
Effect of Jamblang (Syzygium cumini) Seed Extract on ALT and AST
Levels in Isoniazid-induced Male Rats
Ahmad Syukur Hasibuan
1
, Sri Melda Br. Bangun
2
, Romauli Anna Teresia Marbun, Saadah Siregar,
Aminah S.
, Debi Dinha Octora
1
Faculty of Pharmacy, Institut Kesehatan Medistra Lubuk Pakam, Sumatera Utara, Indonesia.
2
Faculty of Public Health, Institut Kesehatan Medistra Lubuk Pakam, Sumatera Utara, Indonesia
Keywords: ALT, AST, Syzygium cumini, Isoniazide
Abstract: Syzygium cumini or known in Indonesia as jamblang fruit which is contain gallic acid, elagic acid, corilagin,
ellagitannin, isoquercetin, quercetin and other antioxidant. Elagic acid (EA) has a function as a free radical
scavenger that can decrease liver function marking enzymes. Use of isonazide can cause side effects such as
an increase in aminotransferase levels that occurs in 10% - 20% of patients several weeks after
consumption. This study aims to determine the effect of Syzygium cumini seed extract in the ALT and AST
values of male rats induced by isoniazid. The research subjects were 30 male white rats strain, weighing
150-200 grams and aged 2-3 months, which were divided into 5 groups. The negative control group was
given aquades, while the positive control was given isoniazid as much as 40 mg on the 12th day until the
25th day. The treatment group was given Syzygium cumini seed extract with multilevel doses (20 mg / rat,
40 mg / rat, and 80 mg / rat) from day 8 to day 25 and isoniazid 40 mg on the 12th day, on the 12
th
, on
26
th
day ALT and AST levels were measured. Data were analyzed using One-Way Anova.The results of the
One-Way Anova test in groups with various doses of extract of jamblang fruit extract (20 mg / rat, 40 mg /
rat, and 80 mg / rat) showed significant results in reducing ALT and AST levels in isoniazid-induced rats,
with p value <0.001 (α = 0.05).The result showed that administration of jamblang fruit extract can reduce
ALT and AST levels of isoniazid-induced mice.
1 INTRODUCTION
The World Health Organization (WHO) mentions
about one third of the world's population or around 2
trillion people with liver disease with a death toll of
1 million. Liver damage can be caused by many
things, as evidenced in previous studies by Prasetyo
(2010) and Kusuma (2010), not only diseases caused
by viruses but can also be from unhealthy lifestyles
such as consumption of foods containing excessive
cooking oil, and consumption alcohol. In addition,
drug consumption can also induce liver damage, for
example anti-tuberculosis drugs. Isoniazid (INH) is
always given to TB cases, the prevalence of TB in
Indonesia is still relatively high, the WHO report in
2010 stated that in 2009 Indonesia's ranking dropped
to fifth with the number of TB sufferers at 292,753
people (WHO, 2010). This shows a considerable
consumption of INH in Indonesia. The use of INH
can cause side effects such as an increase in
aminotransferase levels that occurs in 10% - 20% of
patients several weeks after consumption but does
not cause typical clinical symptoms (
Prasetyo, F. A.,
et a, 2010). Nonetheless 0.1% - 2% of patients
experience acute liver failure (Maddrey,2013). INH
mechanism suspected to cause liver damage can not
be proven with certainty, but hypothetically stated
that the damage was caused by toxic substances in
the form of monoacetylhydrazine (MAH) through
the mechanism of free radicals (oxidative stress)
(
Saukkonen&Jereb, 2012).
Due to its high efficacy, isoniazid (INH) remains
the drug of choice for treatment of latent
tuberculosis (TB) despite the fact that it can cause
liver failure. Although drug-induced liver injury
(DILI) caused by different drugs is somewhat
different , the clinical characteristics of INH-induced
liver injury are fairly typical for idiosyncratic DILI
and include malaise, fatigue, nausea and vomiting.
The duration of therapy before the manifestation of
jaundice can vary between 1–25 weeks with an
average of 12 weeks. Fever Affects on average 20%
Hasibuan, A., Bangun, S., Marbun, R., Siregar, S., S., A. and Octora, D.
Effect of Jamblang (Syzygium cumini) Seed Extract on ALT and AST Levels in Isoniazid-induced Male Rats.
DOI: 10.5220/0009974605430550
In Proceedings of the International Conference on Health Informatics and Medical Application Technology (ICHIMAT 2019), pages 543-550
ISBN: 978-989-758-460-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
543

of the patients and eosinophilia is present in up to
15% of the affected individuals. In most cases, liver
injury is asymptomatic and is only detected by
measuring markers of hepatocyte injury such as
alanine aminotransferase (ALT) and aspartate
aminotransferase (AST). This is especially true for
mild cases of liver injury, which occurs in up to 20%
of patients treated with the drug. However, in most
patients, liver function returns to normal despite
continued treatment with the drug, a phenomenon
referred to as' adaptation 'by hepatologists. Severe
liver injury is seen in up to 1% of the patients.
Elevations in ALT and AST can start as early as 1
week and sometimes as late as 9 months after
starting treatment with INH. However, in more than
half of the patients an ALT increase occurs between
1–6 months. The abrupt increase in ALT that leads
to liver failure is idiosyncratic in nature and is not
clearly related to the duration of treatment, the dose
of the drug, fever or eosinophil count (
Sankhari, et al,
2010).
When liver injury is identified, the first line of
treatment is to stop the drug and monitor the patient
for recovery. In most cases patients recover.
However, the challenge of patients with more severe
liver injury can result in a rapid onset of symptoms
(within hours) and is contraindicated. Histological
characteristics of severe INH-induced liver injury
including hepatocellular injury with multilobular
necrosis and a mononuclear cell infiltrate, which is
generally indistinguishable from viral hepatitis.
Steatosis is unusual in INH-induced liver injury.
However, during active TB treatment, when INH is
given in combination with other agents such as
ethambutol, pyrazinamide and rifampicin (RMP),
there have been reported cases of steatosis and
cholestatic liver injury 7-9. Prolonged treatment
with INH can also lead to a lupus-like autoimmune
reaction with the presence of antinuclear antibodies
hich occurs in up to 20% of patients (
Sankhari, et al,
2010).
INH‐induced liver injury remains a significant
clinical problem. Previous studies suggested that
bioactivation of AcHz was involved in the injury,
but more recent studies point to direct oxidation of
INH as the pathway leading to liver injury. Previous
studies had also suggested that the injury, especially
mild injury, was not immune mediated. However,
recent evidence suggests that INH‐induced liver
injury is indeed immune mediated, but most cases
are mild and resolve with immune tolerance. Severe
injury may include an autoimmune component,
which makes it difficult for patients to recover even
if the drug is stopped, often resulting in liver
transplantation or death. Understanding the
mechanism of INH‐induced liver injury may make it
possible to prevent progression of the injury after the
drug has been stopped. If the injury is immune
mediated, in particular mediated by lymphocytes as
the histology suggests, treatment with agents such as
anti‐thymocyte globulin may be effective (
Metushi &
Phillips, 2016)
However, preventive measures remain a major
concern before the occurrence of severity, including
the use of natural substances hepatoprotectorwith
less side effects. The hepatoprotector substance is
expected to prevent liver damage while reducing the
impact of damage that has already occurred. Of the
various types of medicinal plants that are known to
contain antioxidants, one of the ones that attracts
attention is Syzygium cumini fruit seeds or known in
Indonesia as jamblang fruit. Chemical content of
Syzygium cumini fruit’s seed are gallic acid, elagic
acid, corilagin, ellagitannin, isoquercetin, quercetin,
caffeine acid, ferulic acid, guaiacol,
resorcinaldimethyl ether, lignaglucoside, veratrole,
β-sitosterol, palmitic acid, etc (
Sisodia, 2009).
The plant is well known in many countries due to
its medicinal properties and fruit value. Traditionally,
various parts of the plant were utilized to treat different
ailments of humans and animals (
Thomson, 2000). The
bark has been used for the treatment of sore
throat,bronchitis, asthma, thirst, biliousness, dysentery
and ulcers (
Ayyanar&Subash-Babu, 2012). Leaf juice,
alone or in combination with herbs, goat milk and
honey is reported to be effective in treating diarrhea,
diabetes and stomach-ache (
Nikhat et al, 2008). The
literature review indicated a number of reports
regarding antioxidant activity of extracts, mainly; fruit,
peel, leaves and bark of the plant, using various
models(
Veigas, et al, 2008). The antioxidant activityof
all parts of the plant was attributed to diverse types
ofphytochemical constituents reported previously
(
Banerjee & Narendhirakannan, 2011).
In previous studies, the seeds of this plant can be
used as a drug for diabetes, metrorrhagia, anti-
inflammatory, strengthen teeth and gums and as a
treatment for non-inflamed type Tinea capitis in the
form of a sedion lotion. Elagic acid (EA) has a
function as a free radical decomposition. Elagic acid
has been reported to reduce liver function marker
enzymes on the induction of toxicity with carbon
tetrachloride (CCl4) (
Dalimartha, 2003).
Based on the background the authors wanted to
conduct research to determine the effect of jamblang
seed extract as a hepatoprotector by looking at
decreasing levels of AST and ALT in rats induced
by INH.
ICHIMAT 2019 - International Conference on Health Informatics and Medical Application Technology
544

2 EXPERIMENTAL METHODS
This research used an experimental with post test
only one control groups design.
2.1 Research Sites
Organic chemistry laboratory of the Medical
Institute of Lubuk Pakam Medistra is used for the
screening process of chemical compounds, the
Pharmaceutical Pharmacology Laboratory of the
Medical Institute of Medistra Lubuk Pakam is used
for rat blood collection and the Regional Health
Laboratory for examination of ALT and AST.
2.2 Tools and Materials
The tools used consist of animal balance (GW-
1500), electric balance (Mettler Toledo), laboratory
glassware, mortar and stamfer, surgical tools (Wells
spencer), slide glass, cover glass, parchment paper,
millimeter paper block, watch glass, oral sonde,
dropper and 1 ml syringe (Terumo), microtube,
centrifuge (Velocity 18-R) and microscope
(Olympus). The materials used in this study include
plant, animal and chemical ingredients. Plant
material used is jamblang fruit seeds. The chemicals
used are Na-CMC (sodium carboxy methyl
cellulose), distilled water, sodium chloride 0.9%
(Merck) and EDTA tube. The chemicals used unless
otherwise stated are of technical quality, namely
ethanol (distillation), acetic acid anhydride,
concentrated sulfuric acid, toluene, choral hydrate,
and concentrated hydrochloric acid.
2.3 Ethanol Extract of Jamblang Fruit
Seeds (Syzygium cumini)
The jamblang used should be ripe, with a
blackish purple color on the outside. The seeds are
taken and then dried in the sun. The extract was
obtained by jamblang seeds which were dried,
mashed, and then extracted with ethanol liquid.
Extraction was carried out by the percolation
method. The extraction result is then dissolved with
aquadest plus 0.5% carboxymethyl cellulose (CMC)
and put in a glass bottle stored in the refrigerator.
2.4 Preparation of Experimental
Animals
This research were 30 male white rats (Rattus
norvegicus) strain of Wistar male with a weight of
150-200 grams. Before being approved, the animal
experiment is conditioned for 2 weeks in a good
enclosure to adjust the environment and uniform
food. Large samples of each group are determined
using the Federer formula.
2.5 Sampling Technique
Purposive sampling technique is sampling of the
population is done intentionally according to the
required sample requirements. In purposive
sampling, the characteristics and the number of
samples taken are determined or determined in
advance. Sampling was done by purposive sampling,
with the criteria for selecting subjects based on
characteristics that have been known previously.
Experimental animals were divided into 5 groups,
each group consisting of 6 mice which were
randomly selected. Group 1 as a negative control
group (K-), group 2 as a positive control group (K
+), group 3 as a control treatment for dose 1 (P1),
group 4 as a treatment group for dose 2 (P2), and
group 5 as a treatment group for dose 3 (P3).
2.6 Dose of Jamblang Fruit Seed
Extract
Jamblang seed extract is made by percolation
method. Previously, jamblang seeds were dried,
mashed, and then extracted with 70% ethanol liquid.
The extract is obtained in the form of a solid paste.
Suspension of jamblang fruit seed extract is done by
inserting pasta into the glazing bekker then
weighing, after that it is diluted with distilled water
and added with a suspension agent (CMC 0.5%).
The solution is then homogenized with a manual
stirrer without heating until a suspension is formed.
The weight of the rat used is + 200g (150g - 220g),
then the dose of jamblang seed extract that will be
given to mice is:
a. 100mg / kg body weight / day
= (100mg × 200g) / 1000g / day
= 20 mg / rat / day
b. 200mg / kg body weight / day
= (200mg × 200g) / 1000g / day
= 40 mg / rat / day
c. 400mg / kg body weight / day
= (400mg × 200g) / 1000g / day
= 80 mg / rat / day
Jamblang fruit extract was administered orally
once a day at a dose according to Sisodia and
Bhatnagar (2009) research, 20 mg / rat for the P1
group, 40 mg / rat for the P2 group, and 80 mg / rat
for the P3 group of mice every day starting from the
day 8th to 25th day.
Effect of Jamblang (Syzygium cumini) Seed Extract on ALT and AST Levels in Isoniazid-induced Male Rats
545

2.7 Preparation of Isoniazid
Suspension
Isoniazid (INH) were given in a 300 mg tablet form.
The isoniazid drug tablet were crushed with mortar,
after that it was diluted with distilled water,
homogenized until an isoniazid solution is obtained.
Toxic dose of INH in humans is 30 mg / kg BW.
The conversion factor for humans weighing 70 kg in
mice weighing 200 g is 0.018.
a. Doses in humans weighing 70 kg 30 mg x 70 kg
= 2100 mg / human
b. Conversion in mice weighing 200 g 2100 mg x
0.018 = 37.8 mg / rat. Rounding (40 mg / rat)
2.8 Experimental Procedures
The experimental animals consisted of 30 male
white rats which were divided into 5 groups:
a. Negative control (K-): aquades 1ml / oral rat
b. Treatment 1 (P1): EEBJ 20mg / kg bw
c. Treatment 2 (P2): EEBJ 40mg / kg bw.
d. Treatment 3 (P3): EEBJ 80 mg / kg bw.
e. Positive control (K +): given INH on the 12th day
until the 25th day.
After weighing and determining the dose is
completed then on the eighth day treatment of
experimental animals began. The negative control
group was given aquabides 1 ml orally per rat,
treatment group 1 was given jamblang seed extract
20 mg / rat on the 8th day to the 25th day. Also
given INH on the 12th day until the 25th day. So
that starting on day 12 in one day the rats get
jamblang seed extract jamblang fruit seed extract is
given 1 hour before INH. Treatment group 2 was
given extract of jamblang seeds 40 mg / rat on the
8th day until the 25th day. Also given INH on the
12th day until the 25th day. Starting the 12th day,
jamblang fruit seed extract was given 1 hour before
INH. Treatment group 3 was given 80 mg jamblang
seed extract / rat on the 8th day until the 25th day.
Also given INH on the 12th day until the 25th day.
Starting the 12th day, jamblang seed extract was
given 1 hour before INH. The positive control group
was given INH on the 12th day to the 25th day.
Outside the treatment schedule the rats were given
food pellets and distilled water ad libitum
The Ethanolic Extract of Jamblang Seeds (EEJS)
was given on the 8th day until the 25th day. INH is
given on the 12th day until the 25th day, giving
jamblang seed extract is done 1 hour before INH. On
the 26th day rat blood was taken to measure ALT
and AST levels.
2.9 Measurement of ALT and AST
levels
ALT and AST levels were examined using a
spectrophotomester conducted at the North Sumatra
Provincial Health Laboratory. Blood is drawn from
the heart and arteries as much as 0.5 ml of blood is
inserted into the microtube, allowed to stand at room
temperature for 5 minutes, centrifuged for 10
minutes at a speed of 3000 rpm to produce a clear
serum. Serum was separated and AST and ALT
levels were measured.
2.10 Data Analysis
The data were analysis for normality using the
Shapiro-Wilk test because the sample size was ≤ 50.
Then the variance test was also performed using the
Levene's test. Hypotheses were tested using the One-
Way Anova (Analysis of Variance) test to find out
the existence of mean differences in the five
treatment groups.
3 DISCUSSION
3.1 The Results of Phytochemical
Screening
The results of phytochemical screening of
Jamblang seed ethanol extract showed the content of
alkaloids, flavonoids, quinones, polyphenols,
tannins, and steroids / triterpenoids groups that could
be seen in table 1.
Table 1: Phytochemical Screening Results of Ethanolic
Extract of Jamblang Seed
Compound Group Result
Alkaloids
+
Flavonoids
+
Saponin
-
Quinon
+
Polyphenols
+
Tanins
+
Steroids/ Triterpenoids
+
Notes:
+ :Contains the class of examination compounds
- : Did not contain the class of examination
compounds
The seeds are reported to contain jamboline,
traces of pale yellow essential oil, chlorophyll, fat,
ICHIMAT 2019 - International Conference on Health Informatics and Medical Application Technology
546

resin, albumen, tannins, phenolic compounds such
as ellagic acid, gallic acid, caffeic and ferulic acids
and their derivativesand flavonoids like rutin and
quercetin (
Charles River Laboratories, 2008). Based on
such constituents, seed extracts are expected to
possess excellent astringent and antioxidant
potential, which may be beneficial in relieving
gastroenteritis and liver inflammation.
3.2 Measurement Results of ALT
(Alanin Aminotransferase) and
AST (Aspartate Aminotransferase)
Levels
AST and ALT both are vital transaminase enzymes
and play central role in amino acid metabolism.
Both of these are found in the different body’s
organs such as liver, heart, skeletal muscle, kidneys,
brain, and red blood cells
(Agrawal, 2013). Serum
AST and ALT level, and their ratio (AST/ALT ratio)
are frequently measured clinically as biomarkers for
liver health. Their increased level has been linked
with abnormal liver functions, though these are not
very specific to liver disease. Though, the toxin
treated animals showed increased levels of these
enzymes. Conversely, extract therapies attenuated
the increased level of these enzymes in serum.
Recovery towards the normalization suggests that
these extracts caused parenchymal cell regeneration
in liver, thus protecting membrane fragility and
thereby decreasing enzyme leakage (
Achliya, et al,
2004).
The results of measurements of ALT and AST
levels could be seen on table 2 and table 3 below.
Table 2. Measurement Results of ALT
Goup
Level of
AST(IU/L) ± SD
Negative control 50,90±6,65
EEJS20mg/kg bb 50,80±6,49*
EEJS40 mg/kg bb 48,80±4,14*
EEJS 80 mg/kg bb 45.25±4.78*
Positive control 71,20±10,20
Note:
* = p< 0,05, significant difference with positive
control group
The results of statistical analysis byone way
ANOVA showed that there was a significant
difference (p <0.05) between mice given EEBJ and
an isonazide positive control group. This shows that
the administration of EEBJ has an effect of
decreasing the ALT value on isoniazid-induced test
animals. Based on Charles River Laboratories
(2008) the normal ALT value for white mice is 14-
64 IU / L.
Table 3. Measurement Results of AST
Group
Levels of
AST(IU/L) ± SD
Negative control 207,80±4,55
EEJS 20mg/kg bb 246,00±2,55*
EEJS 40 mg/kg bb 216,80±2,28*
EEJS 80 mg/kg bb 211,75±2,98*
Positive control 246,00±8,33*
The results of statistical analysis using one way
ANOVA showed that there was a significant
difference (p <0.05) between mice given EEBJ and
an isonazide positive control group. This shows that
the administration of EEBJ has an effect of
decreasing AST value on isoniazid-induced test
animals.
Based on Charles River Laboratories (2008) the
normal AST value for white rats is 64-222 IU / L.
The INH used here, is actually an antibiotic drug
but also known to cause oxidative injury particularly
in liver cells(
Achliya, et al, 2004).
INH is a hydrazide that is readily oxidized 11.
Three metabolites have been proposed to be
responsible for INH‐induced liver injury, acetyl
hydrazine (AcHz), hydrazine (Hz) and more recently
a metabolite resulting from the bioactivation of INH
itself.Experiments implicating AcHz and Hz as
hepatotoxic species were performed several decades
ago, mostly in rats where the acute liver injury
correlated with covalent binding of AcHz and with
blood levels of Hz 12. At the time, the parent drug
(INH) was not thought to contribute to liver injury
because its administration did not produce severe
liver injury. However, these experiments utilized
ring‐labelled acetylisoniazid (AcINH) (
Meng, 2015).
This conclusion was not warranted because the
drug that was administered was not INH. It was
AcINH in which the hydrazine is blocked. If
hydrolysis led to AcHz and isonicotinic acid, no
covalent binding of the pyridine ring would occur.
In addition, the characteristics of the liver toxicity in
these studies were different from that in humans. In
particular, it was an acute rather than a delayed onset
idiosyncratic liver injury. Furthermore, the
metabolism in humans may be different from that in
rats. However, the conclusion that direct
bioactivation of INH does not occur has persisted
(
Metushi, 2011). Recently, a reactive metabolite
resulting from bioactivation of INH itself has been
shown to form covalent adducts to liver
Effect of Jamblang (Syzygium cumini) Seed Extract on ALT and AST Levels in Isoniazid-induced Male Rats
547
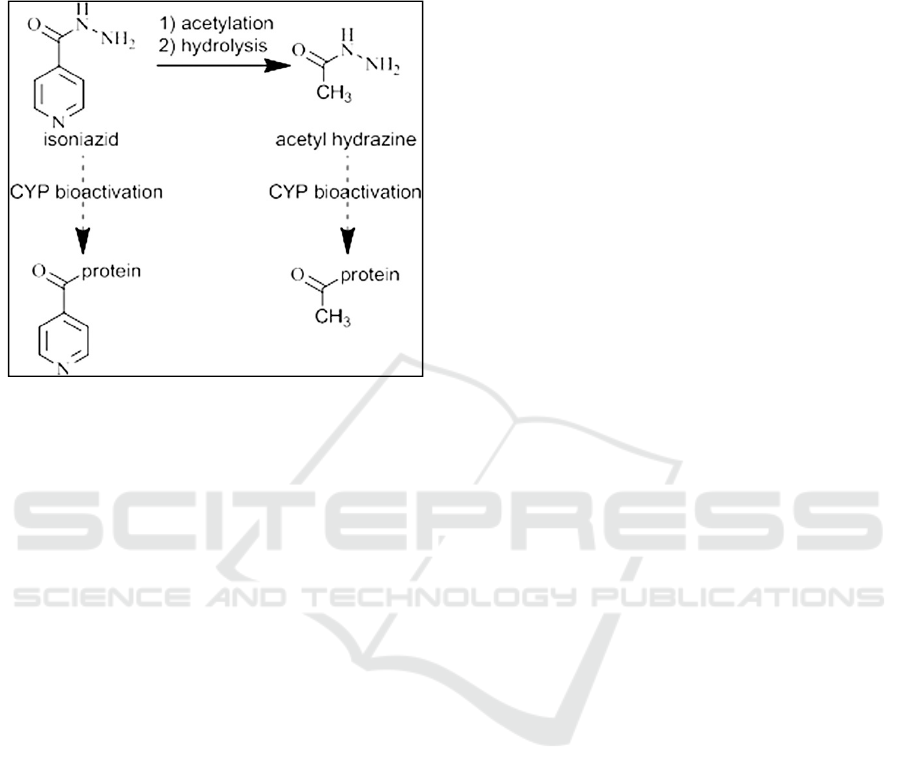
macromolecules 13-17. Covalent binding of this
metabolite is more likely to lead to an immune
response than the reactive metabolite of AcHz which
would only acetylate proteins (Figure 1).
Figure 1. Proposed pathway for an immune mediated
reaction to INH in the liver.
Using western blots and mass spectrometry, it
has been shown that the reactive metabolite of INH
can react with multiple lysine residues on hepatic
proteins. Further characterization using mass
spectrometry revealed drug adducts on
D‐dopachrome decarboxylase, prohibitin 2 and
macrophage migration inhibitory factor 18.
Autooxidation of INH involving free radicals has
also been reported. It is unclear whether this is
significant in vivo where there are many antioxidant
systems.Moreover, INH produces hydrazine
metabolites (nitrogen free radicals) after
metabolism. These reactive free radicals act as
stimulator of lipid peroxidation resulting in cell
death and hepatic necrosis. In this investigation also,
INH treatment showed considerable liver injury.
Which was resembles with earlier investigations
(
Meng, 2015).
The administration of ethanolic extract of seeds
of the plant in two doses lowered the level of
biochemical markers, which were increased by free
radicals of INH. It is probable that the
administration of extract for 14 days increased the
antioxidant capacity of animal to scavenge the free
radicals generated by INH. The free radical
scavenging activity of the plant, under investigation,
has been attributed to the presence of flavonoids and
related compounds. The plant also contained ellagic
acid, a polyphenol having lipid peroxidation
inhibition activity.
The ethanol extracts of E. jambolana seeds
showed hepatoprotective effects in carbon
tetrachloridetreated rats. In addition, another study
has reported on the hepatoprotective and antioxidant
activity of E. jambolana seeds. Hepatoprotective
effects are attributed to its antioxidant activity,
which restores the activity of superoxide dismutase,
catalase, and glutathione peroxidase to normal
levels, and increases glutathione content and levels
of lipid peroxidation and hydroperoxides in the liver.
Seed content includes glycosides, traces of pale
yellow essential oil, fat, resin, albumin, chlorophyll,
the alkaloid jambosine, gallic acid, ellagic acid,
corilagin and related tannins, 3,6-
hexahydroxydiphenoyl glucose and its isomer 4,6-
hexahydroxydiphenoyl glucose, 1-galloyl glucose,
3-galloyl glucose, quercetin, and elements such as
zinc, chromium, vanadium, potassium, and sodium.
Unsaponifiable matter of the seed fat contains b-
sitoterol. Dried seeds of E. jambolana have been
reported, with 11.67% alcohol-soluble extractive
fiber, 3.397% inorganic fiber, 40% water-soluble
gummy fiber, and 15% water-insoluble neutral
detergent fiber. Kumar and colleagues (2009) state
that the ethyl acetate and methanol extracts of the
seeds of S. cumini show the presence of alkaloids,
amino acids, flavonoids, glycosides, phytosterols,
saponins, steroids, tannins, and triterpenoids. Since
the current worldwide morbidity and mortality due
to liver disease is increasing every year, with
corresponding increases in expenditure for drug
treatment, alternative plant therapies may be
beneficial.
Presumably, treatment with ethanolic extracts of
jamblangcan modulate liver cytochrome P-450
enzymes to enhance scavenging of hepatotoxic free
radicals and by increasing antioxidant defense
activities.
4 CONCLUSIONS
Based on the results of the study it can be
concluded that the induction of isoniazid (INH) can
increase levels of ALT and AST, giving EEBJ
20mg, 40mg and 80mg / kg bw in male white rats
which induced by isoniazid gave the effect of
decreasing ALT and AST levels. A moderate to high
dose increase (80 mg / rat / day) did not increased
the effect of increasing ALT and AST due to
INHinduction. So that the use of EEJS was expected
to be developed as an herbal hepatoprotector
product.
ICHIMAT 2019 - International Conference on Health Informatics and Medical Application Technology
548

ACKNOWLEDGEMENT
Thank you to the Medistra Foundation for the
laboratory facilities provided for this study.
REFERENCES
World Health Organization. (2010). World healthstatistics
2010. World Health Organization.
Prasetyo, F. A., Vijaganita, L., Purnami, L. P. S., &
Kusuma, W. (2010). Efek Spider Silk Protein (SSP)
TetragnathaJavanaTerhadap CTBT Dan APTT Pada
Tikus Yang Diinduksi Oleh Heparin Sulfat. Penelitian
PKM, UniversitasSebelasMaret: Solo.
Maddrey, W. C. (2013). Clinical manifestations and
management of drug-induced liver diseases. In Drug-
Induced Liver Disease (pp. 229-240). Academic Press.
Saukkonen, J. J., Powell, K., &Jereb, J. A. (2012).
Monitoring for tuberculosis drug hepatotoxicity:
moving from opinion to evidence.
Sisodia SS, Bhatnagar M. (2009).Hepatoprotective
activity of Eugenia jambolana Lam. in carbon
tetrachloride treated rats. Indian J Pharmacol; 41: 23-
7.
Dalimartha S. (2003). Atlas TumbuhanObat Indonesia
Jilid 3, PuspaSwara, Jakarta.
Jadhav VM, SS Kamble and VJ Kadam, 2009. Herbal
medicine: Syzygium cumini: A Review. J Pharm Res,
2: 1212-1219
Williamson EM, 2002. Major Herbs of Ayurveda.
Churchill Livingstone, China, pp: 279-282.
Sharma B, C Balomajumder and P Roy, 2008.
Hypoglycemic and hypolipidemic effects of flavonoid
rich extract from Eugenia jambolana seeds on
streptozotocin induced diabetic rats. Food Chem
Toxicol, 46: 2376-2383.
Charles River Laboratories. (2008). Clinical Laboratory
Parameters for Crl: (WI) BR Rats. Ballavardvale
Street: Spring.Hal 14.
Agrawal J, Kar A. Synergistic action of phytochemicals
augments their antioxidative efficacy: an in vitro
comparative Study. Asian J Pharmac Clin Res 2013;
6:121-6.
Sarkar S, Chakraverty R, Datta S, Ghosh A. In- vitro
assays for neutralization of snake venom using herbal
drugs: a review. J Crit Rev 2015;3:30-3.
Dharmalingam K, Stalin R, Sachidanandam P, Shanthi P.
Chemotherapeutic efficacy of tridham and 1,2,3,4,6-
penta-o-galloyl-β-dglucose on antioxidants status and
tumor markers in experimental mammary carcinoma
in sprague- dawley rats. Asian J Pharm Clin Res 2016;
9:202- 8
Achliya GS, Wadodkar SG, Dorle AK. Evaluation of
hepatoprotective effect of AmalkadiGhrita against
carbon tetrachloride-induced hepatic damage in rats. J
Ethnopharmacol 2004; 90:229– 32.
Al-Sayed E, Abdel-Daim MM, Kilany OE, Karonen M,
Sinkkonen J. Protective role of polyphenols from
Bauhinia hookeri against carbontetrachloride-induced
hepato- and nephrotoxicity in mice. Ren Fail 2015;
37:1198- 207.
Karavadi B, Suresh MX. Receptor identification and lead
molecular discovery of phage encoded protein in
tch8431/19a strain of streptococcus pneumoniae: a
computational approach. Int J App Pharm 2016; 6:6-
10.
Moresco RN, RL Sperotto, AS Bernardi, RF Cardoso and
P Gomes, 2007. Effect of the aqueous extract of
Syzygium cumini on carbon tetrachlorideinduced
hepatotoxicity in rats. Phytother Res, 21: 793-795.
Abalea V, J Cillard, MP Dubos, O Sergent, P Cillard and I
Morel, 1999. Repair of iron-induced DNA oxidation
by the flavonoid myricetin in primary rat hepatocyte
cultures. Free Radic Biol Med, 26: 1457- 1466.
Thresiamma KC, J George and R Kuttan, 1996. Protective
effect of curcumin, ellagic acid and bixin on radiation
induced toxicity. Indian J Exp Biol, 34: 845-847.
Vishnu KKS, MN Palaksha, K Venkatesh, KY Sandip and
RR Naik, 2013. Antioxidant and hepatoprotective
effects of methanolic extract of Origanummajorana in
CCl4 induced liver injury in rats. Int J Pharm Pharm
Sci, 2: 5898-5912.
Thomson, 2000. PDR for Herbal Medicines. 2nd Ed,
Medical EconomicsCo Inc, Montvale, New Jersey,
USA, pp: 429-430.
Khare CP, 2004. Encyclopedia of Indian Medicinal Plants.
Springer-Verlag, New York, pp: 207-208.
Ayyanar M and P Subash-Babu, 2012. Syzygium
cumini(L.) Skeels: A review of its phytochemical
constituents and traditional uses.Asian Pac J Trop
Biomed, 2: 240-246.
Nikhat F, D Satynarayanaa and BJ Arun, 2008.
Phytochemical and pharmacological investigation of
roots of Syzygium cumini(L) skeel. Asian J Res Chem,
1: 22-25.
Nikhat F, D Satynarayana and EVS Subhramanyam, 2009.
Isolation, characterization and screening of antioxidant
activity of the roots of Syzygium cumini(L) skeel.
Asian J Res Chem, 2: 218-221.
Banerjee A, N Dasgupta and B De, 2005. In vitro study of
antioxidantactivity of Syzygium cuminifruit. Food
Chem, 90: 727-733.
Banerjee J and RT Narendhirakannan, 2011.
Phytochemical analyses,antibacterial, in vitro
antioxidant and cytotoxic activities ofethanolic extract
of Syzygium cumini(L.) seed extract. Int J PharmSci
Res, 2: 1799-1806.
Veigas JM, R Shrivasthava and B Neelwarne, 2008.
Efficient ameliorationof carbon tetrachloride induced
toxicity in isolated rathepatocytes by Syzygium
cuminiskeels extract. Toxicol In Vitro,22: 1440-1446.
Metushi IG, Cai P, Zhu X, Nakagawa T, Uetrecht JP. A
fresh look at the mechanism of isoniazidinduced
hepatotoxicity. Clin PharmacolTher 2011; 89: 911– 4.
Meng X, Maggs JL, Usui T, Whitaker P, Ns F, Dj N, Bk
P. Autooxidation of Isoniazid Leads to
IsonicotinicLysine Adducts on Human Serum
Albumin. Chem Res Toxicol 2015; 28: 51– 8.
Effect of Jamblang (Syzygium cumini) Seed Extract on ALT and AST Levels in Isoniazid-induced Male Rats
549

Sankhari, J. M., Jadeja, R. N., Thounaojam, M. C.,
Devkar, R. V., & Ramachandran, A. V. (2010). Safety
evaluation of Eugenia jambolana seed extract. Asian
Pacific Journal of Tropical Medicine, 3(12), 982-987.
Metushi, I., Uetrecht, J., & Phillips, E. (2016). Mechanism
of isoniazidinduced hepatotoxicity: then and
now. British journal of clinical pharmacology, 81(6),
1030-1036.
El-Shenawy, S. M. A. (2011). Biological Activities of
Eugenia jambolana (Family Myrtaceae) Seeds. In Nuts
and Seeds in Health and Disease Prevention (pp. 685-
691). Academic Press.
ICHIMAT 2019 - International Conference on Health Informatics and Medical Application Technology
550
