
Anti-inflammatory Activity Test for Ethanol Extract Moon Flower
(Tithonia diversifolia) Leaves to Male White Mice
Basyariah Lubis
1
, Ika Nur Saputri
1
, Rony Ajartha
1
,Sri Melda Br Bangun
2
, Chandra Pranata
3
, Novandi
Purba
3
, Nur Ulina M. Br. Turnip
3
1
Faculty of Midwifery, Institut Kesehatan Medistra Lubuk Pakam, Sumatera Utara, Indonesia,
2
Faculty of Public Health, Institut Kesehatan Medistra Lubuk Pakam, Sumatera Utara, Indonesia,
3
Faculty of Pharmacy Institut Kesehatan Medistra Lubuk Pakam, Sumatera Utara, Indonesia.
Keywords: Kembang Bulan Leaf (Tithonia diversifolia), Anti-inflammatory
Abstract: Introduction Hibiscus leaves (Tithonia diversifolia) are wild plants. Leaves, root bark and stems of the moon
are parts that can be used for traditional medicine. This research was conducted with the aim to determine
the effect of ethanol extracts of the moon flower (Tithonia diversifolia) as an anti-inflammatory Methods.
The method used was artificial edema of white male male foot mice with 1% carrageenan induction of 0.05
ml as an edema maker and as a positive control using Na-Diclofenac. This research was a kind of pure
experimental research. Data obtained to find the percentage of inflammation inhibition power. Data
distribution was analyzed by the Kolmogorov-Smirnov test, followed by the ANOVA test and the LSD test
with a confidence level of 95%. The research results showed that lunar leaves have an anti-inflammatory
effect expressed by the% inhibitory inflammation at the lowest dose of 50 mg / kg bw followed by 100 mg /
kg bw and 150 mg / kg bw have the highest percentage of inflammation inhibition. In the ANOVA test
showed a significant difference in each treatment group at the hours of 1 to 6 hours significantly different at
the test level (α ≤ 0.05). Discussion was expected to be able to identify the potential antioxidant activity of
the leaves of the moon flower (Tithonia diversifolia) by chromatography.
1 INTRODUCTION
The use of traditional medicine has become a
habit practiced by almost all countries in the world.
Over the past decade, the use of traditional
medicines has grown rapidly. The development of
traditional medicine continues to be done as health
care for the poor in developing countries (Karamian
et al, 2013). Traditional medicine was widely used
by the community to maintain health and was in
great demand because it is inexpensive and its
availability is affordable for the community,
especially in villages or small towns where health
centers are scarce. Compared to modern medicine,
traditional medicine has several advantages, namely
its side effects were relatively low. It must be
realized that there were dangerous traditional
medicinal ingredients if their use exceeds safe
dosages and concentrations (Katno and Pramono,
2005). The use of traditional medicines including
herbs can be beneficial for health maintenance,
prevention and treatment of diseases (Aditama,
2014).
Indonesia is a tropical country with plant
potential that has been traditionally used as
traditional medicine and has been an Indonesian
culture since centuries ago until now. Indonesia with
a tropical climate causes fertile soil so that many
types of plants can grow. Among the various types,
several types of plants have medicinal properties
(Hariana, 2013). Moon flower (Tithonia diversifolia)
is a plant species that belongs to the Asteraceae
family. The part that was used from lunar plants as a
source of chemicals, which is used for traditional
medicine is usually the leaves, but can also use the
root bark and stem. The leaves of this month's
flower plants contain alkaloids, terpenoids,
flavonoids, saponins, tannins, and polyphenols. The
benefits of lunar leaves are traditionally usually used
as a medicine for stomach aches, bloating, diarrhea
and used as a wound and anti-inflammatory drug
(anti-inflammatory) (Dalimartha, 2000).
Lubis, B., Saputri, I., Ajartha, R., Bangun, S., Pranata, C., Purba, N. and Turnip, N.
Anti-inflammatory Activity Test for Ethanol Extract Moon Flower (Tithonia diversifolia) Leaves to Male White Mice.
DOI: 10.5220/0009974705510557
In Proceedings of the International Conference on Health Informatics and Medical Application Technology (ICHIMAT 2019), pages 551-557
ISBN: 978-989-758-460-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
551

Inflammation is a process that involves a series of
events that can be caused by various stimuli such as
infectious substances, ischemia, antigen-antibody
interactions, and injuries due to heat or other physical
injuries (Goodman and Gilman, 2014). Each type of
stimulus has common signs of inflammation such as
swelling, pain, redness, heat and loss of cell function
that causes discomfort for sufferers so treatment
therapy was needed to overcome them, by using
modern drugs or medicines derived from plants
(Supriyatna et al., 2015).
Therapy that can be given to patients with
inflammatory complaints can be given with Non-
Steroid Anti-Inflammatory Drugs (NSAIDs) Non-
Steroidal Anti-Inflammatory Drugs (NSAIDs) that
can relieve symptoms, maintain cell or tissue
function and slow down or stop processes that
damage tissue (Katzung et al., 2017). Non-steroidal
Anti-Inflammatory Drugs (NSAIDs) which are
widely circulating are modern drugs that can inhibit
various inflammatory mediators (Pinzon, 2007). The
use of NSAIDs orally generally cause various side
effects problems such as central nervous system
(headache), cardiovascular (fluid retention,
hypertension), gastrointestinal tract (abdominal pain,
dysplasia), hemotology, liver, lung, skin and kidney
(Katzung, 2017) . Therefore the use of anti-
inflammatory drugs from plants can be used as an
alternative treatment with relatively smaller side
effects than modern medicine (Kinanti, 2016).
Based on the research of Widia, et al. (2016)
showed that the formulation of ethanol extract of
moon leaves leaves has the potential to inhibit
Staphylococcus aureus using well diffusion method.
Hanifa, (2015) showed that the antioxidant activity
of ethanol extracts from lunar leaves contained total
flavonoid levels of 4.209 mg QE / gram extract.
Meanwhile, Suherman (2013) reported that testing
the antioxidant activity of Tithonia diversifolia leaf
extract produced IC 50 against free radical DPPH of
27.88 μg / ml. The results of the separation of lunar
leaf extract by thin layer chromatography obtained
flavonoid compounds namely 5,7,8,3',4'-
pentahidroksiflavonol or 5,6,7,3',4'-
pentahidroksiflavonol (Aisha, et al., 2015).
Flavonoid compounds have anti-inflammatory
activity by inhibiting the release of serotonin and
histamine to the site of inflammation and inhibiting
the synthesis of prostaglandin from arachidonic acid
by inhibiting the action of cyclooxygenase (COX)
(Hasanah, 2011). Based on Ramadhani and Sri's
research (2017) flavonoid compounds have anti-
inflammatory activity. The strength of the anti-
inflammatory effect was indicated by the percentage
of edema inhibition. Based on the description above,
the moon flower leaves contain flavonoids which
were expected to be used as new drugs in anti-
inflammatory treatment. The author conducted
research to determine the anti-inflammatory effects
of ethanol extracts of lunar leaves in male white
mice carrageenan-induced.
2 RESEARCH METHODS
This type of research used in this study was
purely experimental methods. The research phase
includes sample preparation, sampling, preparation
of experimental animals, simplicia characteristics,
phytochemical screening, extraction methods and
testing of anti-inflammatory effects on white male
mice. The basis of this method was to make an
edema on the sole of the back foot of the mouse
using 1% karegenan. The research data were
analyzed with Analysis of Variance (ANOVA) with
95% confidence so that it can be known whether the
differences obtained are significant or not, if there
are significant differences followed by the Least
Square Difference test (LSD). The research site was
carried out at the Lubuk Pakam Institute of Health
Chemistry Laboratory in the manufacture of reagent
solutions, characteristics of phytochemicals and
phytochemical screening, simplicia extraction
processes and anti-inflammatory testing. The time of
the study was carried out in March - May 2019.
The tools used in this study are laboratory
glassware, UgoBasille®- Plethysmometer, mortar
and stamper, injection syringe, mouse cage, digital
camera, analytical balance, vacuum rotary rotary
evaporator, oral sonde, tissue rolls, labels, blenders,
animal scales. The materials used in this study were
lunar leaves, chemicals used sodium diclofenac
(PT.IndoFarma factory), Carboxy Methyl Cellulose
(CMC), λ-carrageenan, sodium chloride solution
0.9%, Pb (II) acetate, iron (III) chloride P, mercury
(II) chloride, potassium iodide, iodine, α-naphthol,
nitric acid, bismuth nitrate, ether, clofrom,
isopropanolol, ethanol, methanol, sodium sulfate
anhydrous, ethyl acetate, magnesium powder,
bismuth nitrate, ether, chloroform , isopropanolol,
ethanol, methanol, sodium sulfate anhydrous, ethyl
acetate, magnesium powder, zinc powder,
hydrochloric acid P, ether, sulfuric acid P and
distilled water.
The experimental animals used were white male
mice with a body weight of 20-25 g and 8 weeks of
age of 25 animals. Sample was done purposively,
without comparing with the same material from
ICHIMAT 2019 - International Conference on Health Informatics and Medical Application Technology
552

other regions. Samples taken are old and flowering.
Single leaf, carved until half the length of the leaf
bone, jagged, alternating, pointed tip and base,
pinnate, and green. The sample used was the moon
flower leaves (Tithonia diversifolia) obtained from
Tigaras, Kec. Dolok Pardamean, Kab. Simalungun,
North Sumatra Province.
Leaves that have been taken were washed using
running water drained, then weighed wet weight.
Then dried for three days, then sorting was dry and
chopped. Furthermore, it was dried in a drying
cabinet until it was brittle after it was blended into
powder, weighed then put into a tightly closed
plastic bottle container and stored at room
temperature. To see the characteristics of simplicia,
macroscopic and microscopic examination was
performed. The phytochemical screening of samples
as follows:
Alkaloid: 0.5 gram of simplicia powder was
weighed, added 1 ml of 2 N hydrochloric acid and 9
ml of distilled water, heated over a water bath for 2
minutes, cooled and filtered, the filtrate was used for
the alkaloids test. 3 test tubes were taken, then 0.5
ml of filtrate were put into each test tube. In tube I: 2
drops of Mayer's reagent are added, forming white
or yellow lumpy deposits, in tube II: 2 drops of
Dragendrof reagent are added, a brown or orange-
brown precipitate is formed, in tube III: added 2
drops of Bouchardat reagent, will form a brown
sediment to black. The alkaloid is positive if there is
sedimentation or turbidity in two or three of the
above experiments (MOH RI, 1995). Saponin: 0.5
gram of simplicia powder was weighed, put in a test
tube, added 10 ml of hot distilled water, cooled, then
shaken vigorously for 10 seconds. Saponin is
positive if a stable foam is formed not less than 10
minutes as high as 1 to 10 cm and with the addition
of 1 drop of 2 N hydrochloric acid the foam does not
disappear (MOH RI, 1995). Tannin: As much as 0.5
gram of simplicia powder was weighed, then mixed
with 10 ml distilled water, the filtrate was diluted
with water until it was colorless. The solution is
taken as much as 2 ml and added 1-2 drops of
reagent iron (III) chloride 1%. If there is a blue or
blackish green color indicates the presence of
tannins (Farnsworth, 1966).
Preparation of Ethanol Extract Simplicia powder
was extracted by maceration using 70% ethanol
solvent. Procedure: Samples were weighed as much
as 500 grams, then put into maceration containers.
Soaked with solvent until completely submerged and
then covered and stored at room temperature. Stirring
once a day for five days. After that the solvent is
separated from the pulp by pouring the solvent in
another container, and the remaining solvent in the
pulp is mixed and filtered. To ensure the extraction
process takes place perfectly, the pulp that has been
kneaded was soaked again using a new ethanol
solvent. Left for two days while stirring every day,
then kneaded and filtered. Do the same treatment
until the solvent is colorless. All maserates are
combined and evaporated using a rotary evaporator at
± 400C until a thick extract is obtained.
Preparation of Control Test Materials and
Comparative Medicines Hibiscus leaf extract with a
dose of 50 mg / kg bw, 100 mg / kg bw, 150 mg / kg
bw (test material), Diclofenac sodium suspension
dose 6.5 mg / kg bw (positive control), CMC
suspension 0.5 % (negative control), carrageenan
1% (induction). The preparations consist of: Making
a 0.5% CMC suspension: A total of 0.5 g CMC was
sprinkled evenly into a mortar containing 35 ml of
hot distilled water, allowed to stand for 15 minutes
until obtained a transparent mass, crushed to form a
gel and then diluted with a little water, put in a 100
ml flask, then added distilled water to the mark line.
Preparation of diclofenac sodium suspension: 6,5 mg
of diclofenac sodium was added and then crushed
with the addition of 0,5% CMC suspension until
homogeneous, put into 10 ml flask, then added 0,5%
cmc suspension to the mark line. Preparation of
ethanol extract suspension: Weighed 50 mg, 1000
mg, 150 mg extract of moon flower leaves. Each
crushed by adding 0,5% CMC suspension until
homogeneous, put into a 10 ml flask, sufficient to
the mark with 0,5% CMC suspension. Praparation of
inflammation indicator: Weighed 100 mg of
carrageenan lambda, then homogenized with 0,9%
sodium chloride solution, then put in a 10 ml flask,
then supplemented with 0,9% sodium chloride
solution until the mark line was than allowed to
stand and incubated at 37
0
C for 24 hours.
Figure 1: Experiment Sample: (a) Tithonia diversifolia (b)
Simplisia Powder (Tithonia diversifolia), (c) Animal
Research.
Anti-inflammatory Activity Test for Ethanol Extract Moon Flower (Tithonia diversifolia) Leaves to Male White Mice
553

2.1 The Anti-inflammatory Procedure
Were Tested with the following
Scheme
Before testing, mice were fasted for 18 hours while
still being given a drink. Mice were grouped into 5
groups, namely the negative control group (0.5%
CMC suspension), the test material group (three
doses of lunar extract extract suspension) and the
positive control (diclofenac sodium). On the day of
the test, each animal was weighed and marked on its
left leg and tail, then the left leg of the mice was put
in a cell containing a reservoir solution that had been
prepared beforehand until the liquid rose at the
upper boundary line, the pedal was then held,
recorded figures on the monitor as the initial volume
(Vo), which is the foot volume before treatment is
given. The preparation is given orally with a volume
of giving to mice as much as 1 ml in accordance
with the treatment group as follows: Group I: 5 mice
were given a 0.5% w / v Na-CMC suspension orally
as a negative control; Group II: 5 mice were given
orally diclofenac sodium solution (positive control);
Group III: 5 mice were given orally extracts of
hibiscus leaves at a dose of 50 mg / KgBB; Group
IV: 5 mice were given orally extracts of hibiscus
leaves at a dose of 100 mg / kg; dan Group V: 5
mice were given lime leaves extract at a dose of 150
mg / KgBW orally. One hour later, each mouse was
induced with 0.05 ml of carrageenan 1% intraplantar
then measured the initial volume of the mice's feet.
After that measured the volume of mice edema feet
after treatment every interval of 1 hour for 6 hours.
The edema volume is determined based on the
increase in mercury in the plathysmometer.
3 RESULTS AND DISCUSSION
Macroscopic examination results of fresh moon
flowers are leaves single, etched to half the length of
the leaf bone, jagged, alternating, leaf length 26-32
cm, width 15-25 cm, tip and base of pointed leaves,
pinnate, green leaves. Microscopic examination
results showed fresh moon flowers the presence of
multicellular single hair closures, cuticles, upper
epidermis, palisades, spiral trachea, spongy tissue,
lower epidermis, and leaf mouth Diitic type. This
Phytochemical screening of the simplicia leaves of
the moon flower to show the class of secondary
metabolite compounds contained therein. The
examination carried out on the simplified powder of
the moon flower is the examination of the group of
alkaloid compounds, flavonoids, saponins, and
tannins. The results of phytochemical screening of
the simplicia leaves of the moon flower are alkaloids
(-), flavonoids (+), saponins (-), and tannins (+).
Description (+) positive means it contains a class of
compounds and (-) negative means that it does not
contain compounds. Extraction result of moon
flower leaves are 500 gram sample weight, 64 gram
extract weight, solvent volume ( ethanol 70 %) 3
liters, and soaking time 5 x 24 hours.
Inflammation is a disorder that is often
experienced by humans and animals that cause pain
in the surrounding area. So the need for prevention
or treatment to reduce pain, fight or control pain due
to swelling. In this anti-inflammatory study the
method used was the formation of artificial edema
on the soles of mice's feet using carrageenan as an
induction of edema. This method was chosen
because it is one of the methods of testing
anbtiinflamasi activity that is simple, easy to do and
often used. The use of carrageenan as an inducer of
edema has several advantages including not leaving
a scar, not causing tissue damage and giving a more
sensitive response to anti-inflammatory drugs
(Fitriyani, 2008).
Carrageenan as an irritant compound induces cell
injury through the release of mediators that initiate
the inflammatory process. In ssat release of
inflammatory mediators occurs maximal edema and
lasts several hours. Inflammation induced by
carrageenan is characterized by increased pain,
swelling, and prostaglandin synthesis up to 4-5
times. Udem caused by carrageenan induction lasts
for 6 hours and gradually decreases within 24 hours
(Taufiq, 2008). EEDKB anti-inflammatory activity
testing used 25 test animals, with 5 treatment
groups. The group consisted of positive control
given Na diclofenac at a dose of 6.5 mg / kgBW
orally, negative control given CMC Na treatment
0.5% orally, the extract treatment group dose 50 mg
/ kgBW, the extract treatment group dose 100 mg /
kg kgBW, and the 150 mg / KgBW dose extract
group. The mice were fasted for ± 18 hours, then the
mice were weighed marked on the tail and left ankle
of the rat. Before each group was given ethanol
extract of lunar leaves, the volume of mice's feet was
measured first as the initial volume (Vo). After that,
each group was given ethanol extracts of lunar
leaves ie group I was given a 0.5% Na-CMC
suspension, group II was given a diclofenac sodium
suspension of 6.5 mg / KgBW, groups III and IV
and V were each given an EEDKB suspension dose
50, 100, 150 mg / kgBB orally. One hour later, each
foot of the mice's foot was injected intraplantar with
ICHIMAT 2019 - International Conference on Health Informatics and Medical Application Technology
554
0.05 mL of 1% λ-carrageenan solution.
Measurements were made using a pletismometer
with measurement principles based on Archhimedes'
law. After 30 minutes, the measurement is carried
out by dipping the feet of the mice into the
pletismometer cells that contain special fluid until
the solution reaches the upper limit, and the pedal is
held. Numbers are recorded on the monitor. The
change in liquid volume that occurs is recorded as
the volume of the feet of mice (Vt). Measurements
were made every 30 minutes for 360 minutes.
Changes in mice foot volume, can be calculated
percent inflammation in mice feet. Next, a graph of
changes in the average inflammation of the feet of
mice was made. The percent inflammation group in
the feet of mice smaller than the control group
showed that the test material was able to suppress
inflammation caused by carrageenan. The results of
the percent inflammation measurement can be seen
in Figure 2:
Figure 2: Percent graph inflammation of the average foot
mice
In Figure 2 it can be seen that the sodium
diclofenac suspension of 6.5 mg / kgBW has a
smaller inflammation percentage than EEDKB doses
of 50, 100, and 150 mg / kgBW, and the EEDKB
dose of 150 mg / kgBW has a smaller percent
inflammation than EEDKB doses of 100 and 50 mg /
kg body weight. The formation of inflammation by
carrageenan produces acute inflammation, and does
not cause tissue damage, although inflammation can
last for 360 minutes and gradually diminish for one
day.
Carrageenan as the cause of inflammation can be
influenced by anti-inflammatory drugs. Its response
to anti-inflammatory drugs is more sensitive than
other irritants (Juheini, et al., 1990). The percentage
of mice foot inflammation smaller than the control
showed that the diclofenac sodium suspension and
EEDKB suspension were able to inhibit
inflammation in the mouse feet caused by
carrageenan.
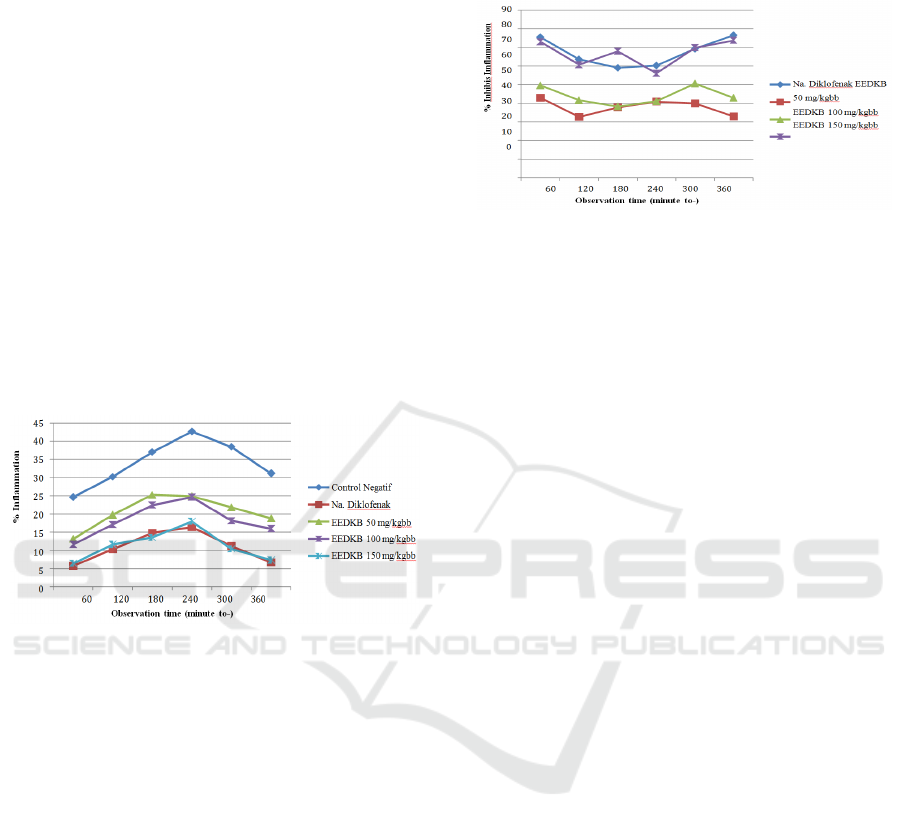
The ability to inhibit this inflammation, called
inflammation inhibition, can be seen in Figure 3.
Figure 3: Percent graph of the average inflammation of the
feet of mice
In Figure 3 it can be seen that EEDKB 50 mg /
kgBW has a smaller percentage of inflammation
inhibition than EEDKB 100, 150 mg / kgBW and
with diclofenac sodium suspension at a dose of 6.5
mg / kgBW, EEDKB 100 mg / kgBW has percent
inflammation inhibition smaller than EEDS 150 mg /
kgBW and with diclofenac sodium suspension 6.5
mg / kgBW, and EEDKB 150 mg / kgBW have a
smaller percentage of inflammation inhibition than
diclofenac sodium suspension at a dose of 6.5 mg /
kgBW.
Data obtained in the normality test by the
Kalmigorov-Smirnov method to see the distribution
of percent data of mice foot inflammation inhibition
to the treatment group showed that all treatment
groups were normally distributed and not
significantly different. Then homogeneity test was
performed using the Levene method to see data on
the percentage of inflammation inhibition of
homogeneous mouse mice or not, the results showed
that all treatment groups were homogeneously
distributed (α≥0.05). Because the data meet the
homogeneity requirements, ANOVA was continued
to look at the average percentage of mice foot
inflammation inhibition in the treatment group to see
significantly different or insignificant values with a
95% confidence level. Least Square Difference
(LSD) test was performed. The results showed that
the percentage of mice foot inflammation inhibition
throughout the initial volume group in each
treatment did not differ significantly, in the
induction volume group EEDKB 50 mg / kgBW and
100 mg / kgBW were significantly different, at hour
to 1-6 for each treatment is significantly different at
the 0.05 test level.
Based on these test results, it can be concluded
that the administration of ethanol extracts of lunar
leaves at a dose of 50 mg / kg bw, 100 mg / kg bw,
150 mg / kg bw can reduce inflammation in the soles
Anti-inflammatory Activity Test for Ethanol Extract Moon Flower (Tithonia diversifolia) Leaves to Male White Mice
555

of male white mice induced by 1% carrageenan.
This research has also been carried out by Verawati,
et al (2011), it has been reported that the ethanol
extract of the moon flower leaves has anti-
inflammatory activity. This is seen from the
decrease in exudate volume in the back
inflammation of female white mice that are given
topically. In the test studies the anti-inflammatory
effects of ethanol extracts of lunar leaves showed
that the effect was dose dependent on increasing
certain doses. Anti inflammatory effects can be seen
from the content of lunar leaves in which flavonoid
compounds have anti-inflammatory activity by
inhibiting the release of serotonin and histamine to
the site of inflammation and inhibiting the synthesis
of prostaglandin from arachidonic acid by inhibiting
the action of cyclooxygenase (COX) (Hasanah,
2011).
4 CONCLUSIONS
Ethanol extract of the moon flower (Thitonia
diversifolia) can provide anti-inflammatory effects.
The obtained extracted ethanol of the moon flower
leaves (Thitonia diversifolia) are 50, 100, and 150
mg / KgBW. The dose of 150mg/kgBW has an anti-
inflammatory effect in inhibiting mouse foot edema
and the dose of 150 mg/kgBW has smallest
percentage of inflammation among the doses used.
REFERENCES
Aisyah, Z, dkk., 2015. Identifikasi senyawa flavonoid dari
daun kembang bulan (tithonia diversifolia) dengan
metode pereaksi geser. Jurnal sains dan teknologi.
Alkandahri, M.Y., dan Surbanas, A., 2017. Kandungan
senyawa kimia dan aktivitas farmakologi ekstrak daun
kembang bulan (tithonia diversifolia (hemsley) A.
Gray) sebagai antimalaria. 3. (15), hal.170- 186.
Brahmachari, G. 2011. Bio-flavonoids with promising
antidiabetic potentials: A critical survey. Research
Signpost, pp.187-212.
Chon, H., Peng, L.Y., Jiang, B., Hon, A.J., Lia, Z.W. and
Sun, H.D. 2000.Chemical constituents from
tithoniadiversifolia.yannanzhiwuyanjiu, 22 pp.361-
364.
Choudhary, M.I. 2001.New α-glukosidase inhibitors from
the mongolian medicinal plant ferula
mongolica.helvecita chimicaacta, 84(8) pp.2409-2416.
Dalimartha, S. (2000). Atlas tumbuhan obat indonesia.
Trubus Agriwidya, Jakarta.
Dewi, R., 2010, Aktivitas anti mikroba dari
elephantropusscober L,tithoniadiversifolia A.Gray,
Tageteserecta L, Skripsi, Sekolah Tinggi Ilmu Farmasi
Indonesia, Padang, 258.
Dheer, R. and Bhatnagar, P. 2010, A study of the
antidiabetic activity of barleriaprionitis linn. Indian
Journal of Pharmacology, 42(2) pp.70-73.
Di Giacomo C, Vanella L, Sorrenti V, Santangelo R,
Barbagallo I, Calabrese G, et al. Effects of Tithonia
diversifolia (Hemsl.) A. Gray extract on adipocyte
differentiation of human mesenchymal stem cells.
PLoS One 2015; 10(4): e0122320.
Domer, F.R., 1971, Animal experiment in
pharmacological basis of therapeutic, Charles C.
Thomas Publiser, Springfield, IIIonis, USA.
Elufioye,T., Alatise, O.I., Fakoya, F.A., Agbedahunsi
,J.M., dan Houghton,P.J .(2009). Toxicity Studies of
Tithonia diversifolia A.Gray (Asteraceae) in Rats.
Journal of Ethnopharmacology 122. Halaman 410-
415.
Gama RM, Guimarães M, Abreu LC, Armando-Junior J.
Phytochemical screening and antioxidant activity of
ethanol extract of Tithonia diversifolia (Hemsl) A.
Gray dry flowers. Asian Pacific Journal of Tropical
Biomedicine. 2014;4:740–742
Gryglewsky, J.R., 1997, Some experimental models for
the study of inflamation and anti inflamatory drugs,
Departemen of Pharmacology, Copernicus Academy
of Medicine, Cracow, Poland.
Goffin E, Ziemons E, De Mol P, Madureira MC, Martins
AP, Da Cunha AP, Philippe G, Tits M, Angenot L,
Frederich M. In vitro antiplasmodial activity of
Tithonia diversifolia and identification of its main
active constituent: Tagitinin C. Planta Med.
2002;68:543–545.
Goodman & Gilman. (2014). Dasar farmakologi terapi.
Edisi 10 Volume 2., Jakarta: Penerbit Buku
Kedokteran EGC. Halaman 666-675, 688-689.
Hanum, I. Fridah and van der Masen, LIG, 2002,
Auxiliary Plants, J.Nat.Prod p.297-298.
Harborne, J.B. (1987). Metode fitokimia penuntun cara
modren menganalisa tumbuhan. Edisi 1. Penerjemah:
Kosasih Padmawinata dan Iwang Soediro. Bandung:
ITB. Halaman152.
Harvey, Richard, A., dan Pamela, C, Champe. (2014).
Farmakologi ulasan bergambar. Edisi 4. Jakarta: Buku
Kedokteran EGC. Halaman 595, 598.
Jagtap, U.B. and Bapat, V.A. 2010. Artocarpus: A review
of its traditional uses, phytochemistry and
pharmacology. Journal of Ethnopharmacology, 129
(2010) pp.142–166.
Karamian, Roya., dan Ghasemlou, Fatemeh. (2013).
Screening of total phenol and flavonoid content,
antioxidant and antibacterial activities of the
methalonic extract of three Silence species from Iran.
International Journal of Agriculture and Crop
Science, 5 (3) : 305-312.
Kinanti, Rianti, Putri. (2016). Skripsi. Uji aktivitas
antiinflamasi topikal fraksi etil asetat dari ekstrak
metanol daun sirih merah (Piper crocatum Ruiz &
Pav.) pada mencit diinduksi karagenan. Universitas
Sanata Dharma. Yogyakarta.
ICHIMAT 2019 - International Conference on Health Informatics and Medical Application Technology
556

Lumbanraja, L.B. (2009). Skrining fitokimia dan uji efek
antiinflamasi ekstrak etanol daun tempuyung (Sonchus
arvenis L.) terhadap radang pada tikus. Skripsi.
Fakultas Farmasi Universitas Sumatra Utara.
Manjoer, Arif., et.al. (1999). Kapita selekta kedokteran
Edisi III Jilid 1. Jakarta : Media Aeculapius Fakultas
Kedokteran Universitas Indonesia.
Mwanauta, R.W., Mtei, K.A., and Ndakidemi, P.A.
(2014). Prospective bioactive compounds from
vernonia amygdalina, lippia javanica, dysphania
ambrosioides and tithonia diversifolia in controlling
legume insect pests. Agricultural Sciences. 5. pp.1129-
1139.
Pinzon, R., Lucas, M., (2007), Breakhrough in
management of acute pain, Jurnal Kedokteran dan
Farmasi, 20(4), 151-155.
Ramadhani, Nur., dan Sri, Adi, Sumiwi. (2017). Aktivitas
antiinflamasi berbagai tanaman diduga berasal dari
flavonoid. Jurnal Farmasi Universitas Padjadjaran.
Vol. 4. No.4. Suplemen. 1.
Rowe, R.C., Paul, J.S., dan Marian, E.Q. (2009).
Handbook of pharmaceutical excipient. Edisi 6. USA:
Pharmaceutical Press. Halaman 623.
Supriyatna, Febriyanti, R, Dewanto, Wijaya, I., dan
Ferdiansyah, F., (2015), Fitoterapi sistem organ:
pandangan dunia barat terhadap obat herbal Global,
Ed. 2, Cet. 2, CV Budi Utama, Yogyakarta, 223-224.
Suralkar, A.A. (2008). In vivo animal models for
evaluation of antiinflammatory activity. Article
Review. Vol 6. Issue 2
Winter, C.A., Risley, E.A., dan Nuss, G.W. (1962).
Carrageenin-induced oedema in the hind paw of the
rat as an assay to antiinflammatory drugs. Proc Soc
Exp Biol Med.111:544-547.
Witko-Sarsat, V., Friedlander, M., and Khoa, T. N. 1998.
Advanced oxidation protein products as novel
mediators of inflammation and monocyte activation in
chronic renal failure. The Journal of Immunology,
161(5) pp.2524–2532.
Yang, L., Liang, M., and Zhou, Q. 2010. Advanced
oxidation protein products decrease expression of
nephrin and podocin in podocytes via ROS-dependent
activation of p38 MAPK. Science China Life
Sciences, 53(1) pp.68–77.
Zhang, X.F., and Tan, B.K. 2000. Antihyperglycemic
and anti-oxidant properties of Andrographispaniculata
in normal and diabetic rats. J. Clin. Exp. Pharmacol. J.
Physiol, 27(3) pp.58-63.
Zhao, G., Li, X., Chen, W., Xi, Z., and Sun, L. 2012.
Three new sesquiterpenes from tithonia diversifolia
and their anti-hyperglycemic activity.fitoterapia, 83
pp.1590–1597.
Anti-inflammatory Activity Test for Ethanol Extract Moon Flower (Tithonia diversifolia) Leaves to Male White Mice
557
