
Antimicrobial Label from Lemongrass Oil Incorporated with
Chitosan/Ascorbic Acid
Retno Yunilawati
1
, Windri Handayani
2
, Agustina Arianita C.
3
, Bunda Amalia
3
and Cuk Imawan
*1
1
Departemen Fisika, Fakultas Matematika dan Ilmu Pengetahuan Alam (FMIPA), Universitas Indonesia, Depok
16424, Indonesia
2
Departemen Biologi, Fakultas Matematika dan Ilmu Pengetahuan Alam (FMIPA), Universitas Indonesia, Depo
k
16424, Indonesia
3
Badan Penelitian dan Pengembangan Industri, Kementerian Perindustrian, Indonesia
Keywords: Antimicrobial Label, Lemongrass Oil, Chitosan, Ascorbic Acid
Abstract: Lemongrass oil is one of the essential oil which potential to be used as an antimicrobial agent in active
packaging. The aim of this research is to prepare antimicrobial labels and assess their activity. Antimicrobial
labels are made from a matrix of chitosan/ascorbic acid and lemongrass oil as active ingredients with various
concentrations ranging from 1% to 10%. Lemongrass oil was characterized using Gas Chromatography-Mass
Spectrometry (GC-MS) to determine compounds suspected of having antimicrobial activity. The GCMS
chromatograms have shown that lemongrass oil contains 73.21% citral compounds composed of 29% neral
(beta-citral) and 44.21% geranial (alpha-citral) as antimicrobial agents. Lemongrass oil was tested on Gram-
positive bacteria Staphylococcus aureus and Gram-negative bacteria Escherichia coli using direct method and
vapor method. This test has shown that lemongrass oil has antimicrobial activity in both bacteria. The labels
provide optimum antimicrobial activity for the lemongrass oil concentration of 10%. These results conclude
that the lemongrass oil incorporated with chitosan/ascorbic acid has the potential to be an active packaging.
The abstract should summarize the contents of the paper and should contain at least 70 and at most 200 words.
It should be set in 9-point font size, justified and should have a hanging indent of 2-centimenter. There should
be a space before of 12-point and after of 30-point.
1 INTRODUCTION
Antimicrobial label is one form of active packaging
application, where the packaging made with the aim
to maintain the quality of the material it is packaged.
Antimicrobial labels are made by combining
antimicrobial materials into a polymer. Essential oil
has been widely used as an antimicrobial agent
considering its safe, natural, environmentally
friendly, and has a broad spectrum. One of the
essential oils is lemongrass oil. Lemongrass oil
contains several compounds such as neral and
geranial which can function as antimicrobials
(Argyropoulou et al., 2007).
In this research lemongrass oil is incorporated
with chitosan which is a biodegradable polymer
forming an antimicrobial label. Chitosan is a polymer
that insoluble in neutral pH, but soluble in acidic
environment, such as acetic acid, formic acid, and
hydrochloric acid. Acetic acid has an unpleasant and
pungent odor that can later affect food products in the
label application. Likewise, formic acid and
hydrochloric acid which has a pungent odor and can
penetrate (Ozdemir Kubra S, 2017). Therefore, this
research uses ascorbic acid as an alternative to
chitosan, which has safer than acetic acid and
hydrochloric acid.
This research aims to prepare antimicrobial label
using lemongrass oil incorporate with
chitosan/ascorbic acid and investigate their
antimicrobial activity
2 MATERIALS AND METHODS
2.1 Materials
Lemongrass oil was used in this experiment obtained
from Nusaroma, a local essential oils company in
Indonesia. The chemical materials used in this
Yunilawati, R., Handayani, W., Arianita C., A., Amalia, B. and Imawan, C.
Antimicrobial Label from Lemongrass Oil Incorporated with Chitosan/Ascorbic Acid.
DOI: 10.5220/0009968501470152
In Proceedings of the 2nd International Conference of Essential Oils (ICEO 2019), pages 147-152
ISBN: 978-989-758-456-5
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
147

experiment were ascorbic acid (Merck), chitosan in
powder form (PT. Biokitosan Indonesia), and tween
80.
2.2 Methods
2.2.1 Lemongrass Oil Characterization
Lemongrass oil compounds were identified by gas
chromatography with a mass spectrometer detector
(GC-MS) Agilent 6890 series with capillary column
HP-5MS, 30 m x 0.25 mm id x 0.25 µm film
thickness. Helium gas was used as the carrier gas at
constant pressure of 65 kPa. The lemongrass oil was
injected with a volume of 1 µL in split ratio of 1:25.
The increasing of oven temperature was programmed
from 60-240°C with step of 3°C per minute until
reaching 240°C.
2.2.2 Antimicrobial Assay of Lemongrass
Oil
Direct Contact Agar Diffusion Tests.
This
method used the method carried out by (Handayani et
al., 2019). The antimicrobial activities determined by
the paper disc diffusion method using type strain of
Staphylococcus aureus NBRC 100910 and
Escherichia coli NBRC 3301 in The Mueller Hinton
Agar. 10 ml of molten media poured into sterile Petri
plates (d=90 mm) and allowed to solidify for 5
minutes. After that, in a tube, 10 µl of bacteria culture
10-6 CFU/mL added with 10 ml of medium and
mixed gently with the inoculate before poured on the
top of molten media before and allowed to dry for 5
minutes. The negative control (sterile distilled
water), positive control (tetracycline 15 µg/mL),
lemongrass oil with concentration 1000 µg/mL
loaded on 6 mm disc, whereas the volume for each
disc was 10 µl. The loaded disc placed on the surface
of the medium then incubates at 35°C for 18 hours.
After the end of incubation, a clear zone formed
around the disc was measured.
Vapor Phase Agar Diffusion Test. This vapor
method used the method carried out by (Wang et al.,
2016). The vapor phase agar diffusion test was
technically similar to the direct contact diffusion test.
However, the filter discs were placed at the top in
centre of the inner side of the Petri dish cover. The
dishes were then sealed using laboratory parafilm to
avoid evaporation of the test compounds, followed by
incubation at 37°C for 24 h. The diameter of the
inhibition zone was recorded.
2.2.3 Antimicrobial Labels Preparation
The chitosan solution was prepared by dissolving 2 g
of chitosan powder into 100 mL of 1% (w/v) ascorbic
acid and stirring at 200 rpm for 2 h at 50 °C using a
magnetic stirrer. The antimicrobial label was
prepared by mixing lemongrass oil with 30 mL of the
chitosan solution in four different concentrations (1
% v/v, 3%v/v, 5% v/v and 10% v/v) with the added
of tween 80 as surfactant (0.2% v/v) and stirring the
resultant mixture for 10 min at room temperature
using a magnetic stirrer. The label solution was
poured onto a 10 × 15 cm acrylic board and left for
48 h at room temperature to form the film.
2.2.4 Antimicrobial Labels Characterization
A uv vis spectrophotometer (Shimadzu UV-2450)
was used to measure the reflectance of the chitosan
label and lemongrass-chitosan labels. A Fourier
Transform Infrared (FTIR) spectra were collected for
the chitosan label dan the lemongrass-chitosan labels
using a double-beam spectrophotometer (Thermo
Nicolet iS5) to determine the functional group
2.2.5 Antimicrobial Assay of Labels
The antimicrobial activities of labels were tested in
direct contact agar diffusion test and vapor phase agar
diffusion test. Labels are cut in a circle with a
diameter of 6 mm and then placed in a petri dish to
test antimicrobial activity with the technique as
described previously.
3 RESULTS AND DISCUSSION
3.1 Chemical Compounds of Lemongrass
Oil
Characterization using GC-MS showed the
chromatogram profile detected 6 peaks in lemongrass
oil (Figure 1) which indicated there were 6
compounds in lemongrass oil. The compounds were
identified based on comparison of mass spectrum
ICEO 2019 - 2nd International Conference of Essential Oil Indonesia
148
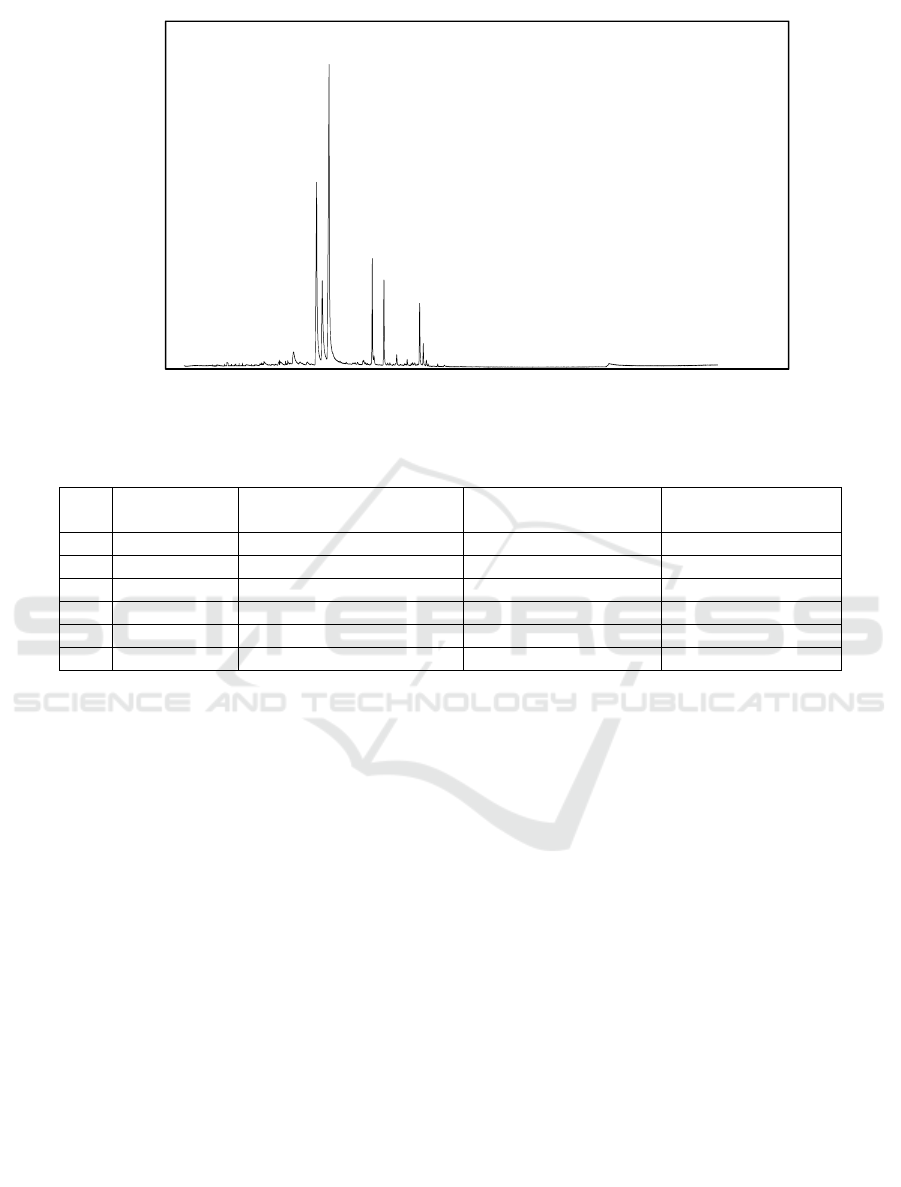
Figure 1: GCMS chromatogram of lemongrass oil
Table 1: Chemical compound identified of lemongrass oil with GC-MS
No Retention
time
Identified compound Molecular formula Relative percentage
area (%)
1 17.101 Neral (beta-citral) C
10
H
16
O 29.00
2 17.753 Geraniol C
10
H
18
O 10.80
3 18.524 Geranial (Alpha citral) C
10
H
16
O 44.21
4 23.302 Geranyl acetate C
12
H
20
O
2
6.50
5 24.588 Beta-caryophyllene C
15
H
24
5.67
6 28.589 Gamma-cadinene C
15
H
24
3.83
with reference data from the database (Wiley 7).
Based on this, lemongrass oil was known contain 6
compounds, namely neral (beta-citral), geraniol,
geranial (alpha-citral), geranyl acetate, beta-
caryophyllene and gamma cadinene (Table 1) with
the main compounds being citral and geraniol. These
results appropriated with previous finding reported in
literature, citral and geraniol has been described as the
main compounds of lemongrass oil (Ganjewala,
2009). Citral as the major component of lemongrass
oil present at level of approximately 65%-85%
(Saddiq and Khayyat, 2010). The content of citral in
this research was 73.21%.
Citral (3,7 dimethyl-2-6-octadienal) is mixture of
two isomers geometric, neral (beta-citral) and
geranial (alpha-citral) which are monoterpene
aldehyde. Citral has an activity antibacterial against
Gram-positive bacteria and Gram-negative bacteria,
both on oil form and vapor form (Argyropoulou et al.,
2007) Geraniol (3,7-dimethyl-octa-trans-2,6-dien-1-
ol) is an acyclic monoterpene alcohol with the
chemical formula C
10
H
18
O (Ternus ZR, 2015).
Geraniol is reported to have activity against several
pathogenic bacteria (Ternus ZR, 2015). The aldehyde
groups in citral and alcohol groups in geraniol that
play a role in antibacterial activity. Aldehydes,
phenols, esters, oxygenated terpenoids, ketones, and
amines are the principle components responsible for
the antimicrobial activity of essential oil (Ju et al.,
2019).
3.2 Antimicrobial Activity of
Lemongrass Oil
The result of antimicrobial assay showed that the
clear zone was formed in positive control and sample
(lemongrass oil) both in Gram-positive Bacteria S.
aureus and Gram-negative bacteria E. coli (Figure 2).
The diameter of clear zone/inhibition zone in S.
aureus is lower than in E. coli (Table 2). Generally,
essential oils are more active in Gram-positive
bacteria than in Gram-negative bacteria
(Bhavaniramya et al., 2019) (Huang et al., 2014).
Gram-negative bacteria have a rigid outer membrane,
composed of a double layer of phospholipids
(lipopolysaccharide),
0
2000000
4000000
6000000
8000000
10000000
12000000
14000000
16000000
0 10203040506070
Abundance
Time(minutes)
Antimicrobial Label from Lemongrass Oil Incorporated with Chitosan/Ascorbic Acid
149
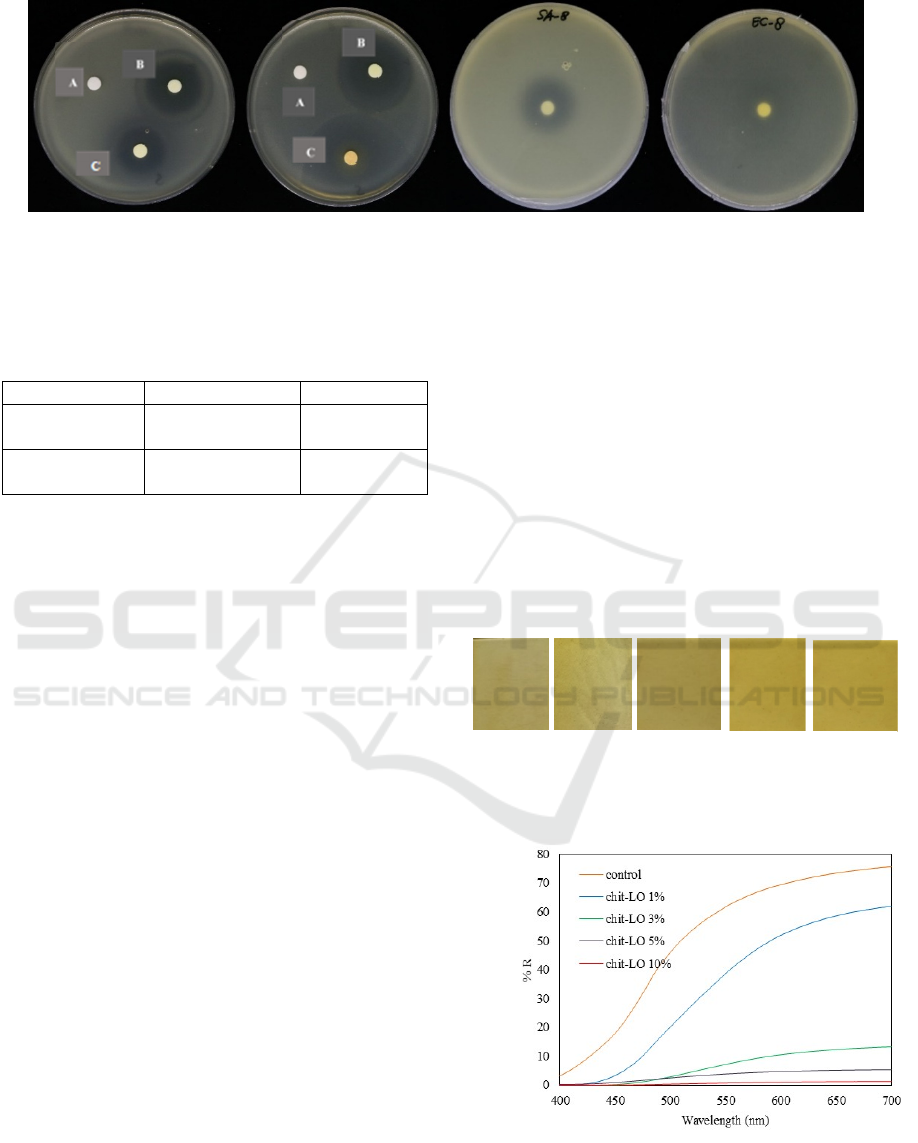
Figure 2: Antimicrobial activities of lemongrass oil against Gram-positive bacteria S. aureus and Gram-negative
bacteria E. coli; A = negative control; B=positive control; C=sample
Tabel 2: Diameter of Inhibition zone of lemongrass oil
Samples S. aureus (mm) E. coli (mm)
Lemongrass
oil
25 47
Lemongrass
oil (vapor)
22 36
thereby limiting the diffusion of hydrophobic
compounds through it. In this experiment, lemongrass
oil is more active in Gram-negative bacteria E. coli,
contrary to that statement. The antimicrobial activity
of essential oil is influenced by many factors, such as
the respective composition of the essential oils, the
structural configuration of the constituent
components, their functional groups and possible
synergistic interactions between components
(Dorman and Deans, 2000). The lemongrass oil has
two functional groups (aldehydes and alcohol) which
expected have synergistic interactions in
antimicrobial activity.
The antimicrobial activity of lemongrass oil in
vapor form was lower compare with in oil form.
Some experiments have indicate that lemongrass oil
in vapor phase is more effective than in the liquid
phase (Tyagi and Malik, 2010) (Hyun et al., 2015),
contrary with this experiment. It can be explained that
the antimicrobial activity in vapor contact was
influence by the concentration of vapor, and the major
constituent (Inouye, Takizawa and Yamaguchi,
2001).
3.3 Labels Characterization
The antimicrobial labels made of lemongrass oil and
chitosan/ascorbic acid were shown at Figure 3. The
colour of control label (chitosan/ascorbic acid
without lemongrass oil) was yellowish and the label
was transparent. When lemongrass incorporated in
matrix, the more lemongrass oil was added, the label
was opaquer and less transparent. The transparency of
the label was optically expressed as a reflectance and
determined using a UV spectrophotometer. The
reflectance of each label was shown in Figure 4. The
reflectance value decreases with increasing
concentration of lemongrass oil.
FTIR spectroscopy was performed to explore the
intermolecular interaction between lemongrass oil
and chitosan. The FTIR spectra of the control
(chitosan/ascorbic acid matrix) along with the
lemongrass oil incorporated chitosan/ascorbic acid
are shown in Fig.5.
Figure 3: Antimicrobial label from lemongrass oil
incorporated with chitosan/ascorbic acid
Figure 4: Reflectance spectra of antimicrobial labels
control LO LO 3% LO 5%
LO 10%
S.aureus E.coli
S.aureus
E.coli
direc
t
va
p
o
r
ICEO 2019 - 2nd International Conference of Essential Oil Indonesia
150
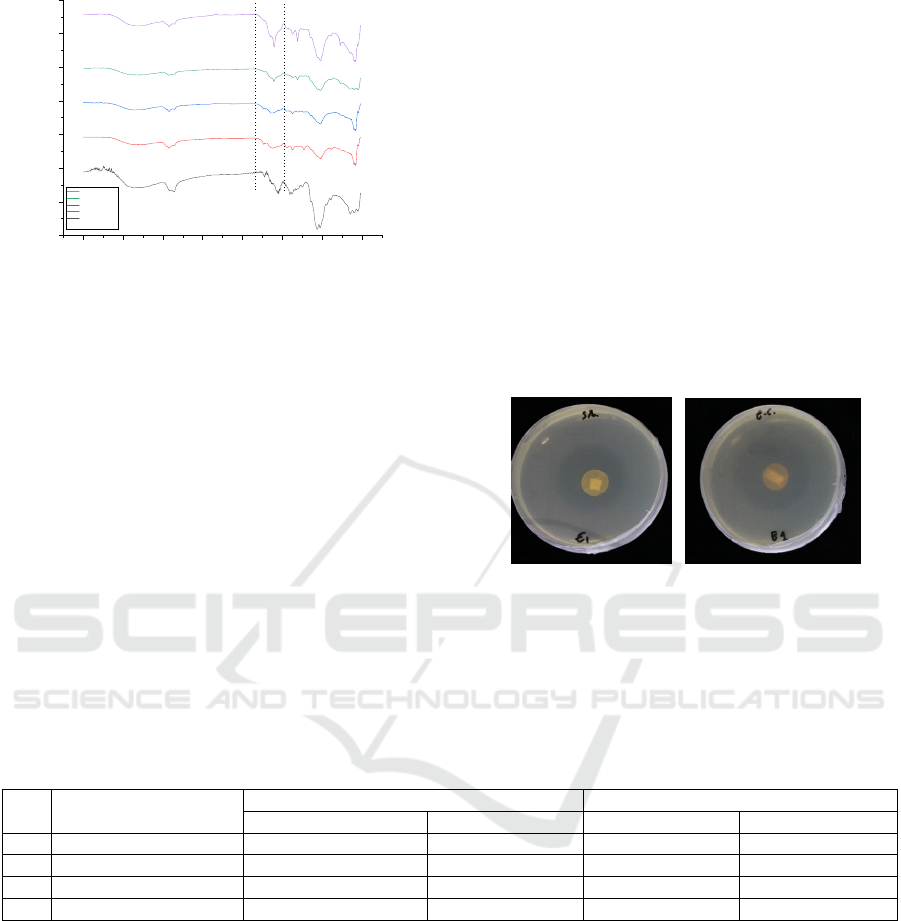
4000 3500 3000 2500 2000 1500 1000 500
%T
Wavenumber (cm-1)
Chit + LO 10%
Chit + LO 5%
Chit + LO 3%
Chit + LO 1%
Control
Figure 5: FTIR spectra of chitosan and all of the labels
FTIR spectroscopy was performed to
explore the intermolecular interaction between
lemongrass oil and chitosan. The FTIR spectra of the
control (chitosan/ascorbic acid matrix) along with the
lemongrass oil incorporated chitosan/ascorbic acid
are shown in Fig.5. The FTIR spectra of chitosan and
all of the labels gave a broad peak in the range of
3200–3500 cm
-1
indicate the stretching vibration of
hydroxyl group (O-H) (Zhang et al., 2018). When the
lemongrass oil was incorporated into
chitosan/ascorbic acid, the major peak of the infrared
spectrum did not change very much, suggested that
there was no significant change in the
chitosan/ascorbic acid. There were no significant
changes was due the lemongrass oil didn’t form
covalent bonding with chitosan. These results were
appropriate with several previous studies that used
chitosan as a matrix for essential oils (Gursoy et al.,
2018) (Li et al., 2019). However, there was the peak
in 1722 cm
-1
in the labels indicating the presence of
citral, the major component of lemongrass oil
(Natrajan et al., 2015). The intensity of this peak was
greater when more lemongrass oil was added.
3.4 Antimicrobial Activity of the
Labels
The antimicrobial test results from the label showed
that the label has antimicrobial activity on the
lemongrass oil concentration was 10% as
summarized in the diameter of inhibition zone were
shown in Tabel 5. The clear zone/inhibition zone was
formed both in Gram-positive bacteria S. aureus and
Gram-negative bacteria E. coli (Figure 6).
Figure 6: Antimicrobial activities of antimicrobial labels
with lemongrass oil concentration 10% against Gram-
positive bacteria S. aureus (a) and Gram-negative bacteria
E. coli (b)
Tabel 3: Diameter of inhibition zone of the antimicrobial labels
No. % lemongrass oil (v/v) Direct contact test Vapor test
S. aureus (cm) E. coli (cm) S. aureus(cm) E. coli (cm)
1. 1 - - - -
2. 3 - - - -
3. 5 - - - -
4. 10 2.6 3.0 2.5 2.7
Labels with lemongrass oil concentrations above 10%
have been tried in this experiment but the results show
that the labels are not compatible. There was a
separation between lemongrass oil and chitosan
matrix when the concentration of lemongrass was
above 10%. Therefore, the optimal concentration of
lemongrass oil on the label is 10%. Previous studies
that have been conducted have shown that lemongrass
concentrations below 10% have had antimicrobial
activity (Ali, Noh and Mustafa, 2014) but in
difference of the solvent of chitosan and difference
of microbe.
4 CONCLUSIONS
The lemongrass oil was used in this study contained
73.21% of citral as the major compound which is an
antimicrobial agent. The lemongrass oil has the
antimicrobial activity in Gram-positive bacteria S.
aureus) and Gram-negative bacteria E. coli. The
labels from lemongrass oil incorporated with
chitosan/lemongrass oil shown the antimicrobial
activity in Gram-positive bacteria S. aureus and
Gram-negative bacteria E. coli with the optimal
lemongrass oil concentration of 10% (v/v).
(
a
)
(
b
)
Antimicrobial Label from Lemongrass Oil Incorporated with Chitosan/Ascorbic Acid
151

ACKNOWLEDGEMENT
This research is supported by PSNI (Penelitian
Strategis Nasional Institusi) from Kementerian Riset,
Teknologi, dan Perguruan Tinggi Republik Indonesia
No NKB-1798/UN2.R3.1/HKP.05.00/2019. We also
thank the Center of Excellence Biology Resources
Genome Study (CoE IBR-GS) FMIPA UI and the
Center for Chemical and Packaging (CCP) for the
facilities and equipment to support this research.
REFERENCES
Ali, A., Noh, N. M. and Mustafa, M. A. (2014)
‘Antimicrobial activity of chitosan enriched with
lemongrass oil against anthracnose of bell pepper’,
Food Packaging and Shelf Life. Elsevier Ltd., 3, pp. 56–
61. doi: 10.1016/j.fpsl.2014.10.003.
Argyropoulou, C. et al. (2007) ‘Chemical composition of
the essential oil from leaves of Lippia citriodora H.B.K.
(Verbenaceae) at two developmental stages’,
Biochemical Systematics and Ecology, 35(12), pp. 831–
837. doi: 10.1016/j.bse.2007.07.001.
Bhavaniramya, S. et al. (2019) ‘Role of essential oils in
food safety: antimicrobial and antioxidant
applications’, Grain & Oil Science and Technology.
doi: 10.1016/j.gaost.2019.03.001.
Dorman, H. J. D. and Deans, S. G. (2000) ‘Antimicrobial
agents from plants: Antibacterial activity of plant
volatile oils’, Journal of Applied Microbiology, 88(2),
pp. 308–316. doi: 10.1046/j.1365-2672.2000.00969.x.
Ganjewala, D. (2009) ‘Cymbopogon essential oils :
Chemical compositions and bioactivities’, International
Journal of Essential Oil Therapeutics, 3, pp. 56–65.
Gursoy, M. et al. (2018) ‘False flax (Camelina sativa) seed
oil as suitable ingredient for the enhancement of
physicochemical and biological properties of chitosan
films’, International Journal of Biological
Macromolecules. Elsevier B.V., 114, pp. 1224–1232.
doi: 10.1016/j.ijbiomac.2018.04.029.
Handayani, W. et al. (2019) ‘Coriandrum sativum l . (
apiaceae ) and elettaria cardamomum ( l .) maton (
zingiberaceae ) for antioxidant and antimicrobial
protection Coriandrum sativum l . ( apiaceae ) and
elettaria cardamomum ( l .) maton ( zingiberaceae ) for
antioxidant and antimi’, Journal of Physiscs:
Conference Series. doi: 10.1088/1742-
6596/1317/1/012092.
Huang, D. F. et al. (2014) ‘Chemical constituents,
antibacterial activity and mechanism of action of the
essential oil from Cinnamomum cassia bark against
four food-related bacteria’, Microbiology (Russian
Federation), 83(4), pp. 357–365. doi:
10.1134/S0026261714040067.
Hyun, J. E. et al. (2015) ‘Preservative effectiveness of
essential oils in vapor phase combined with modified
atmosphere packaging against spoilage bacteria on
fresh cabbage’, Food Control. Elsevier Ltd, 51, pp.
307–313. doi: 10.1016/j.foodcont.2014.11.030.
Inouye, S., Takizawa, T. and Yamaguchi, H. (2001)
‘Antibacterial activity of essential oils and their major
constituents against respiratory tract pathogens by
gaseous contact’, Journal of Antimicrobial
Chemotherapy, 47(5), pp. 565–573. doi:
10.1093/jac/47.5.565.
Ju, J. et al. (2019) ‘Application of essential oil as a
sustained release preparation in food packaging’,
Trends in Food Science and Technology
, 92(1800), pp.
22–32. doi: 10.1016/j.tifs.2019.08.005.
Li, Z. et al. (2019) ‘Preparation, characterization and anti-
aflatoxigenic activity of chitosan packaging films
incorporated with turmeric essential oil’, International
Journal of Biological Macromolecules. Elsevier B.V.,
131, pp. 420–434. doi: 10.1016/j.ijbiomac.2019.02.169.
Ozdemir Kubra S, G. V. (2017) ‘Extending the shelf-life of
pomegranate arils with chitosan-ascorbic acid coating’,
Food Science and Tachnology 76 (2017) 172-180, 76,
pp. 172–180. doi: 10.1016/j.lwt.2016.10.057.
Saddiq, A. A. and Khayyat, S. A. (2010) ‘Chemical and
antimicrobial studies of monoterpene: Citral’, Pesticide
Biochemistry and Physiology. Elsevier Inc., 98(1), pp.
89–93. doi: 10.1016/j.pestbp.2010.05.004.
Ternus ZR, Z. M. (2015) ‘Microbiological Characterization
of Pure Geraniol and Comparison with Bactericidal
Activity of the Cinnamic Acid in Gram-Positive and
Gram-Negative Bacteria’, Journal of Microbial &
Biochemical Technology, 07(04), pp. 186–193. doi:
10.4172/1948-5948.1000203.
Tyagi, A. K. and Malik, A. (2010) ‘In situ SEM, TEM and
AFM studies of the antimicrobial activity of lemon
grass oil in liquid and vapour phase against Candida
albicans’, Micron, pp. 797–805. doi:
10.1016/j.micron.2010.05.007.
Wang, T. H. et al. (2016) ‘Evaluation of the antibacterial
potential of liquid and vapor phase phenolic essential
oil compounds against oral microorganisms’, PLoS
ONE, 11(9), pp. 1–17. doi:
10.1371/journal.pone.0163147.
Zhang, Z. et al. (2018) ‘Preparation and characterization of
biocomposite chitosan fi lm containing Perilla
frutescens ( L .) Britt . essential oil’, Industrial Crops &
Products, 112(December 2017), pp. 660–667. doi:
10.1016/j.indcrop.2017.12.073.
ICEO 2019 - 2nd International Conference of Essential Oil Indonesia
152
