
Esterification of Rhodinol Fraction with Acetic Anhydride using
Zeolite Catalyst
Gadis Dian Anggreini
1
, Mafud Cahayo
1
,
Masruri
2
, and
Warsito
1,2
1
Essential Oil’s Institute, Brawijaya University, Jl. Veteran Malang 65145, Malang, Indonesia
2
Department of Chemistry, Faculty of Science, Brawijaya University, Jl. Veteran Malang 65145, Malang, Indonesia
Keywords: Esterification, Rhodinol, Java Citronella Oil, Acetic Anhydride, Zeolite.
Abstract: This research has been conducted on the effect of esterification reaction in the chemical composition of
rhodinol fraction from java citronella oil (Cymbopogon winterianus). The reaction process in this research is
done at 230 ° C by using rhodinol fraction and acetic anhydride with zeolite as a catalyst. Based on the
research, the optimum reaction time is 1 hour and the optimum mole ratio of reactants is 1: 1. The% yield of
citronellyl acetate and geranyl acetate are 74.06% and 95.92%.
1 INTRODUCTION
Indonesia is a country rich in the diversity of essential
oil-producing plants. As many as 40 types of essential
oils produced from these plants have been traded and
one type of essential oil that has the potential to be
developed commercially is citronella oil (Gunawan,
2009).
Citronella oil consists of 40 components, but the
identity of citronella oil scent is only determined by
three compounds namely citronellal, citronellol, and
geraniol (Kaul et al., 1997).
Citronellal, citronellol, and geraniol are single
components that have a higher selling price than
fragrant citronella essential oils in the form of crude
oil (Aldrich, 2019). Separation of fragrant citronella
oil using batch scale vacuum fractionation distillation
has been able to separate the citronellal fraction and
rhodinol fraction (a mixture of citronellol and
geraniol) (Eden et al., 2018).
Citronellol and geraniol can be further enhanced
for its selling value by converting them into
compounds that are widely used in the food,
cosmetics and pharmaceutical industries, namely
citronellyl acetate and geranyl acetate (Claon and
Akoh, 1993).
Citronellyl acetate and geranyl acetate are ester
compounds that can be synthesized through an
esterification reaction between an acidic compound
and alcohol using an acid catalyst (Fessenden and
Fessenden, 1999). The HZSM-5 zeolite catalyst was
used in a previous study to synthesize isopentyl
acetate and succeeded in obtaining a yield of 95.1%
(Ma et al., 1996)
Therefore, to increase the higher selling value of
the rhodinol fraction obtained from citronella oil, it is
necessary to esterify the rhodinol fraction to obtain
citronellyl acetate and geranyl acetate.
2 METHOD
2.1 Esterification of Rhodinol Fraction
Rhodinol of 10 mL (citronellol = 0.02 mole and
geraniol = 0.01 mole) were taken into a 20 mL boiling
flask flat and then added 2.92 mL of acetic anhydride
(0.03 mole) and 0.14 g of zeolite. after that, the flask
is heated at 130°C with stirring using a magnetic
stirrer and after 1 hour the catalyst can be separated
by filtering.
The organic liquid from the previous reaction is
washed with distilled water repeatedly until the pH of
the water phase is equal to 7. after that, the organic
phase is separated and weighed.
The same method is used to find out the optimum
reflux times by repeating the previous method with
the variation of reflux time (2 hours and 3 hours). The
reflux time method that produces optimum citronellyl
acetate and geranyl acetate products is used to find
out the optimum mole ratio of acetic anhydride (0.06
mole and 0.9 moles) for this esterification reaction.
Dian Anggreini, G., Cahayo, M., Masruri, . and Warsito, .
Esterification of Rhodinol Fraction with Acetic Anhydride using Zeolite Catalyst.
DOI: 10.5220/0009957901230126
In Proceedings of the 2nd International Conference of Essential Oils (ICEO 2019), pages 123-126
ISBN: 978-989-758-456-5
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
123
2.2 Characterization using Gas
Chromatography Mass
Spectrometry (GC-MS)
Each sample was dissolved in n-hexane solvent in a
ratio of 1: 100, then 0.1 μL was taken and injected
using a syringe on GCMS-QP 2010S Shimadzu
instruments to obtain chromatogram and compound
prediction.
3 RESULT
3.1 Component Analysis of Rhodinol
Fraction
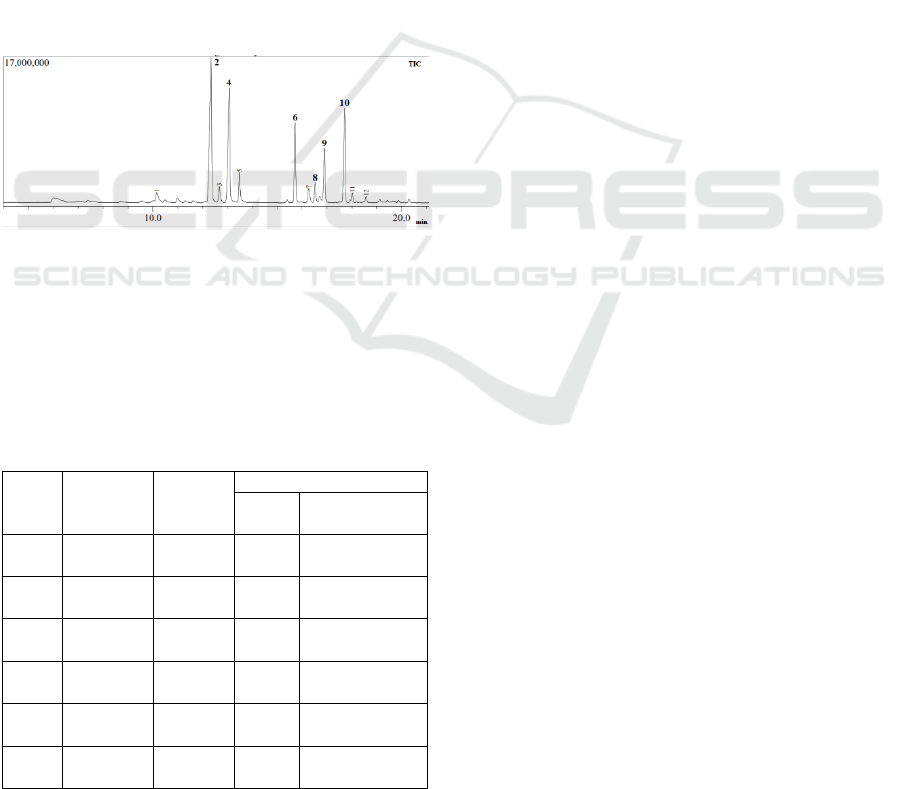
Figure 1 is a chromatogram that we obtained from the
GC-MS instrument, from that figure we can observe
that there are 12 peaks found in the rhodinol fraction.
Figure 1: Chromatogram of rhodinol fraction.
Six peaks in figure 1 is a component that we
should focus on because we would observe the
different before and after esterification reaction, the
effect of variation time reaction and mole ratio acetic
anhydride, for more details look at table 1.
Table 1: Tabulation from chromatogram of rhodinol
fraction.
Peak
Time
Retenion
(min)
Area
(%)
Estmation
SI Compound
2 12,34 31,51%
SI:
97
Citronellol
4 13,08 21,50%
SI:
97
Geraniol
6 15,71 11,10%
SI:
97
Citronellyl
Acetate
8 16,51 2,26%
SI:
97
Geranyl
Acetate
9 16,89 7,19%
SI:
96
β-Elemen
10 17,71 14,18%
SI:
97
Caryophyllene
3.2 Synthesis of Esters (Citronellyl
Acetate and Geranyl Acetate)
The synthesis of ester compounds (Citronellyl acetate
and geranyl acetate) is based on the Fischer
esterification reaction, which is the reaction between
the acetyl group (-COCH
3
) on the anhydride acetate
and the alcohol group (-OH). According to Fracotte
the formation of ester compounds using acetic
anhydride will produce a high% yield compared to
using acetic acid because the carbonyl group of acetic
acid is not strong enough as an electrophile to be
attacked by alcohol (Fracotte and Lohmann, 1989).
In the synthesis of citronellyl acetate and geranyl
acetate, the nucleophilic acyl substitution reaction
occurs. The use of the zeolite catalyst aims to reduce
the activation energy by changing the reaction
mechanism, which is to add the reaction steps.
Although the catalyst participates in the reaction
stage, at the end of the reaction process will be formed
again. With the lower value of the activation energy,
effective collisions that produce the product will
occur more frequently so the reaction goes faster. In
the reaction process, zeolite produces acylium ions
which act as electrophiles in the substitution reaction,
so that the acylium ion is easily attacked by O atoms
which are attached to hydroxyl groups from both
citronellol and geraniol. The hydroxyl groups in
citronellol and geraniol act as nucleophiles in the
presence of free electron pairs on the O atom, then the
hydroxyl group attacks the C atom of the carbonyl
group in the acylium ion to form oxonium ions. The
existence of this attack by nucleophiles causes the
substitution of H atoms in the hydroxyl groups from
citronellol and geraniol with acyl groups from acetic
anhydrides to form citronellyl acetate and geranyl
acetate.
At the end of the synthesis process, the liquid and
solid phases are produced. The solid phase is a zeolite
catalyst and can be separated by filtering. Meanwhile,
the liquid phase is containing esters (citronellyl
acetate and geranyl acetate) and acetic acid
compounds as byproducts.
3.3 Effect of Time on Rhodinol
Esterification Reaction with Acetic
Anhydride
Table 2 and table 3 are a tabulation of the data
produced by the esterification reaction with a fixed
number of mole of acetic anhydride but the varying
reflux time which is: 1 hour, 2 hours and 3 hours.
ICEO 2019 - 2nd International Conference of Essential Oil Indonesia
124
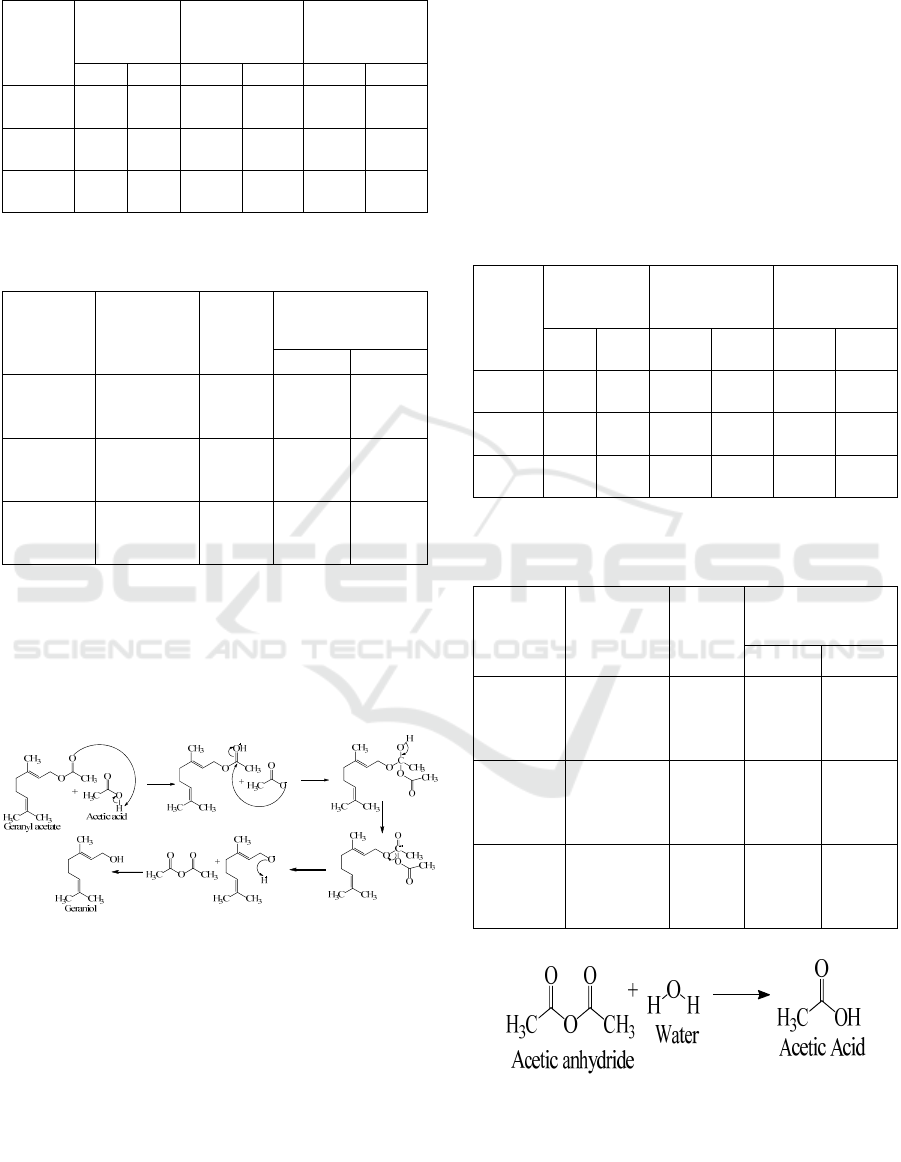
Table 2: Effect of Reflux time on Citronellyl Acetate (CA)
and Geranyl Acetate (GA) percentage.
Reflu
x time
(hour)
Before
Synthesis
(%)
After
Synthesis
(%)
synthesis
results (%)
CA GA CA CA CA GA
1
11,
1
2,2
6
48,3
3
26,0
4
37,2
3
23,7
8
2
11,
1
2,2
6
45,4
9
27,2
3
34,3
9
24,9
7
3
11,
1
2,2
6
47,1
1
10,4
9
36,0
1
8,23
Table 3: Effect of Reflux time on Citronellyl Acetate (CA)
and Geranyl Acetate (GA) %yield.
Quantity
Of
Rhodino
l
Quantity
Of Acetic
Anhydrid
e
Reflux
Time
(Hour
)
%Yield
CA GA
10 mL
(0,03
mol)
2,92 mL
(0,03 mol)
1
74,06
%
95,92
%
10 mL
(0,03
mol)
2,92 mL
(0,03 mol)
2
66,25
%
97,45
%
10 mL
(0,03
mol)
2,92 mL
(0,03 mol)
3
65,49
%
30,61
%
From Table 2 and Table 3 we could see that The
optimum reflux time to produce the highest %yield
citronellyl acetate and geranyl acetate yield is 1 hour.
Hydrolysis of esters by acetic acid is possible so that the
formed ester product can converts back into an alcohol
compound as the reaction time increases (Figure 2).
Figure 2: Reaction mechanism of hydrolysis ester (geranyl
acetate)
3.4 Effect of Mole Ratio on Rhodinol
Esterification Reaction with Acetic
Anhydride
Table 4 and table 5 are a tabulation of the data
produced by the esterification reaction with a fixed
reflux time but the varying number of mole of acetic
anhydride which is: 0.03 mole, 0.06 mole, and 0.09
mole.
From Table 4 and Table 5 we could see that The
optimum mole ratio between rhodinol and acetic
anhydride to produce the highest %yield citronellyl
acetate and geranyl acetate yield is 1:1.
There are water molecules in rhodinol so that the
reaction of acetic anhydride to acetic acid is possible
(Figure 3), after that hydrolysis of ester by acetic acid
is possible (Figure 2).
Table 4: Effect of Reflux time on Citronellyl Acetate (CA)
and Geranyl Acetate (GA) percentage.
Acetic
Anhy-
-dride
(mole
)
Before
Synthesis
(%)
After
Synthesis
(%)
synthesis
results (%)
CA GA CA CA CA GA
0,03
11,
1
2,2
6
48,3
3
26,0
4
37,2
3
23,7
8
0,06
11,
1
2,2
6
49,9
9
20,1
8
38,8
9
17,9
2
0,09
11,
1
2,2
6
45,0
9
21,2
5
33,9
9
18,9
9
Table 5: Effect of Reflux time on Citronellyl Acetate (CA)
and Geranyl Acetate (GA) %yield.
Quantity
Of
Rhodino
l
Quantity
Of Acetic
Anhydrid
e
Reflux
Time
(Hour
)
%Yield
CA GA
10 mL
(0,03
mol)
2,92 mL
(0,03 mol)
1
74,06
%
95,92
%
10 mL
(0,03
mol)
5,84 mL
(0,06 mol)
1
79,60
%
74,49
%
10 mL
(0,03
mol)
8,76 mL
(0,09 mol)
1
71,29
%
80,61
%
Figure 3: reaction mechanism of hydrolysis ester (geranyl
acetate)
Esterification of Rhodinol Fraction with Acetic Anhydride using Zeolite Catalyst
125

4 CONCLUSIONS
Based on research by the author, it can be concluded
that:
1. The optimum ratio of rhodinol to acetic anhydride
is 1: 1, with the yield of citronellyl acetate
obtained is 74.06% while the % of geranyl acetate
yield is 95.92%. Based on the results of the GC-
MS analysis obtained 37.23% citronellyl acetate
and 23.78% geranyl acetate.
2. The optimal reflux reaction time for esterification
of rhodinol with acetic anhydride is 1 hour.
REFERENCES
Aldrich, S. (2019) Catalogue Product.
Claon, P. and Akoh, C. 1993. Enzymatic synthesis of
geranyl acetate in n-hexane with Candida antarctica
lipase. Biotechnology Letters, 15(12), 1211–1216.
Eden, W. T. et al. 2018. Fractionation of Java Citronella Oil
and Citronellal Purification by Batch Vacuum
Fractional Distillation. The 12th Joint Conference on
Chemistry. Semarang: IOP Publishing, p. 349.
Fessenden, R. J. and Fessenden, J. s. 1999. Kimia Organik
Jilid 2. 3rd edn. Jakarta: Erlangga.
Fracotte, E. and Lohmann, D. 1989. Helv. Chim. Acta, 114,
647.
Gunawan, W. 2009. Kualitas dan Nilai Minyak Atsiri,
Implikasi pada Pengembangan Turunannya. Booklet
Seminar Nasional Kimia Bervisi SETS. Semarang:
Himpunan Kimia Indonesia Jawa Tengah, 18–29.
Kaul, P. N. et al. 1997. Chemical Composition of the
Essential Oil Of Java Citronella (Cymbopogon
Winterianus Jowitt) Grown in Andhra Pradesh, Pafai
Journal, 19, 29–33.
Ma, Y. et al. 1996. Zeolite-Catalyzed Esterification I.
Synthesis of Acetates, Benzoates, and Phthalates,
Applied Catalyst A: General, 139, 51–57.
ICEO 2019 - 2nd International Conference of Essential Oil Indonesia
126
