
The Effect of NAA Concentration and Different Parts of Stem on
Growth of Patchouli (Pogostemon cablin Benth.)
Mardhiah Hayati
1, 2
,
Nurhayati
1
and
Revira Sari
3
1
Department of Agrotechnology, Faculty of Agriculture, Universitas Syiah Kuala, Banda Aceh, 2311, Indonesia
2
Atsiri Research Center, Universitas Syiah kuala, Banda Aceh, 2311, Indonesia
3
Student of Department of Agrotechnology, Faculty of Agriculture, Universitas Syiah kuala, Banda Aceh, 2311,
Indonesia
Keywords: NAA concentration, cutting, patchouli. Stem, growth
Abstract: The need for patchouli is increasing with the increase of population and the development of the cosmetics
industry. The supply of healthy and of high production patchouli cutting is necessary to ensure the optimum
of production. The purpose of this study was to determine the effect of NAA concentration and the right parts
source of cuttings and the interaction between the two on the growth of patchouli. The study was conducted
in the Experimental Field and Plant Physiology Laboratory of the Faculty of Agriculture, Universitas Syiah
Kuala, Banda Aceh, in January to April 2019. The study used a factorial randomized block design with a 4x3
factorial pattern with three replications. NAA was applied using Growtone, a brand with an NAA
concentration of 3%. Factors studied were Growtone concentration at 0, 4.0, 8.0, and 12.0 g L
-1
water, and
parts of stem source (shoots, middle, and base). The results showed that the best growth of patchouli cuttings
was at Growtone concentration of 4.0 g L
-1
water, while the best shoot length and leaf area was found in the
treatment of Growtone concentration of 12.0 g L
-1
water. Meanwhile, the best growth of patchouli cuttings
was found in the stem taken from the shoot’s part. There was no significant interaction between NAA
concentration and the source of the different parts of stem on the growth of patchouli.
1 INTRODUCTION
Patchouli (Pogostemon cablin Benth.) is a highest
ranked essential oil producing plants (Singh et al.,
2015). It has a strategic potential in the world market
where the oil is used as a scent binding agent in
perfumes, cosmetics, medicines and aromatherapy
(Swamy and Sinniah, 2016; Yang et al., 2013).
Patchouli oil can also be used as insect repellent
(Maia and Moore, 2011) and antiseptic (Haryudin and
Maslahah 2011). Recently, no synthetic ingredients
or substitutes have been found to match the benefits
of patchouli oil. The largest quantity of patchouli oil
is produced in Indonesia (Swamy and Sinniah, 2016).
Patchouli cultivation in Indonesia was originally
developed in Aceh, North Sumatra, West Sumatra
and Bengkulu (Haryudin and Maslahah 2011). Three
superior quality of patchouli varieties (Tapak Tuan,
Lhokseumawe, and Sidikalang varieties) have been
resealed by the Indonesian Research Institute of
Spices and Medical Plants, Bogor, Indonesia. Tapak
Tuan varieties is superior for its production,
Lhokseumawe varieties has high oil content, while
Sidikalang varieties tolerant to bacterial wilt and
nematode (Nuryani, 2006).
In recent years, according to Kementerian
Pertanian Republik Indonesia (2019), Indonesian
patchouli production is unstable and does not show
any progress (2.207 tons in 2017 and 2.211 tons in
2019). The problem of unincreased production and
quality of Indonesian patchouli is caused by many
factors such as plant genetic quality, non-intensive
cultivation, poor seedlings, limited seed sources,
varied seedling, reduced planting area, decreased
level of soil fertility, harvest and postharvest
mechanism, and patchouli oil distillation that is far
from perfect (Nuryani, 2006; Setiawan and Rosman,
2013).
The formation of adventitious roots of plant is
controlled by genetic and environmental factors,
among which phytohormone auxin plays a major role
(Zhao et al., 2014). Exogenous auxin application
(e.g., naphthalene acetic acid, NAA) can increase
adventitious root formation in cuttings of most plant
Hayati, M., Nurhayati, . and Sari, R.
The Effect of NAA Concentration and Different Parts of Stem on Growth of Patchouli (Pogostemon cablin Benth.).
DOI: 10.5220/0009958001270133
In Proceedings of the 2nd International Conference of Essential Oils (ICEO 2019), pages 127-133
ISBN: 978-989-758-456-5
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
127

species (Damiano et al., 2008; Ragonezi et al., 2010.
The growth of patchouli cuttings can be stimulated by
the application of growth regulators containing auxin
(exogenously) to stimulate root growth and can
physiologically influence plant growth.
Currently, there are many growth regulators in the
market, including Growtone with Naphthalene acetic
acid (NAA) content of 0.3% and acetamide 1-
naphthalene of 0.75%. NAA serves to stimulate root
growth and reduce the risk of cuttings decay. Faizin
(2016) found that the number of leaves, shoot length,
number of roots, and root length of patchouli plant
cuttings were best shown at Growtone concentration
of 6.0 g L
-1
water. This shows that Growtone
concentration of 6.0 g L
-1
water has been able to
increase the growth of patchouli cuttings more than
any other treatment. Handriyano (2007) states that the
length of cuttings of 25 cm with a soaking time of 45
minutes at Growtone concentration of 0.8 g L
-1
water,
increase the growth of Jatropha cuttings where the
root length, root volume and buds of Jatropha cuttings
appear better than in other treatments.
Propagation by cuttings is one of vegetative
propagation technique that is widely used in
horticultural crops such as ornamental plants (Oinam
et al., 2011) and forestry plants (Nakhooda et al.,
2016) or for the propagation of elite genotypes on a
large scale. As patchouli plants are rarely produce
seeds, it is mostly propagated using stem cutting or in
vitro multiplication (Swamy et al., 2010; Saravanan
et al., 2015). The success of vegetative propagation
mainly depends on the efficient selection of stem
cuttings. Rathnayake et al. (2015) found in
Pogostemon heyneanus, two nodal hardwood cuttings
performed better in rooting parameters when
compared to semi-hardwood and softwood cuttings.
Cuttings must be available in good conditions,
because it is likely that cuttings will decay after
planting. For the best result, cuttings are suggested to
be prepared in nurseries before planting them directly
in the field (Nuryani et al., 2007). Differences in stem
cuttings affect plant growth, while cuttings for
patchouli plants can be used at the shoots, the middle
and the base of the stem. Melati et al., (2006) found
that almost all growth parameters (plant height,
number of branches, number of leaves) observed
showed that the growth of leafy cuttings of patchouli
was better than non-leaf cuttings. Conversely,
Iskandar (2014) found that the suitable planting
material was base cuttings compared to other parts of
stem. The results of his study showed that the plant
height, number of leaves, and the number of shoots
were higher.
The length of time for a stem to produce root was
a problem faced by patchouli farmers in patchouli
planting (Pandji and Sofyan, 1986). The capacity to
form adventitious roots in stem cuttings varies
between cuttings and within plant species or even
genotypes (De Klerk et al., 1999). Stuepp et al. (2014)
suggested that age, size, juvenility and maturity levels
of vegetative cuttings play a key role in the
establishment of better rooting. The cuttings are taken
from woody stems and parts of plants that are not too
old, and the cuttings chosen for seedlings must be free
from pests and diseases.
The use of suitable patchouli cuttings along with
a combination of IBA concentration provide a high
percentage of cuttings life, initially buds emerge,
number of shoots, root length, root volume, biomass
dry weight and root dry weights, exceeding the base
cuttings and middle cuttings of patchouli (Purba et al.,
2017).
Based on the description above, this study used
several concentrations of Growtone as a source of
NAA and different parts of stem cutting to determine
the highest growth of patchouli plant. This study aims
to determine the effect of Growtone concentration
and different parts of stem as the best part of stem
cutting and the interaction between the two factors on
the growth of patchouli plant.
2 MATERIALS AND METHODS
2.1 Experimental Site
The research was carried out at the Experimental
Field and Plant Physiology Laboratory of the Faculty
of Agriculture, Universitas Syiah Kuala, Banda Aceh,
which was carried out in January to April 2019.
2.2 Tools and Materials
The tools used in this study were analytical scales,
100 ml measuring cups, calipers, Tapak Tuan
varieties patchouli cuttings from different parts of
stem (shoots, middle stem and base), Growtone of 72
g, polybags with a size of 25 cm x 30 cm as much as
108 sheet, and Urea fertilizer as much as 216 g.
Paranet was used as a shading material.
2.3 Research Implementation
Planting media used were soil and manure with a ratio
of 3:1 (based on volume). Patchouli cuttings from
different plant parts with a length of 25 cm were
soaked in Growtone with a concentration according
ICEO 2019 - 2nd International Conference of Essential Oil Indonesia
128

to treatment for 24 hours. Patchouli cuttings control
treatment soaked with water without Growtone. Each
patchouli cutting was planted in each polybag with a
depth of 5 cm. Maintenance of patchouli plant
includes watering, fertilizing with Urea of 2 g per
polybag at one week after planting (DAP), weeding
and losing the soil was carried out at 25, 45 and 55
DAP. Plant revocation was done at the age of 75
DAP.
2.4 Experimental Design
This study used Randomized Block Design with a
4x3 factorial pattern with 3 replications. There were
2 factors studied, the first factor was Growtone
concentration consists of 4 levels (0, 4.0, 8.0 and 12.0
g L
-1
water). The second factor was cuttings from
different parts of stem (shoots, middle and base parts
of stem). Each experiment unit consists of three
polybags. Data were analyzed with analysis of
variance (ANOVA), and analysis of differences in
mean values using Tukey Test at 5% significant level.
2.5 Observation Parameters
Observations were made on the number of shoots,
shoot length, shoot diameter, number of leaves at 15,
30, 45, 60 and 75 DAP. Measurement of leaf length,
leaf width, leaf area, fresh and dry weight of biomass
(using an oven for 3x24 hours with a temperature of
60ºC to a constant weight), number of roots, root
length and root volume of patchouli plant were
performed at 75 DAP.
3 RESULT AND DISCUSSION
3.1 Effect of Growtone Concentration
on Growth of Patchouli Cuttings
The results of analysis of variance showed that
Growtone concentration had a very significant effect
on all observed growth variables. The average growth
of patchouli cuttings due to the treatment of
Growtone concentration were as indicated in Table 1.
The results showed that the highest number of shoots
at 15, 30 and 75 DAP were obtained at Growtone
concentration of 4.0 g L
-1
water and the highest
number of shoots at 45 and 60 DAP at Growtone
concentrations of 8.0 g L
-1
water. The largest shoot
diameter at 15 and 30 DAP were obtained in control
treatment, while at 45 and 60 DAP at Growtone
concentrations of 8.0 g L
-1
water, and at 75 DAP was
the largest at Growtone concentrations of 12.0 g L
-1
water. The highest shoot lengths at 15, 30, 45 and 60
DAP were found at Growtone concentrations of 12.0
g L
-1
water, while at 75 DAP was at Growtone
concentrations of 8.0 g L
-1
water. The highest number
of leaves at ages 15, 30, 45 and 60 DAP were found
at Growtone concentration of 8.0 g L
-1
water but not
significantly different from Growtone concentrations
of 4.0 g L
-1
water. The highest number of leaves at 75
DAP was found at Growtone concentration of 4.0 g
L
-1
of water, and was not significantly different from
control. The highest leaf length and leaf width were
found at Growtone concentration of 8.0 g L
-1
water,
while the largest leaf area was found at Growtone
concentration of 12.0 g L
-1
water. Fresh and dry
biomass weights, the highest number of roots and root
volume were found at Growtone concentration of 4.0
g L
-1
water, while the highest root length was found
at Growtone concentration of 8.0 g L
-1
water.
The best Growtone treatment was found at a
concentration of 4.0 g L
-1
water. This fact indicated
that the use of Growtone concentration of 4.0 g L
-1
water had been able to provide a better growth of
patchouli cuttings. Faizin's (2016) found that
Growtone concentrations of 6.0 g L
-1
water which
was applied to patchouli plants showed the best
results compared to other treatments. This fact
indicated that Growtone concentrations of 6.0 g L
-1
water was optimal enough to stimulate the formation
of new patchouli plant roots, cell division, formation
and growth of patchouli plant cuttings. This also
indicated that the lower the use of Growtone
concentration, the better the growth of patchouli
cuttings. According to Zhao (2010) and Heddy (2006)
auxin as a plant growth regulator (PGR) play its role
in plant growth and development by affecting
membrane proteins, so protein synthesis and nucleic
acid can be faster and auxin influence the formation
of new roots, cell division and the formation of new
shoots. The best shoot length and leaf area parameters
were found in the treatment of Growtone
concentration of 12.0 g L
-1
water. The increasing
concentration of Growtone containing auxin
(naphthalene acetic acid) play a role in stimulating
growth, thus Growtone at the base of plant cuttings
increased the speed of growth of patchouli shoots and
enlarge the leaf area. Purba et al. (2017) suggested
that auxin hormone in cuttings was active enough to
divide the plant plus exogenous PGR to provide
optimal auxin conditions in the growth and
development of patchouli cuttings. The formation of
adventitious roots is the main condition for its success
in propagation.
The Effect of NAA Concentration and Different Parts of Stem on Growth of Patchouli (Pogostemon cablin Benth.)
129
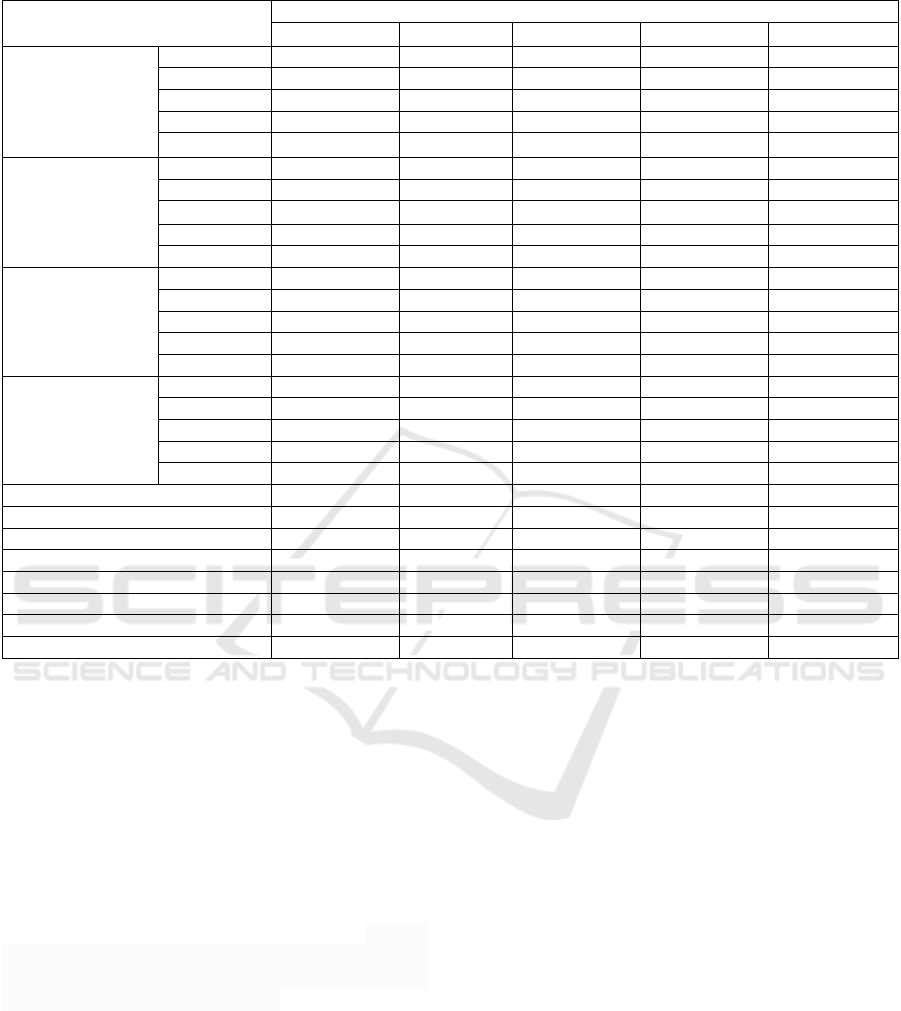
Table 1. Average growth of patchouli cuttings due to different Growtone concentrations.
Parameters
Concentration of Growtone (g L
-1
water)
Control
4.0
8.0
12.0
Tukey (5%)
Number of shoot
15 DAP
1.19 a
1.41 b
1.07 a
1.19 a
0.22
30 DAP
2.07 a
2.33 ab
2.26 ab
2.48 b
0.38
45 DAP
4.93 a
5.41 a
6.22 b
5.41 a
0.47
60 DAP
7.11 a
7.48 a
8.26 b
7.30 a
0.68
75 DAP
6.44 a
9.52 c
8.85 c
7.93 b
0.78
Shoot’s diameter
(mm)
15 DAP
0.55 b
0.47 ab
0.53 ab
0.43 a
0.11
30 DAP
1.00 ab
0.96 a
1.06 b
0.95 a
0.16
45 DAP
1.67 a
1.60 a
1.82 b
1.87 b
0.14
60 DAP
2.58 a
2.49 a
2.71 ab
2.74 b
0.14
75 DAP
3.09 a
3.16 a
3.10 a
3.59 b
0.21
Shoot’s length
(cm)
15 DAP
3.03 a
3.51 b
4.20 c
5.43 d
0.42
30 DAP
6.03 a
6.67 a
7.47 b
9.61 c
0.76
45 DAP
9.18 b
7.76 a
8.99 ab
11.30 c
1.39
60 DAP
10.68 ab
10.11 a
11.35 b
13.28 c
0.82
75 DAP
19.75 b
18.49 a
21.50 c
21.35 c
1.14
Number of leaves
15 DAP
0.44 a
0.67 b
0.70 b
0.67 b
0.14
30 DAP
0.85 a
1.93 c
2.04 c
0.67 b
0.14
45 DAP
6.19 a
9.26 bc
10.11 c
8.41 b
1.25
60 DAP
13.63 a
19.59 c
20.19 c
16.81 b
1.85
75 DAP
39.41 b
47.48 b
46.44 b
26.11 a
11.23
Length of leaves (cm)
8.09 a
9.33 b
9.77 c
10.17 c
0.42
Width of leaves (cm)
6.58 a
7.37 b
7.82 c
7.79 c
0.29
Area of leaves (cm
2
)
38.74 a
55,85 b
65.18 c
71.87 d
4.33
Fresh weight of biomass (g)
43.51 a
62.21 b
55.38 b
67.35 b
9.58
Dry weight of biomass (g)
8.90 a
14.51 c
10.76 b
12.09 b
1.76
Number of roots
22.58 a
25.67 b
23.56 a
24.67 ab
3.96
Length of roots (cm)
44.56 a
43.44 a
49.78 b
46.44 a
4.12
Volume of roots (ml)
17.11 b
20.67 c
12.78 a
14.67 ab
2.93
Notes: Numbers followed by the same letter in the same line are not significantly different at the 0.05 Tukey test level.
DAP= Day After Planting.
Pacucar et al. (2014) argued that adventitious root
growth is stimulated by interactions between
phytohormones and external growth regulators. The
stimulation of adventitious root formation is also
shown to be positively influenced by ethylene, which
may be through modulation of auxin transport
(Druege et al., 2014; Wei et al., 2019), thus the
production of ethylene induced by indole acetic acid
can be a factor involved in the stimulation of
adventitious root formation (Pan et al., 2002). Several
hormones such as auxins, cytokines, and ethylene
have long been known to regulate adventitious root
formation (De Klerk et al., 1999). Adventitious roots
can develop either from pericyclic cells or from
various types of cells and tissues, which depend on
the plant species and environmental stimuli involved
(Druege et al., 2016). The synthesis of auxin-induced
ethylene can play a role in the adventitious root
initiation and is associated with increased cellulose
activity (Kemmerer and Tucker, 1994).
The growth of patchouli cuttings in the control
treatment was very low compared to other treatments.
Control treatment that was not given Growtone was
not able to stimulate the speed of cell division in the
formation of plant organs such as roots, stems and
leaves. Pasetriyani research (2014) stated that the
control treatment or Growtone concentration of 0
mg/plant shows the lowest growth.
3.2 Effect of Different Parts of Stem
Cutting on Growth of Patchouli
The results of the analysis of variance showed that
cutting from different parts of stem had a very
significant effect on the number of shoots at 30, 45,
60 and 75 DAP and the number of leaves at 15, 30,
45 and 60 DAP. The average growth of patchouli
plants due to the treatment of cuttings from different
part of stem is shown in Table 2. The table shows that
the number of shoots at 30, 45, 60, 75 DAP and the
number of leaves at 15, 30, 45 and 60 DAP were
mostly found in shoot cuttings, and significantly
different from other cuttings treatment. The number
ICEO 2019 - 2nd International Conference of Essential Oil Indonesia
130
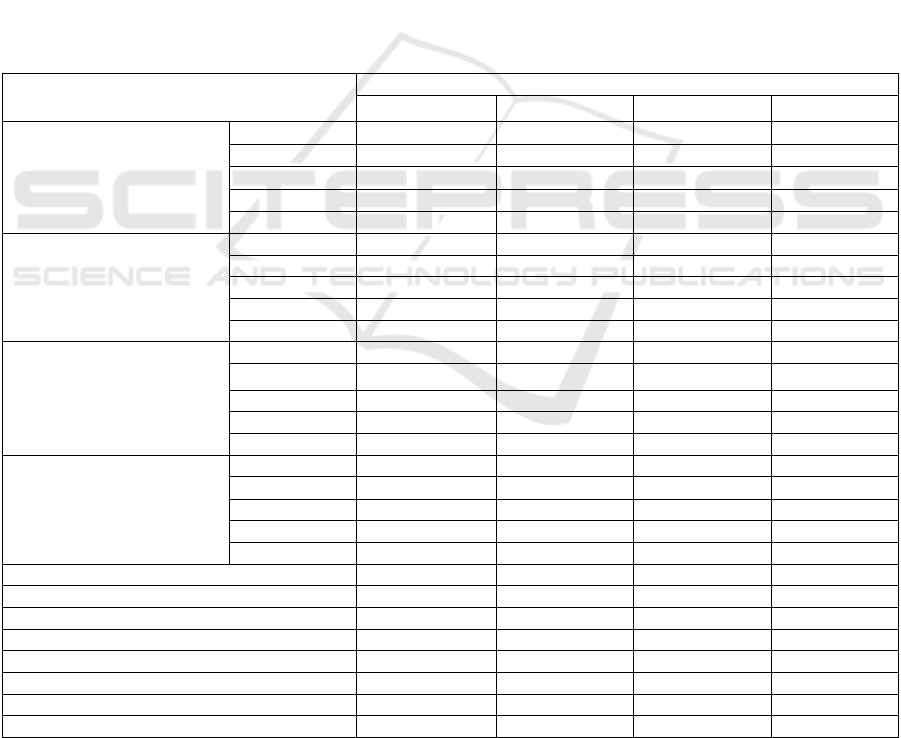
of shoots at 15 DAP, shoot diameter at 15, 30, 45, 60
and 75 DAP, the length of shoots at 15 and 75 DAP,
the number of leaves at 75 DAP significantly
different from the treatment of the middle and base
stem cuttings.
The shoot lengths at 30, 45 and 60 DAP tend to be
longer in the treatment of middle stem cuttings,
although statistically not significantly different from
the treatment of shoot and the base cuttings. Biomass
fresh and dry weight tend to be higher in base stem
cuttings, although was not significantly different from
the treatment of shoot and middle stem cuttings.
The results showed that shoot’s stem cutting with
leaves provide the highest growth compared to the
middle and base stem cuttings. At the shoot cuttings
of patchouli, several leaves were present compared to
middle and base stem cuttings, where in the presence
of leaves also get the number of roots (Garbuio et al.,
2007). In patchouli, stem cuttings with leaves are
preferred for vegetative propagation because of the
higher rooting and shooting capacity (Swamy and
Sinniah, 2016). Shoot cutting contains a lot of
carbohydrates and auxin to trigger the formation of
shoots and leaves.
The minimum percentage of leaves in cuttings of
patchouli occurred as the consequence of low
carbohydrate availability, as well as low reserve
tissue, and higher ABA content (Kojima et al., 1993).
Carbohydrates have been considered as one of the key
factors that contribute to adventitious root formation
(Shang et al., 2019). Faizin (2016) used shoot, middle
and base stem cuttings and found that the best result
was found on shoot treatment compared to other
treatments. Shoot cuttings has been optimal enough
to stimulate the speed of root formation, the
emergence of early shoots and more leaf formation.
Table 2. The average growth of patchouli cuttings due to different parts of stem cuttings.
Parameters
Stem cutting
Shoot
Middle
Base
Tukey 5%
Number of shoots
15 DAP
1.56
0.97
0.97
-
30 DAP
3.42 b
1.72 a
1.72 a
0.50
45 DAP
8.42 b
3.78 a
4.28 a
0.63
60 DAP
11.78 b
4.97 a
5.86 a
0.91
75 DAP
13.78 b
5.67 a
5.11 a
1.05
Shoot’s diameter (mm)
15 DAP
0.65
0.42
0.42
-
30 DAP
1.22
0.89
0.87
-
45 DAP
1.84
1.68
1.70
-
60 DAP
2.70
2.61
2.59
-
75 DAP
3.45
3.10
3.15
-
Shoot’s length (cm)
15 DAP
4.18
3.93
3.97
-
30 DAP
7.11
7.77
7.46
-
45 DAP
8.15
10.87
8.90
-
60 DAP
11.20
11.69
11.18
-
75 DAP
21.13
19.62
20.07
-
Number of leaves
15 DAP
1.86 b
0.14 a
0.11 a
0.18
30 DAP
3.67 b
0.78 a
0.44 a
0.55
45 DAP
15.06 b
5.64 a
4.78 a
1.66
60 DAP
28.58 b
12.72 a
11.36 a
2.47
75 DAP
42.47
39.00
38.00
-
Length of leaves (cm)
9.70
8.69
9.63
-
Width of leaves (cm)
7.73
7.46
7.58
-
Area of leaves (cm
2
)
60.68
52.92
60.13
-
Fresh weight of biomass (g)
57.32
48.75
65.27
-
Dry weight of biomass (g)
11.87
9.88
12.95
-
Number of roots
25.42
23.25
23.67
-
Length of roots (cm)
51.67
40.50
46.00
-
Volume of roots (ml)
19.17
11.92
17.83
-
Notes: Numbers followed by the same letter in the same line are not significantly different at the 0.05 Tukey test level.
DAP= Day After Planting.
The Effect of NAA Concentration and Different Parts of Stem on Growth of Patchouli (Pogostemon cablin Benth.)
131

Abidin (1990) argued that shoot cuttings contain
a lot of auxin when compared to other parts, as
endogenous auxin from a plant is produced from
meristem tissue and causes apical dominance so that
the formation of roots is faster and stimulates the
emergence of shoots. Heddy (2006) argued that the
role of carbohydrates to form roots and shoots is very
large. The growth of good shoots and roots will lead
to good leaf formation and increases the
photosynthetic process, thus more carbohydrates are
produced. Purba et al. (2017) found that the use of
shoot cuttings in the provision of PGR IBA with a
concentration of 100 ppm increased the growth of
patchouli cuttings in all variables, such as the
percentage of live cuttings, age of buds, number of
shoots, root length, root volume, and root dry weight.
3.3 Effect of Interaction of Growtone
Concentration and Difference Parts
of Stem on the Growth from
Patchouli
The results showed that there was no significant
interaction between the concentration of Growtone
and the stem cuttings on all patchouli growth
variables. The growth of different patchouli stem
cuttings due to differences in the concentration of
Growtone applied was not affected by different parts
of stem cuttings, and the treatment of stem cuttings
were not affected by differences in concentration of
Growtone.
4 CONCLUSION
The results showed that Growtone concentration had
a very significant effect on all growth variables. The
best growth of patchouli cuttings was found at
Growtone concentration of 4 g L
-1
water, while the
highest shoot length, shoot diameter at 75 DAP and
the leaf area was found in the treatment of Growtone
concentration of 12 g L
-1
water. Different stem
cuttings have a very significant effect on the number
of shoots at 30, 45, 60 and 75 DAP and the number of
leaves at 15, 30, 45, and 60 DAP. The best growth of
patchouli cuttings was found in the shoot cutting.
There was no significant interaction between
Growtone concentration and the different part of stem
cutting on all patchouli growth variables that were
observed.
REFERENCES
Abidin, Z., 1990. Dasar-dasar pengetahuan zat pengatur
tumbuh, Penerbit Angkasa, Bandung, Indonesia.
Damiano, C. Padro, M.D.A., Frattarelli, A., 2008.
Propagation and establishment in vitro of myrtle
(Myrtus communis L.), pomegranate (Punica granatum
L.) and mulberry (Morus alba L.). Propag. Ornam.
Plants. 8, 3–8.
De Klerk, G.J., Van der Krieken, W., de Jong, J.C., 1999.
Review the formation of adventitious roots: New
concepts, new possibilities. In Vitro Cell. Dev. Plants.
35, 189–199.
Druege, U., Franken, P., Lischewski, S., Ahkami, A.H.,
Zerche, S., Hause, B., Hajirezaei, M.R., 2014.
Transcriptomic analysis reveals ethylene as stimulator
and auxin as regulator of adventitious root formation in
petunia cuttings. Front. Plant Sci. 5,1-19.
Druege, W., Franken, P., Hajirezaei, M.R., 2016. Plant
hormone homeostasis, signaling, and function during
adventitious root formation in cuttings. Front. Plant
Sci. 7, 1-14.
Faizin, R., 2016. Pengaruh jenis setek dan konsentrasi zat
pengatur tumbuh Growtone terhadap pertumbuhan
tanaman nilam (Pogostemon cablin Benth). J. Agrotek
Lestari. 2(1), 39-50.
Garbuio, C., Biasi, L.A., Kowalski, A.P.J., Signor, D.,
Machado, E.M., Deschamps, C., 2007. Propagação por
estaquia em patchouli com diferentes números de folhas
e tipos de estaca. Scientia Agraria. 8, 435-438.
Handriyano, A., 2007. Pengaruh panjang setek dan lama
perendaman dalam Growtone terhadap
pertumbuhan stek jarak pagar (Jatropha curcas L.).
Tesis. University of muhammadiyah Malang.
Indonesia.
Haryudin, W., Maslahah, N., 2011. Karakteristik
morfologi, anatomi dan produksi terna aksesi nilam asal
Aceh dan Sumatera Utara. Bul. Littro. 22(2), 115-126.
Heddy, S., 2006. Hormon Tumbuhan, Raja Grafindo
Persada, Jakarta.
Iskandar, S., 2014. Pengaruh asal bahan setek dan dosis
pupuk N terhadap pertumbuhan bibit Nilam
(Pogostemon caplin Benth). Skripsi. Sekolah Tinggi
Pertanian Dharma Wacana, Lampung. Indonesia.
Kementerian Pertanian Republik Indonesia. 2019. Data
lima tahun terakhir. Retrieved on October 25, 2019
from: pertanian.go.id.
Kemmerer, E.C. Tucker, M.L., 1994. Comparative study of
celluloses associated with adventitious root initiation,
apical buds, and leaf, flower, and pod abscission zones
in soybean. Plant Physiol. 104, 557-62.
Kojima, K., Kuraishi, S., Sakurai, N., Itou T., Tsurusaki, K.
1993. Spatial distribution of abscisic acid and 2-trans-
abscisic acid in spear, buds, roots and roots of asparagus
(Asparagus officinalis L.). Sci. Hort. Amsterdam. 54,
177-89.
Maia, M.F., Moore, S.J., 2011. Plant-based insect
repellents: a review of their efficacy, development and
testing. Malaria J. 10.
ICEO 2019 - 2nd International Conference of Essential Oil Indonesia
132

Melati, Rusmin, D., Sukarman, 2006. Pengaruh lama
penyimpanan setek berakar terhadap pertumbuhan
nilam (Pogostemon cablin Benth.). Jurnal Littri. 12(4),
135-139.
Nakhooda, M., Jain, S.M., 2016. A review of eucalyptus
propagation and conservation. Propag. Ornam. Plants.
16, 101–119.
Nuryani, Y., 2006. Karakterisasi empat aksesi nilam.
Buletin Plasma Nutfah. 12(2), 45-49.
Nuryani, Y., Emmyzar, Wiratno, 2007. Budidaya
Tanaman Nilam. Badan Penelitian dan
Pengembangan. Balai Penelitian Tanaman Obat dan
Aromatik. J. Penelitian Sirkuler. 12, 1-27.
Oinam, G., Yeung, E., Kurepin, L., Haslam, T., Lopez-
Villalobos, A., 2011. Adventitious root formation in
ornamental plants: I. General overview and recent
successes. Propag. Ornam. Plants. 11, 78–90.
Pan, R., Wang, J., Tian, X., 2002. Influence of ethylene on
adventitious root formation in mung bean hypocotyl
cuttings. Plant Growth Regulation. 36, 135-139.
Pandji, M., Sofyan, R., 1986. Tanaman nilam sebagai
sumber minyak atsiri, Balai Penelitian Tanaman Obat
dan Rempah, Bogor.
Pasetriyani, E.T., 2014. Pengaruh macam media tanam dan
zat pengatur tumbuh Growtone terhadap pertumbuhan
stek batang tanaman jarak pagar (Jatropha curcas
Linn). J. Agroscience. 7, 82-88.
Pacurar, D.I., Perrone, I., Bellini, C., 2014. Auxin is a
central player in the hormone cross-talks that control
adventitious rooting. Physiol. Plant. 151, 83–96.
Purba, R.S., Ginting, J., Ginting, J. 2017. Respons
pertumbuhan setek nilam (Pogostemon cablin Benth.)
pada berbagai bahan tanam dan konsentrasi IBA. J.
Agroteknologi FP USU. 5(4), 799-805.
Ragonezi, C., Klimaszewska, K., Castro, M.R., Lima, M.,
de Oliveira, P., Zavattieri, M.A., 2010. Adventitious
rooting of conifers: Influence of physical and chemical
factors. Trees. 24, 975–992.
Rathnayake, R.M.D.H., Dharmadasa, R.M., Abeysinghe,
D.C., 2015. Suitable maturity stage, type of cuttings and
potting media for vegetative propagation of
Pogostemon heyneanus Benth. World J. Agric. Res. 3,
203-207.
Saravanan, R., Kumar, V.S., Maiti, S., 2015.
Standardization of single eye cutting in patchouli
(Pogostemon cablin). Current Hort. 3(1), 19-23.
Setiawan dan R. Rosman. 2013. Produktivitas nilam
nasional semakin menurun. Warta Penelitian dan
Pengembangan Tanaman Industri. 19(3), 8-11.
Shang, C., Yang, H., Ma, S., Shen, Q., Liu, L., Hou, C.,
Cao, X., Cheng, J., 2019. Physiological and
transcriptomic changes during the early phases os
adventitious root formation in mulberry stem hardwood
cuttings. Int. J. Mol. Sci. 20, 1-20.
Singh, R., Singh, M., Srinivas, A., Rao, E.P., Puttanna, K.,
2015. Assessment of organic and inorganic fertilizers
for growth, yield and essential oil quality of industrially
important plant patchouli (Pogostemon cablin)
(Blanco) Benth. J. Essent. Oil Bear. Plants. 18(1), 1–
10.
Stuepp, C.A., Zuffellato-Ribas, K.C., Wendling, I.,
Koehler, H.S., Bona, C., 2014. Vegetative propagation
of mature dragon trees through epicormic shoots.
Bosque. 35(3), 337–345.
Swamy, M.K., Balasubramanya, S., Anuradha, M., 2010. In
vitro multiplication on Pogostemon cablin Benth.
through direct regeneration. African J. Biotech. 9(14),
2069-2075.
Swamy, M.K., Sinniah, U.R., 2016. Patchouli (Pogostemon
cablin Benth.): Botany, agrotechnology and
biotechnological aspects. Industrial Crops and
Products. 87, 161-176.
Wei, K., Ruan, L., Wang, L., Chang, H., 2019. Auxin-
induced adventitious root formation in nodal cuttings of
Camellia sinensis. Int. J. Mol. Sci. 20(19), 4817.
Wu L, Wu Y, Guo Q, Li S, Zhou K, Zhang J. 2011.
Comparison of genetic diversity in Pogostemon cablin
from China revealed by RAPD, morphological and
chemical analyses. J. Med. Plants Res. 5, 4549-4559.
Yang, X., Zhang, X., Yang S.P., Liu, W.Q., 2013.
Evaluation of the antibacterial activity of patchouli oil.
Iran J. Pharm. Res. 12, 307-316.
Zhao, X., Zheng, H., Li, S., Yang, C., Jiang, J., Liu, G.,
2014. The rooting of poplar cuttings: A review. New
Forest. 45, 21-34.
Zhao, Y., 2010, Auxin biosynthesis and its role in plant
development. Annu. Rev. Plant Biol. 61, 49-64.
The Effect of NAA Concentration and Different Parts of Stem on Growth of Patchouli (Pogostemon cablin Benth.)
133
