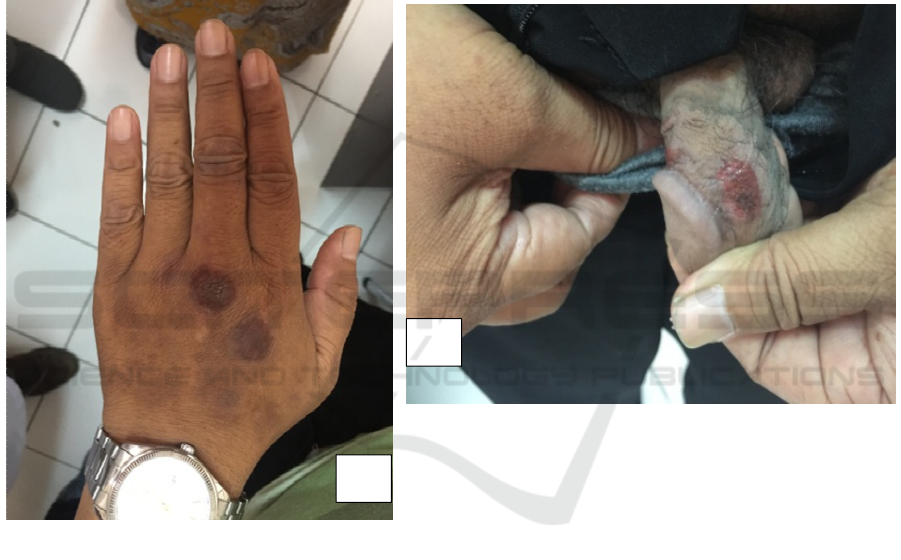
Picture 1. (A) two similar violaceous macular lesions, circumscribed, annular, on the dorsum manussinistra. (B)
Erythematosus macular lesion, circumscribed, annular with a central necrotic on the corpus penis region
From a dermatology examination we found two
similar violaceous macular lesions, circumscribed,
annular, on the dorsum manussinistra, and single
erythematosus macular lesion, circumscribed,
annular with a central necrotic in corpus penis
region. From a physical examination, we found the
awareness is compos mentis, blood pressure was
110/60 mmHg, heart rate was 90x/minute,
respiratory rate was 22x/minute and the body
temperature was 37
o
C. The nutritional status was
good and no abnormalities was found in other
physical status.
This patient was given an education to avoid the
offending drug (ambroxol) because this has been the
third times he was experienced. Then he was given
cetirizine 10 mg once daily and topical
desoximetasone cream applied on the lesion twice
daily.
3 DISCUSSION
The number of diagnosed FDE cases are increasing
steadily, due in part to increased awareness by
physicians as well as increased requests by patients
to identify the precise cause of repeated eruptions
and pigmentation.(Lee, 2000) Fixed drug eruption is
the second most common adverse cutaneous
A
B
Fixed Drug Eruption Due to Ambroxol
417

eruption reported in both in- and outpatients units,
occurring at all ages and in all races. (Ayanlowo,
2015;Sehgal et al., 2006)
Acute FDE lesions can develop within 30
minutes to 8 hours after drug administration. Lesions
are clinically characterized as single or multiple,
sharply demarcated, round or oval erythematous
patches or plaques that may become vesicular or
bullous. Because of the diversity of clinical pictures,
the correct diagnosis may sometimes be difficult to
achieve. But the FDE is characterized by recurrence
the same lesion at the same site after repeated
exposure to a causative drug. With repeated
exposure, new lesions can appear, and the previous
lesions may increase in size. In our case, this patient
has complained an itchy, two similar violaceous
macular lesions on the left back of the hand and
erythematosus macular lesion in his genital part.
These lesions appearone day after he tookambroxol
that he buy over-the-counter. The lesions in the left
back of the hand appeared firstfollowed by the
lesion in his genital. He recalled a history of two
similar episodes in the same location 1 years and 6
months ago due to the same medication
(ambroxol).Fixed drug eruption are commonly
found on the genitalia and in the perianal area,
although they can occur anywhere on the skin
surface.(Shear NH&Knowles SR,2012)
The diagnosis of a cutaneous drug eruption
involves the precise characterization of reaction
type. Some cutaneous reaction such as FDE, are
almost always due to drug therapy. Timing of drug
exposure and reaction onset, course of reaction with
drug withdrawal or continuation, timing and nature
of recurrent eruption on rechallenge, a history of a
similar response to a cross-reacting medication and
previous reports of similar reactions to the same
medication are helpful to diagnosed.(Shear
&Knowles,2012)Clinical history is most important
in diagnosing FDE, but patch tests and drug
challenge tests are also helpful and are used
frequently for a more objective diagnostic
approach.We didn’t do a drug challenge test in this
case because our patient clearly remembered that the
cutaneous lesions appeared after he ingested
ambroxol.
Ambroxol belongs to a group of medications
called mucolytic. Ambroxolworks by thinning down
the mucus in the airway passages, thus making the
mucus less sticky and it also facilitates the removal
of the mucus from the airways. Ambroxol is
available for pain relief of sore throats and available
over-the-counter in many countries includes
Indonesia. The review of ambroxol safety was
initiated following post marketing reports of
hypersensitivity reactions including anaphylactic
reactions and accumulating evidence from case
reports and literature demonstrating that ambroxol is
potentially responsible for severe cutaneous adverse
reactions (SCARs). (Sehgal et al.,
2006;Shear&Knowles, 2012). The European
Medicines Agency’s Pharmacovigilance Risk
Assessment Committee (PRAC) has completed a
review of the safety of ambroxol and bromhexine-
containing medicines. The PRAC considered that
ambroxol and bromhexine are associated with a
small increased risk of hypersensitivity reactions and
possibility of a risk of SCARs. The PRAC was
considered that the risk of SCARs should be
addressed by its inclusion in the product information
accompanied by a warning for patients and
caregivers to recognise the prodromes of SCARs and
to discontinue treatment immediately in the event of
such signs.(Shear &Knowles,2012)
4 CONCLUSION
There is an increased risk of cutaneous drug
reactions with ambroxol. We must increase
awareness of fixed drug reaction cases due to the
medication that are often used freely especially an
over-the-counter medication.
REFERENCES
Ayanlowo OO. 2015. Fixed drug eruption at a
dermatology clinic in Lagos, Nigeria.Journal of
Clinical Science. 12(2):108-12. DOI : 10.4103/1595-
9587.169692.
Breathnach SM. 2004. Drug reactions: Fixed drug
eruption. In: Burns T, Breathnach S, Cox N, Griffiths.
Editors. Rooks Textbook of Dermatology. 7
th
Ed. Vol
4. United Kingdom: Blackwell Science. p20-9, 73.
Butler DF, Ilse JR, Schwartz RA, Vinson RP, Callen JP,
Quirk CM, et al. 2015. Fixed drug eruption. Available
from
:http://www.emedicine.medscape.com/article/1336702
-followup#showall.
European Medicines Agency. 2015. Ambroxol and
bromhexineexpectorants : safety information to be
updated. Available from : https://www.ema.europa.eu/
en/documents/press-release/ambroxol-bromhexine-
expectorants-safety-information-be-updated-risk-
allergy-skin-reactions-be_en.pdf
Health Products Regulatory Authority. 2015. Risk of
severe allergic reactions with ambroxol and
bromhexine-containing medicines considered
small.Availablefrom:
ICTROMI 2019 - The 2nd International Conference on Tropical Medicine and Infectious Disease
418

https://www.hpra.ie/docs/default-source/3rd-party-
documents/mims-articles/hpra-april-2015-respiratory-
supplement-ambroxol.pdf?sfvrsn=2
Lee AY. 2000. Fixed drug eruptions.Incidence,
recognition and avoidance. Am J ClinDermatol. 1:
277-85
Schulz M, Hammerlein A, Hinkel U, Weis G, Gillissen A.
2006. Safety and usage pattern of an over-the-counter
ambroxol cough syrup: a community pharmacy-based
cohort study.
Sehgal VN, Srivastava G. 2006. Fixed drug eruption
(FDE): changing scenario of incriminating drugs. Int J.
Dermatol. 45: 897-908
Shear NH & Knowles SR. 2012. Cutaneous reactions to
drug. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller
AS, Leffell DJ, Wolf K, editors. Fitzpatrick’s
dermatology in general medicine.8th Ed. New York:
McGraw Hill. p.449-57.
Fixed Drug Eruption Due to Ambroxol
419
