
Late Diagnosis Merkel Cell Carcinoma
with History of Basal Cell Carcinoma
Adelia Hanung Puspaningtyas
1*
, Lydia Kurniasari
1
, Renni Yuniati
1
, Buwono Puruhito
1
, Puji Sriyani
1
1
Department of Dermatovenereology,
5
Department of Surgery Faculty of Medicine
Diponegoro University / Dr. Kariadi General Hospital
Keywords: Merkel Cell Carcinoma, Basal Cell Carcinoma, Excision, Split Thickness Skin Graft, Chemotherapy
Abstract: Merkel cell carcinoma (MCC) is a rare and aggressive neuroendocrine tumor. The etiopathogenetic remains
unclear, associated with Merkel cell polyomavirus.
MCC presents as an asymptomatic lesion or dome-
shaped nodules that clinically benign. Diagnosis is based on histopathology and immunohistochemistry
assay. This therapy includes excision, radiotherapy, immunotherapy, and chemotherapy. This case report is
aimed to give more understanding of the diagnosis and management of MCC. A 47-year-old man presented
with multiple pale-reddish tumors for eight months previously. Initially, the clinical feature was reddish,
scaly, and dry patches spread over the extremities. The biopsy two years ago showed BCC. There was no
regional lymph node involvement. Physical examination found verrucous tumors, with the largest size of 5
centimeters, erythematous macules, papules, erythematous plaques, crusts, and scales on the right elbow and
leg. Excision was followed by split-thickness skin graft and chemotherapy. One month post-surgery, the
patient had tetraparesis, and he died due to distant metastases one month later. The diagnosis of MCC was
established on history, clinical, histopathological, and immunohistochemistry examinations.
Sun exposure,
elderly age, and fair-skin type are the major factors that can cause MCC. Patient sustains advanced stage
and distant metastatic with a mortality rate between 33%- 46 %. MCC generally occurs on elderly fair-
skinned men with high UV exposure and poor prognosis at an advanced stage.
1 INTRODUCTION
Merkel cell carcinoma is a rare and aggressive
neuroendocrine tumor (Schadendorf et al, 2017;
Harms, 2017), high risk of metastasis, recurrence,
and death (Schadendorf et al, 2017; Pulitzer, 2017),
known as the second largest cause of death from
skin cancer after melanoma (Harms, 2017).
Although MCC is rarely found, it is estimated that
around 1500 new cases are found every year in the
United States with incidence rate tripled than the
same cases in the last 20 years (Schadendorf et al,
2017). The aetiopathogenesis of MCC is still
unclear, with tumor cells showing similar
histological and morphological features with Merkel
cell even though Merkel cells are postmitotic cells
and mostly located in the area of palms and soles,
oral and genital mucosa, and nail plates (not
predilection areas of MCC) (Xue et al, 2019). MCC
is associated with Merkel cell polyomavirus
(MCPyV) infection tumor (Schadendorf et al, 2017;
Harms, 2017) which is found in 80% cases of MCC,
exposure to ultraviolet light, and
immunosuppression condition (Xue et al, 2019;
Femia et al, 2018). Several factors that are thought
to increase the risk of MCC include sunlight,
prolonged exposure to UV light and
photochemotherapy, 81% MCC occurs in the sun-
exposed area (Tegeder et al, 2017). Human
polyomavirus infection is usually asymptomatic,
except in immunocompromised individuals, with
symptoms of progressive multifocal neuropathy or
leukoencephalopathy (Femia et al, 2018). The latest
pathogenesis model divided MCC into two groups:
positive virus group (80% found in the northern
hemisphere) and negative virus groups found most
frequently in the southern hemisphere (Schadendorf
et al, 2017; Harms, 2017). In the positive virus
group, MCPyV will encode the modifying gene (T
antigen), so that tumor cell proliferation occurs,
whereas in the negative virus group UV light is
thought to induce genetic changes and acts as a
driver mutation (Xue et al, 2019; Femia et al, 2018).
The risk of MCC is influenced by ethnicity, age,
Puspaningtyas, A., Kurniasari, L., Yuniati, R., Puruhito, B. and Sriyani, P.
Late Diagnosis Merkel Cell Carcinoma with History of Basal Cell Carcinoma.
DOI: 10.5220/0009989203710374
In Proceedings of the 2nd International Conference on Tropical Medicine and Infectious Disease (ICTROMI 2019), pages 371-374
ISBN: 978-989-758-469-5
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
371

gender, and history of treatment (Harms, 2017).
More than 95% of MCC patients have light skin
type, occur in men, age above 50 years (Xue et al,
2019).
MCC is characterized as a soft red or violet
nodule, overgrowing, in sun-exposed area (Harms,
2017). Crusting and ulceration are rarely found in
the early stages of the disease (Xue et al, 2019).
Abbreviations of AEIOU are used to show clinical
changes in MCC: asymmetrical, expanding rapidly,
immune system suppression, older than 50, UV-
exposed site in light-skin patients (Schadendorf et al,
2017; Harms, 2017), with 89% of patients show 3 or
more characteristics of AEIOU (Schadendorf et al,
2017).
Based on their histological features, MCC is
divided into 3 subtypes: trabecular, intermediate,
and small cell. The trabecular type is usually found
in mixed tumors, and the standard type is the most
common type characterized by a basophilic nucleus
with high mitotic activity. While the small cell type
is undifferentiated and indistinguishable from small
cell carcinoma in other locations. Histopathological
features in MCC include the hyperchromatic nucleus
and high mitotic activity (Schadendorf et al, 2017;
Tegeder et al, 2017). Immunohistochemical
examination show positive staining for cytokeratin
20 and neuron-specific enolase and gives negative
results for thyroid transcription factor-1
(Schadendorf et al, 2017; Ko et al, 2016; Harms et
al, 2016).
Staging MCC based on AJCC (American
Joint Committee on Cancer) criteria with four
significant division stages based on tumor size,
lymph node involvement, and presence/absence of
metastases (Harms et al, 2016).
Several treatments for MCC include surgery,
radiotherapy, chemotherapy, and immunotherapy
tumor (Schadendorf et al, 2017; Harms, 2017; Femia
et al, 2018; Cassler et al, 2016; Tello et al, 2018). In
cases with primary lesions are generally performed
wide excision and SLNB (Sentinel Lymph Node
Biopsy) with 1-2 cm free margin. In high-risk tumor
conditions (location of the head and/or neck, size
more than 1 cm, positive excision margin,
lymphovascular invasion) or high-risk patient
(immunocompromised condition) (Xue et al, 2019;
Femia et al, 2018) surgery is followed by 50Gy to
66Gy radiotherapy in the primary lesions and
regional lymph nodes as an adjuvant after the wound
healing process (Xue et al, 2019; Femia et al, 2018).
In small primary tumor lesions (less than 1 cm), no
involvement of lymph nodes, lymphovascular
invasion, or immunosuppression, postoperative
radiotherapy is not recommended (Krispinsky et al,
2018). Radiotherapy can be used as monotherapy in
conditions that surgical therapy cannot be
performed, either due to unresectable tumors, patient
rejection or due to the risk of morbidity in surgery
(Femia et al, 2018). Chemotherapy can be useful as
palliative therapy in conditions of patients who are
not operable or in metastatic conditions.
Chemotherapy regimens for MCC based on the
protocol for small cell lung cancer by giving
carboplatin / cisplatin-etoposide as the first line (Xue
et al, 2019; Femia et al, 2018). However, in the case
of patients who have received surgical therapy or
radiotherapy or both, chemotherapy as an adjuvant is
not recommended because it can increase morbidity
(associated with neutropenia) and mortality,
decreased quality of life, resistance to
chemotherapy, and suppressing the immune system
(Tegeder et al, 2017). Although chemosensitive, the
effectiveness of chemotherapy in MCC cases tend
not to be durable (Xue et al, 2019).
The aim of this case report is to give more
understanding of the diagnosis and management of
Merkel cell carcinoma.
2 CASE
A 47-year-old Chinese male came with complaints
of pale-reddish tumor in his right arm and leg eight
months ago. Soft rapidly enlarged and quickly bled
tumor, in some parts covered with pus and foul-
smelling, painful (+). Initially, skin disorders were
reddish, scaly, and dry patches spread over the
extremities. At his initial disease, he denied having a
fever, fatigue, weakness in limbs, unintentional
weight loss, and other systemic symptoms. Two
years ago, the patient had taken a surgery on the
right hand with biopsy result showed basal cell
carcinoma. History of sunlight exposure (+) because
he was a foreman. History of family members with
the malignant disease was denied.
Generalist status: the patient was compos mentis,
good nutritional status with 167 cm height, 75 kg
body weight, 120/80 mmHg blood pressure, pulse 80
x/minute, respiratory frequency 18 x/minute, and
36
o
C axillary temperature. From dermatological
status, there was a variety size of verrucous tumors,
the largest tumor was 5 cm, with crust, papules,
erythematous macules, scally, erythematous plaques
on the elbow and right leg. There was no
involvement of regional lymph nodes. Blood
laboratory test showed mild anemia; gamma GT 108
U / L; urea 41 mg / dL; creatinine 1.6 mg / dL;
chloride 108 mmol / L; non-reactive anti HIV
screening; ASTO qualitative negative, HsCRP 1.28
ICTROMI 2019 - The 2nd International Conference on Tropical Medicine and Infectious Disease
372
mg/L; HBsAg negative; anti HCV 0.15. From
peripheral blood smear testing, erythrocytes were
obtained: mild anisocytosis (normocytic,
microcytic), mild poikilocytosis (ovalocyte, pear
shape, teardrop), polychromatic +; leukocytes:
normal amounts estimation, monocytosis,
neutrophilia, atypical lymphocytes +; platelets:
estimated quantities are difficult to analyze,
clumping ++, were dominated by normal forms,
large forms +.
Biopsy specimens showed fragments of tumor
tissue coated with a flattened squashed epithelium,
keratinized, some of them were ulcerative with
swollen and hyperemic connective tissue stroma
caused of lymphocytes, leukocytes, histiocytes
contained tumor masses in the form of nests of
malignant cells with pleomorphic, hyperchromatic
round, oval nuclei, provide salt and pepper picture,
some vesicular, eosinophilic cytoplasm, arranged
molding, uninterrupted, in rows around blood
vessels, and also groups of cells with a pseudo
rosette structure. Immunohistochemical staining
showed the most positive cells for synapthopicin,
NSE, CK 7, and CK 20. Differential diagnosis
included basal cell carcinoma (BCC) and squamous
cell carcinoma (SCC).
Excision was followed by split-thickness skin
graft and chemotherapy using cisplatin 50 mg and
paclitaxel 300 mg/50 cc protocol. One month after
surgery, the patient had tetraparesis, and he died due
to distant metastases one month later.
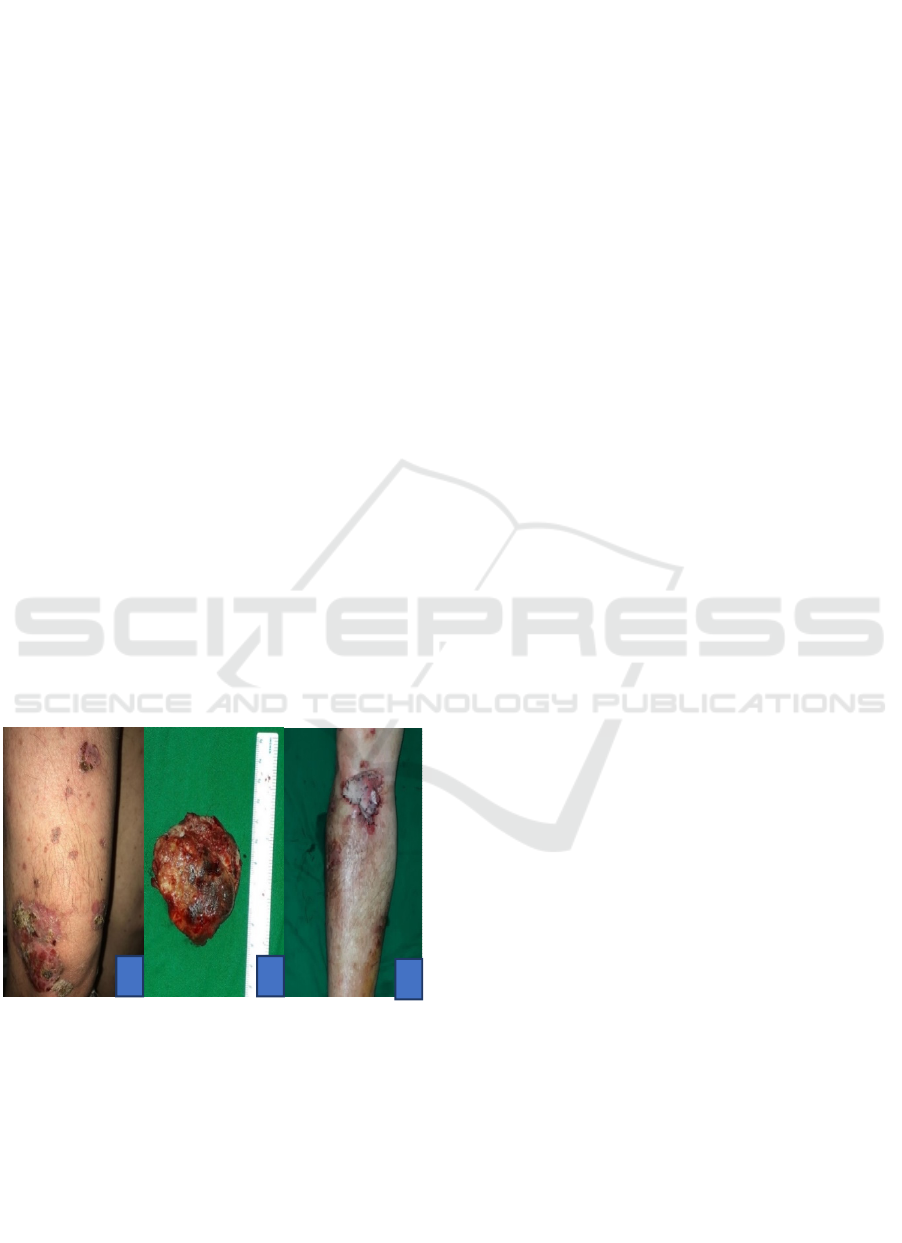
Figure 1. A. The verrucous flesh-red tumor partially is
covered by crust, painless in suppression, soft on touch; B.
Mass of tumor cells after excision; C. Post split-thickness
skin graft surgery
3 DISCUSSION
The incidence of Merkel cell carcinoma correlates
with exposure to UV light in light-skin individuals
along with age and mostly found at the location of
the head, neck, and upper extremities (Schadendorf
et al, 2017)About 10% of patients are found younger
than 50 years old. (Tegeder et al, 2017) The UV
exposure is thought as a cause of cellular mutations
which is resulting in uncontrolled proliferation,
especially in the virus-negative group.(Kneiling et
al,2011) MCC more often attacks men (62%) than
women (38%). (Harms et al,2017).
MCC lesions mostly appear as nodules that are
red-violet, soft, rapidly enlarged, and painless in
suppression. (Schadendorf et al, 2017) In the patient
has found 3 characteristics of abbreviation AEIOU
(asymptomatic, expanding rapidly, and lesions at the
location of the body exposed to UV) show suspicion
towards MCC. (Schadendorf et al, 2017). 65% of
MCC patients only have limited skin disease, 26%
of patients are accompanied by regional lymph node
involvement and 8% of patients with distant
metastases.(Xue et al,20019; Harms et al,2017).
Histopathological features of MCC at low
enlargement in the form of large nodules that are
infiltrating the dermis or subcutaneous, with stromal
changes including mucin, inflammation, and
increased vascularity. At high magnification, the
tumor consists of small round cells with a few
cytoplasms and pale, smooth neuroendocrine
chromatin (salt and pepper image), can also be found
hyperchromasia, accompanied by epidermal
involvement in the form of scattered pagetoid
images, also rosette. (Schadendorf et al, 2017;
Harms et al,2017). In addition to histopathological
examination with hematoxylin-eosin staining,
enforcement of the diagnosis of MCC requires
immunohistochemical staining including keratin-20
(CK 20), thyroid transcription factor-1 (TTF-1), CD
56, chromogranin A, synaptophysin, and
neurofilament protein (NFP). CK 20 is a sensitive
marker for MCC, with positive results are found in
89% -100% cases of MCC. (Femia et al, 2018)
In
addition to CK-20, the presence of at least one
marker which is derived from neuroendocrine
differentiation (ex synaptopodin) can confirm
neuroendocrine tumor origin. (Pulitzer, 2017) MCC
and BCC provide similar morphological features
caused by mucin in stromal or intratumor, but the
presence of CK20 and dotlike keratin expression can
distinguish MCC from BCC. (Pulitzer, 2017;
Harms,2017). In SCC with strong neuroendocrine
differentiation, it is often difficult to distinguish
from MCC because of the discovery of CK20 and
neuroendocrine granules that are also positive for
SCC biopsy. However, SCC always gives negative
results for MCV. (Pulitzer, 2017)
A
B
C
Late Diagnosis Merkel Cell Carcinoma with History of Basal Cell Carcinoma
373

Patients were referred to the plastic surgery
department for excision and split-thickness skin
graft. The choice of therapy in MCC cases is based
on several characteristics of the disease, including
clinical stage, the involvement of regional lymph
nodes, comorbid factors, and general condition of
the patient. Surgical excision with 1-3 cm borders at
the location of the primary tumor is the foremost
step in the treatment of MCC, where patients with
high-risk tumors should be followed by radiotherapy
as an adjuvant. (Schadendorf et al, 2017; Xue et al,
2019; Femia et al, 2018) Split thickness skin graft is
a skin-tight technique to cover the defect of
extensive skin and epithelial failure. The donor
location is usually chosen from the lateral thighs,
with the skin to be draped with holes using No. 11
and sewn to the side of the wound. This technique
provides faster healing results (between 4-6 weeks)
and a more acceptable appearance.(Kneiling et
al,2011;Yi et al,2015) Clinical output in MCC
patients with the latest therapy is still weak, with the
incidence of disease progression and metastasis
occur in the first three years after the diagnosis is
established. The size of the primary tumor is the
most reliable indication of the occurrence of
metastases, where small tumor (less than 1 cm) has a
risk of metastases 10-20% (Harms,2017). The most
common metastases are lymph node glands,
followed by metastases at a distant location of the
skin, lungs, nervous system center, bone, and liver.
Chemotherapy with carboplatin / cisplatin-etoposide
is recommended for stage IV MCC. (Schadendorf et
al, 2017; Xue et al, 2019;Harms,2017; Femia et al,
2018) 5-year survival rates are 51% for patients with
localized disease, 35 % in diseases with lymph node
involvement, and only 14% in distant metastatic
disease (Harms,2017).
4 CONCLUSION
A case of Merkel cell carcinoma has been reported
in a 47-year-old male Chinese patient, with a
dermatological examination in variety size of
verrucous tumor, the largest tumor is 5 cm,
accompanied by crusting, papules, erythematous
macules, squares, erythematous plaques on the
elbows and right foot, without the involvement of
regional lymph nodes. The results of
histopathological and immunohistochemical
examination supported the diagnosis of Merkel cell
carcinoma. Therapy modalities of this patient are
excision surgery and split-thickness skin graft
followed by cisplatin-paclitaxel chemotherapy, but
the patient died due to distant metastases to the
central nervous system.
REFERENCES
Cassler NM, Merrill D, Bichakjian CK, Brownell I. 2016.
Merkel Cell Carcinoma Therapeutic Update. Curr
Treat Options Oncol. 17 (7): 36.
Femia D, Prinzi N, Anichini A, Mortarini R, Nichetti F,
Corti F, et al. 2018. Treatment of Advanced Merkel
Cell Carcinoma : Current Therapeutic Options and
Novel Immunotherapy Approaches. Springer Nature
Switzerland AG. 13 (5): 567-82.
Harms PW. 2017. Update on Merkel Cell Carcinoma. Clin
Lab Med. 37 (3): 485-501.
Harms KL, Healy MA, Nghiem P, Sober AJ, Johnson TM,
Bichakjian CK, et al. 2016. Analysis of Prognostic
Factors from 9387 Merkel Cell Carcinoma Cases
Forms the Basis for the New 8th Edition AJCC
Staging System. Ann Surg Oncol. 23(11): 3564-71.
Kneilling M, Breuninger H, Schippert W, Hafner HM,
Moehrle M. 2011. A modified , improved , easy and
fast technique for split-thickness skin grafting. British
Journal of Dermatology. (165) :581–4.
Ko JS, Prieto VG, Scolyer A, Reynolds JP, Piliang M,
Ernstoff MS, et al. 2016. Histologic pattern of merkell
cell carcinoma sentinel lymph node metastasis
improves stratification of Stage III patients. Mod
Pathol. 29 (2): 122–30.
Krispinsky AJ, Massick S. 2018. Typically atipical:
Merkel cell carcinoma. Am J Med. 18: 31050-7.
Pulitzer M. 2017. Merkel Cell Carcinoma. Surg Pathol. 10
(2): 399-408
Schadendorf D, Hariharan S, Bharmal M, et al. 2017.
Merkel cell carcinoma : Epidemiology, prognosis ,
therapy and unmet medical needs. European Journal of
Cancer 71: 53-69.
Tegeder A, Afanasiev O, Nghiem P.Merkel cell
carcinoma. Dalam: Gold Smith L, Katz S, Gilchrest,
Palle A, Leffel D, Wolf K (Editor). 2017. Fitzpatrick’s
Dermatology in General Medicine. Edisi ke-8. Vol.2.
New York: Mc Graw Hill. Hal 1362-71.
Tello TL, Coggshall K, Mas SSY, Yu SS. 2018. Merkel
cell carcinoma: An update and review current and
future therapy. J Am Dermatology. 78 (3): 445–54.
Xue Y, Thakuria M. 2019. Merkel Cell Carcinoma
Review. Hematol Oncol Clin NA. 33 (1): 39–52.
Yi JW, Kim JK. 2015. Prospective Randomized
Comparison of Scar Appearances between Cograft of
Acellular Dermal Matrix with Autologous Split-
Thickness Skin and Autologous Split-Thickness Skin
Graft Alone for Full-Thickness Skin Defects of the
Extremities. Plast. Reconst. Surg. (135); 609–16.
ICTROMI 2019 - The 2nd International Conference on Tropical Medicine and Infectious Disease
374
