
Quality Characteristics and Antibacterial Activity of Transparent
Solid Soap with Addition of Cananga Oil (Cananga odorata)
Rulita Maulidya
1
, Yuliani Aisyah
1,2
and Dewi Yunita
1
1
Agricultural Industry Technology Department, Agriculture Faculty, Universitas Syiah Kuala,
Banda Aceh, Indonesia 23111
2
Atsiri Research Center, Universitas Syiah Kuala, Banda Aceh, Indonesia, 23111
Keywords: Transparent solid soap, cananga, virgin coconut oil, palm oil, alkali.
Abstract: Cananga oil (Cananga odorata) is a natural source of fragrances that can be used as an antibacterial agent, so
cananga oil can be added to the formulation for making antibacterial soap. Therefore, the aim of this study is
to determine the formulation of cananga soap using different types of oil and to characterize the quality of
transparent solid soap. This study uses a completely randomized design (CRD) with a factorial pattern
consisting of two factors and three replications. Oil type (VCO and palm oil) and cananga oil concentration
(0% (control), 0.5%, 1%, and 1.5%; w / v) were factors in this study. Moisture content, free alkali content,
pH, hardness, foam stability and antibacterial activity were analyzed. Staphylococcus aureus and Escherichia
coli were used to test antibacterial activity. The results showed that soap made from VCO oil and 1.5%
cananga oil was the best formulation. The characteristics of transparent solid soap are water content 1.81-
4.39%, free alkali content 0.63-0.96%, pH 11.33-11.81, hardness 0.042 - 0.065 mm / g / s, and foam stability
69.70-85.45%. However, soaps made from VCO were only able to inhibit the growth of Staphylococcus
aureus with inhibitory diameters of 8.1-11.0 mm. Further research is needed to reduce the levels of free alkali
in soap and to increase the concentration of cananga oil so that it can inhibit the growth of Escherichia coli.
1 INTRODUCTION
Soap is a product of fatty acids and strong alkali salts
(sodium or potassium) hydrolysis. There are two
forms of soaps which are bar and liquid. Bar soap is
divided into 3 types, namely opaque, translucent and
transparent solid soaps. Transparent solid soap has
the highest level of clarity where this soap can be
penetrated by light (Prihandana et al., 2007).
Transparent solid soap has more excellence compared
to opaque soap specifically in its clear appearance and
its softer foam because diethanolamine cocoamide,
alcohol and sugar solution were added during
production. Also, high concentration of glycerine was
added giving the transparent solid soap moister.
The types of fatty acid of the raw materials used
in production of transparent solid soap influence the
characteristics of the soap produced (Momuat et al.,
2017). Fatty acids are the major component which is
made up from fat so selection of the fat in the soap
production is very important. The types of fatty acids
used in making transparent soap can come from VCO
oil and palm oil (Widyasanti, 2016), coconut oil
(Rozi, 2013), used cooking oil (Priani, 2010), VCO
and olive oil (Febriyenti, 2014).
Nowadays, transparent solid soap produced with
addition of natural ingredients is in great demand by
consumers especially because of beneficial effects on
skin health. Many synthetic antibacterial ingredients
such as triclosan and chloroxylenol are used to
produce antibacterial soaps (Wijana et al., 2019).
Unfortunately, the use of chemical soap continuously
can cause antibiotic resistance (Roslan et al., 2009).
Natural antibacterial alternatives are needed in soap
production.
In this research, cananga oil was added as
essential oil because cananga is a local flowering
plant in Aceh Province, Indonesia. In Indonesia, in
addition to being used as flowers for the ceremonial
cananga become the identity flora of the Province of
Nanggroe Aceh Darussalam and North Sumatra
Province (Sotyati, 2016). It has a distinctive and
fragrant flower aroma. The chemical composition of
cananga oil is -humulene (7.1%), germacrene D
(8.1%), -farnesene (12.6%), farnesol (5.6%) and
benzyl benzoate (3.8%). The main components that
112
Maulidya, R., Aisyah, Y. and Yunita, D.
Quality Characteristics and Antibacterial Activity of Transparent Solid Soap with Addition of Cananga Oil (Cananga odorata).
DOI: 10.5220/0009957701120118
In Proceedings of the 2nd International Conference of Essential Oils (ICEO 2019), pages 112-118
ISBN: 978-989-758-456-5
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

contribute to the aroma of cananga oil are linalool
(8.7%), dan -caryophyllene (26.8%) (Giang and
Son, 2016). Cananga has also been used for
antibacterial, anti-inflammatory and local anesthetic
activity (Erindyah, 2002).
Cananga oil has the ability to inhibit the growth of
Staphylococcus aureus bacteria. Activity against
bacteria continued to increase in accordance with the
amount of antibacterial compounds in the oil
(Maulidya, 2016; Anggia et al., 2014). The
components of O-methylmoschatoline, liriodenine
(24%), 3,4-dihydroxybenzoic acid, germacrene D
(11%), and ߚ-caryophyllene (12%) have been
investigated to contribute in antimicrobial activity
(Tan et al., 2015). The use of cananga oil serves not
only as a substitute for synthetic antibacterial
substances, but also as a fragrance in transparent solid
soap.
Therefore, the purpose of this study was to
determine the cananga soap formulation as well as to
characterise the quality of the transparent solid soap.
The raw materials used were palm oil and virgin
coconut oil (VCO). Also, cananga oil was used in
various concentration (0.5%, 1%, 1.5%).
2 MATERIALS AND METHODS
2.1 Material
The materials used in this study were cananga oil,
virgin coconut oil (VCO), palm oil, stearic acid,
NaOH, glycerin, ethanol, sugar solution, NaCl,
diethanolamine cocoamide, aquadest, nutrient agar
(Merck®), commercial antibacterial soap,
Staphylococcus aureus and Escherichia coli.
2.2 Research Design
This study used a completely randomized design
(CRD) with a factorial pattern consisting of two
factors. The first factor was types of oils (M)
consisting of two levels (VCO (M1) and palm oil
(M2)). The second factor was cananga oil
concentration (K; w/v) consisting of four levels (0%
(K1; control), 0.5% (K2), 1% (K3) and 1.5% (K4).
2.3 Transparent Soap Production
The oils (coconut oil and palm oil) were heated at
70°C. Stearate acid and NaOH 30% were added and
mixed until homogeneous to produce soap stocks.
Ethanol, glycerin, sugar solutions, sodium chloride,
and diethanolamine cocoamide were added to the
soap stock and stirred constantly for 10 minutes until
the mixture became homogeneous and clear solution
was formed. Cananga oil (0.5%, 1%, 1.5%) was
added to the soap mixture at 40°C and was stirred
until homogeneous. The soap mixture was molded in
a transparent solid soap mold. Furthermore, the
curing process took for 3 weeks.
2.4 Analysis of Transparent Soap
The chemical (water content, free alkali level, pH)
and physical (hardness and foam stability) properties
were examined following Indonesia National
Standard (SNI 06-4085-1996). The soap was
examined for antibacterial testing on Escherichia coli
and Staphylococcus aureus (Widyasanti, 2016). The
antibacterial ability was observed by measuring the
inhibitory area around the media which had been
placed on disc paper, which was marked by the
presence of a clear zone. The clear zone formed is
measured using a callipers.
2.5 Statistical Analysis
Data from water content, free alkali content, pH test,
hardness and foam stability were analysed with
analyse of variance (ANNOVA). The level used in
this analysis was 5%. If there is a significant effect
between treatments, Least Significance Different
(LSD) was used as the post hoc test to find out the
differences between treatments.
3 RESULTS AND DISCUSSION
3.1 Chemical Properties of
Transparent Solid Soap
3.1.1 Water Content
Based on SNI 06-3532-1994, the maximum moisture
content in soap is 15%. The amount of water
contained in soap can affect the characteristics of the
soap during the storage period. Soap with a high
water content or > 15% will experience a decrease in
weight and dimensions (Fachmi, 2008). Based on the
analysis of variance, it is known that the type of oils
has a very significant influence on the water content
of the transparent solid soap produced. The
percentage of water content can be seen in Figure 1.
This result showed that the moisture content of
transparent solid soap made from VCO and palm oil
met the SNI.
Quality Characteristics and Antibacterial Activity of Transparent Solid Soap with Addition of Cananga Oil (Cananga odorata)
113
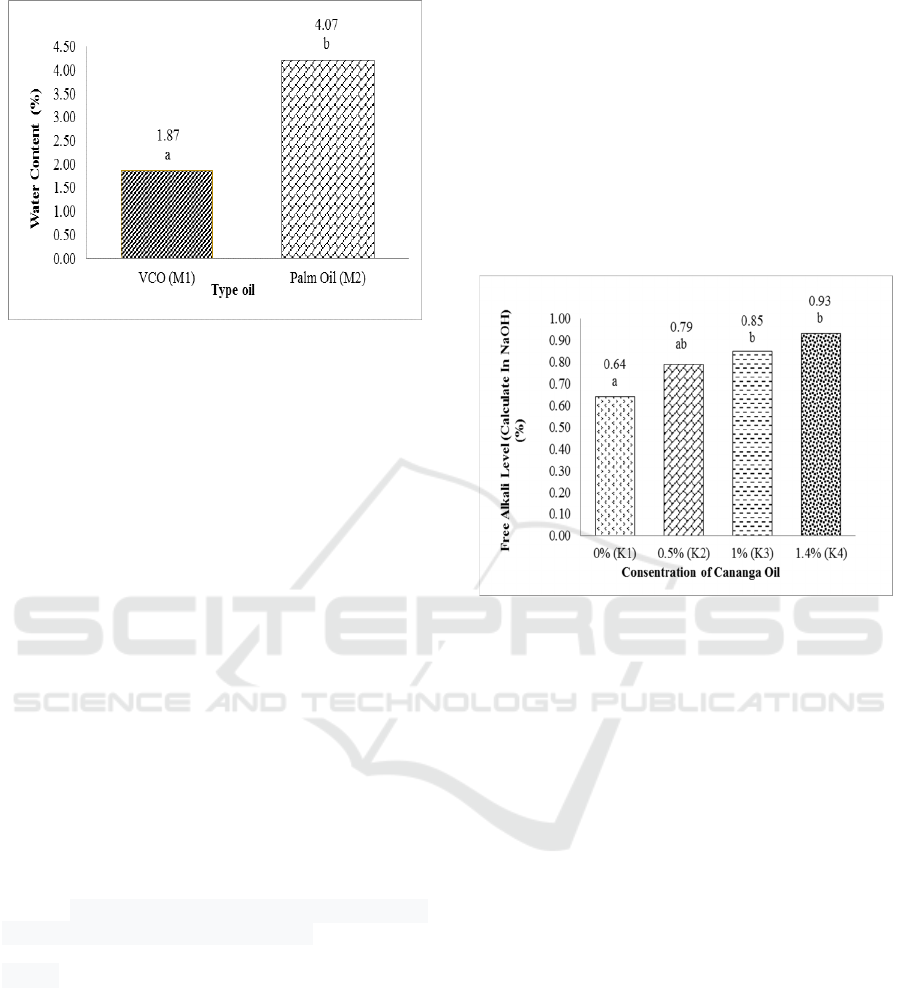
Figure 1: Percentage of water content of transparent solid
soap at different type of oils. The notation followed by the
same letter shows no difference (LSD
0.05
= 0.41 and
coefficient of variation = 15.60%).
Water content is an important quality parameter
on transparent soaps. High water content causes an
increase in rancidity in transparent soap products. The
type of oil in each treatment is sensitive to water
content. The amount of water and volatile substances
in soap will affect the solubility of soap in water when
used (Karo, 2011). The transparent soap produced has
a water content of 1.47% (VCO) and 4.07% (palm
oil).
The results of the diversity analysis (α = 0.05)
showed that the treatment of oil type had a very
significant effect on the water content of transparent
soap. Duncan's further test results show that the water
content of soap in this type of coconut oil is different
from soap made from VCO oil. Fatty acids that react
with NaOH will form soap and water. In addition, the
increase in water content can be caused by the end
result of oxidation of fatty acids contained in soap
which produces volatile aldehyde and ketone
compounds (Karo, 2011). So that soap from VCO oil
has a lower moisture content value than soap from
palm oil.
The highest saturated fatty acid in palm oil is
palmitate acid, and VCO is lauric acid.
3.1.2 Free Alkali Level
Free alkali is alkali in soap which is not needed during
the sapling process (SNI, 1996). Free alkali levels
obtained from this study were 0.64% -0.93%, so as to
increase the pH of the soap. The maximum free alkali
level is 0.1% (SNI, 1996). Soaps that have high free
alkali levels or > 0.1% can cause skin irritation
(Fachmi, 2008). Based on the analysis of variance, it
is known that the concentration of canaga oil has a
very significant influence on the free alkali level of
the transparent solid soap produced. The percentage
of free alkali level can be seen in Figure 2.
Based on Figure 2, the value of alkali levels
increases with increasing cananga oil concentration.
The excess alkali in soap is thought to be caused by
the chemical component of ylang oil containing
alkaloid compounds. The typical chemical
composition of cananga oil generally consists of five
main components, caryophyllene (29.60%),
germacrene-D (19.22%), geraniol acetate (10.79%),
bergamotene (7.97%), α-humulent (7.97%) 7.77%).
Figure 2: Free alkali levels of transparent solid soap at
different cananga oil concentrations. The notation followed
by the same letter shows no difference (LSD
0.05
= 0.16 and
coefficient of variation = 15.81%).
Free alkali levels of soap products produced are
quite high, this is presumably because cananga oil
contains alkaloid compounds. Alkaloids are organic
compounds that are basic or alkaline (Lenny, 2006).
Most alkaloids at room temperature are generally in
the form of colourless crystals and are volatile.
Alkaloids are generally soluble in water, but some are
soluble in organic solvents. Most alkaloids are weak
bases, and some are amphoteric. (Babbar 2015). The
main components that contribute to the aroma of
cananga oil are linalool (8.7%) and β-caryophyllene
(26.8%). This is because linalool is a compound that
gives a distinctive aroma (Oktapiyani, 2004).
3.1.3 pH
The results of pH measurements can be seen in Figure
3. The type of oil has a very significant effect on the
value of pH. The pH value obtained in VCO oil is
around 11.34 and palm oil is 11.69. The pH values
have met the quality criteria for bath soap ranging
from 9-11 (Hambali, 2005). The final pH value of the
product is strongly influenced by the basic ingredients
ICEO 2019 - 2nd International Conference of Essential Oil Indonesia
114
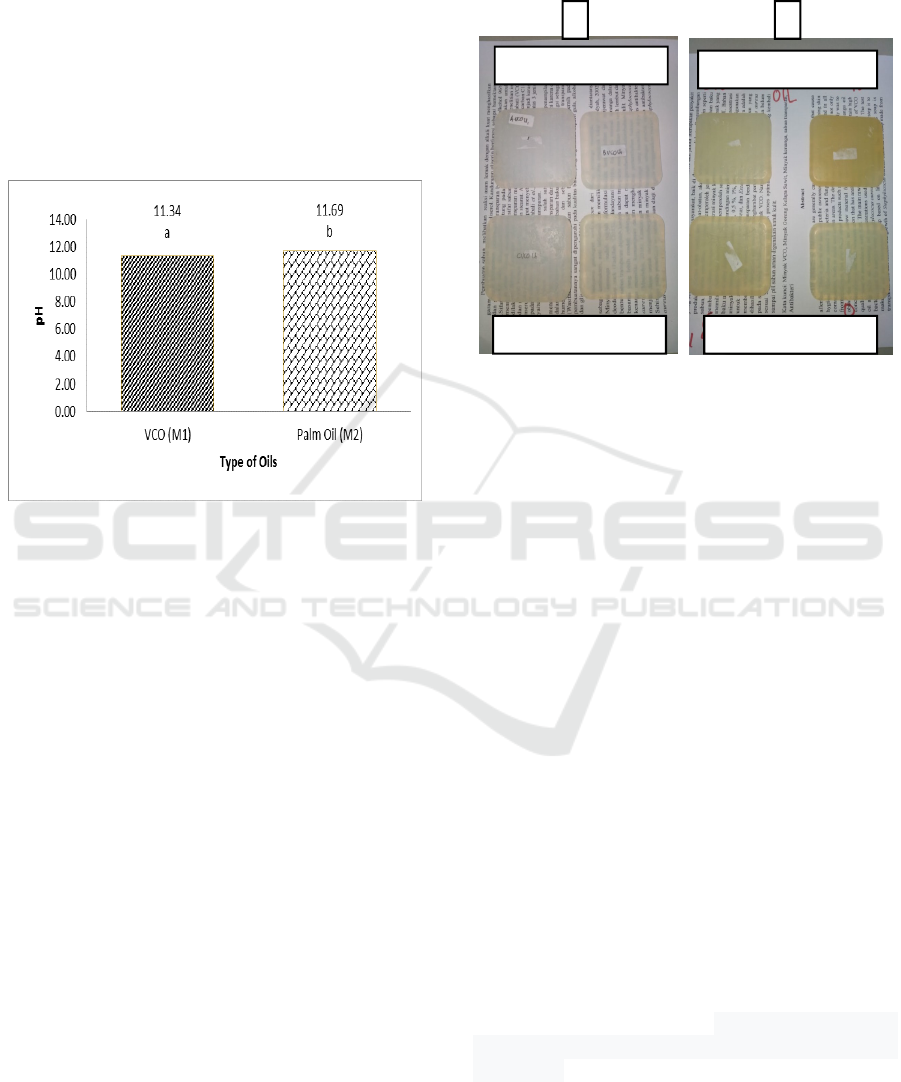
used (Rahmanto, 2011). In addition, pH
measurements in the range 9-11 are relatively safe for
the skin (Edoga, 2009).
This pH instability can most likely be caused by a
heating factor, due to the hydrolysis of the active
ingredient of sodium ester with fatty acids so that it
can cause free alkali which can increase the pH of
soap (Nurhadi, 2012). The pH of alkaline soap can
help the skin in opening pores and dirt that sticks to
the skin, bound by foam contained in the soap
(Setyoningrum, 2010).
Figure 3: pH value of transparent solid soap at different type
of oils. The notation followed by the same letter shows no
difference (LSD
0.05
= 0.26 and coefficient of variation =
2.65%).
The pH on VCO is lower than that of palm oil. It
is thought that the difference in the fatty acid carbon
chain can affect the low VCO pH value. Addition of
weak fatty acids, such as citric acid, can reduce the
pH of soap (Wasitaatmadja, 1997). Fatty acids in
VCO (lauric acid) have shorter chains when
compared to fatty acids in palm oil (palmitic acid).
This pH instability is most likely caused by a heating
factor, due to the hydrolysis of the active ingredient
of sodium ester with fatty acids so that it can cause
free alkali which can increase the pH of soap
(Nurhadi, 2012).
3.2 Physical Properties of Transparent
Solid Soap
The production of transparent soap made with various
concentration of cananga oil were made on the basis
of 300 g. During the production, the soap loses 100g.
This was expected due to the amount of foam
produced before the printing process so that a lot of
foam was removed when the foam was separated with
the soap mixture. The resulting soap can be said
transparent if when the soap is placed on paper with
12 font size, the letters can be read clearly. The
transparent soap produced in each treatment can be
seen in Figure 4.
Figure 4: Transparent soaps made from: 1) virgin coconut
oil and 2) palm oil at various concentration of cananga oil.
Based on Figure 4 the VCO soap is more
transparent compared to the palm oil soap.
Transparent soap can be produced in several different
ways. One of the oldest methods is by dissolving the
soap in alcohol with gentle heating to make a clear
solution which is then given a fragrance and coloring.
The color of the bar soap depends on the choice of
starting material and if good quality soap is not used,
it is likely that the final product will be very yellow
in color (Williams, 2002). The basic ingredients of
VCO soap have a clear color while palm oil has a
yellowish color. This is thought to be the cause of the
transparent soap from VCO becoming more clear
when compared to palm oil.
3.2.1 Hardness
The hardness of transparent solid soap can be
influenced by saturated fatty acids which are used as
raw materials in making transparent solid soap. The
results of variance indicate that the type of oil affects
the hardness in soap. Hardness of transparent solid
soap can be seen in Figure 5.
From Figure 5, the type of oil in this study affects
the value of soap hardness. Factors affecting the
hardness of saturated fatty acids and water content
values (Widyasanti, 2016). The highest saturated
fatty acid in palm oil is palmitate acid, and VCO is
lauric acid. Saturated fatty acids are fatty acids that
do not have double bonds, saturated fatty acids are
usually solid at room temperature, so it will produce
a harder soap (Gusviputri et al., 2013). The longer the
A 0% and B 0.5%
A 0% and B 0.5%
C 1% and D 1.5% C 1% and D 1.5%
1 2
Quality Characteristics and Antibacterial Activity of Transparent Solid Soap with Addition of Cananga Oil (Cananga odorata)
115
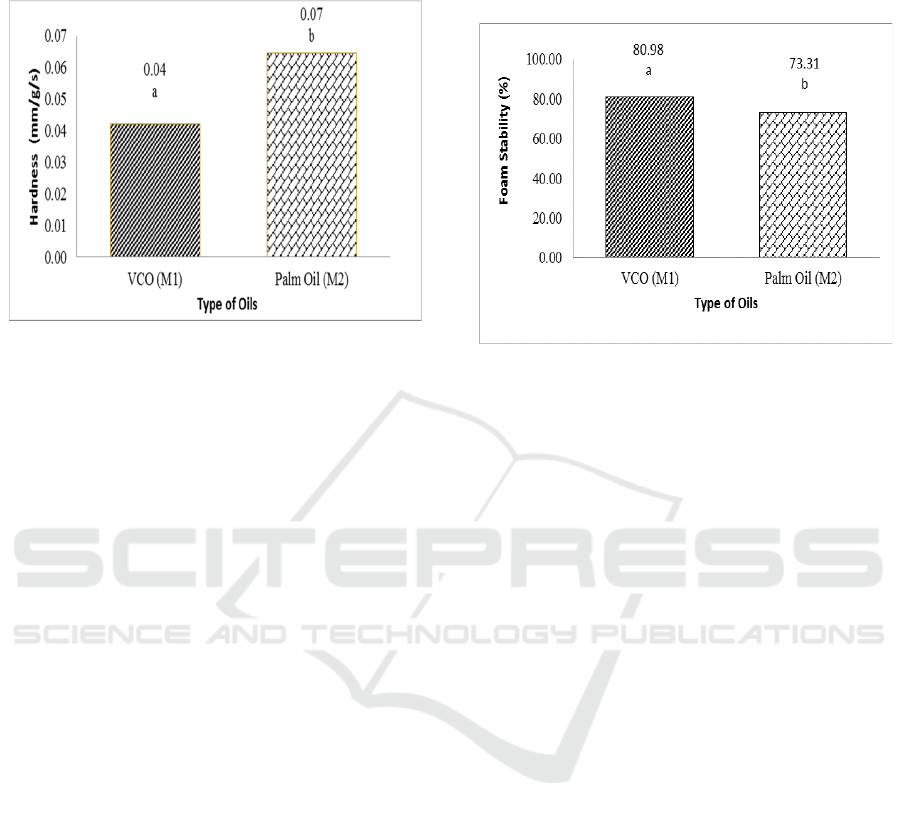
carbon chain of fatty acids, the fatty acids tend to be
solid.
Figure 5: Hardness of transparent solid soap at different
type of oils. The notation followed by the same letter shows
no difference (LSD
0.05
= 0.02 and coefficient of variation =
37.94%).
The value of water content from the research
results is higher palm oil (4.07%) and lower VCO
(1.87%). The higher the amount of water content
contained in soap, the higher the level of hardness
shown by the penetrometer scale. If the penetrometer
scale shows a high number, the soap will be soft
(Widyasanti, 2016). If the soap is too soft, it will
cause the soap to dissolve easily and become easily
damaged (Steve, 2008).
3.2.2 Foam Stability
Foam is one of the important parameters in
determining the quality of bath soap. In its use, foam
plays a role in the cleansing process on the skin. The
results of various analyses show that the
concentration of cananga oil added to transparent
solid soap does not show a significant difference in
the stability value of the foam. While the type of oil
used in this study showed a significant effect on the
5% test level on the stability of the soap foam. Foam
stability can be seen in Figure 6.
Palm oil contains palmitic acid which is good in
maintaining foam stability. The saturated fatty acids
found in palm oil are palmitic acid which can function
for foam stability (Widyasanti, 2010). Saturated fatty
acids contained in soap make foam more stable when
compared to unsaturated fatty acids (Gromophone
1983).
However, the water content of products made
from palm oil tends to be high, making the foam on
the product unstable. So that the foam is more stable
in VCO-based soap products. Foam characteristics
are also influenced by the presence of soap active
ingredients or surfactants, foam stabilizers and soap
making materials (Amin, 2006).
Figure 6: Foam stability of transparent solid soap at
different type of oils. The notation followed by the same
letter shows no difference (LSD
0.05
= 4.47 and coefficient
of variation = 6.69%).
3.3 Antibacterial Activity of
Transparent Cananga Oil
Gram positive bacteria Staphylococcus aureus and
Gram negative bacteria Escherichia coli were used to
test the antibacterial effect of transparent solid soap
containing cananga oil. These bacteria were selected
because these pathogenic bacteria are often found on
the hands and skin. The results showed that addition
of cananga oil until 1.5% in transparent solid soap
production made from VCO and palm oil could not
inhibit E. coli. The inhibitory effect of S. aureus was
shown on the soap made from VCO only (Figure 7).
From Figure 7, there is a very significant
influence on inhibitory diameter of S. aureus because
of the interaction between type of oil and cananga oil
concentration ranging from 8.07 - 11.00 mm. The
inhibition occurred in the VCO oil because this oil
contains lauric acid which also has antibacterial effect
(Febriyenti, 2014). Antibacterial compounds in soap
provide activity in inhibiting bacteria caused because
the soap is hydrophilic-lipophilic. Nonpolar groups
on soap are -R and -COONa groups which are
polar in nature. The hydrophilic nature of soap causes
antimicrobial compounds to be able to diffuse in polar
agar media, while the lipophilic nature of soap will
help the penetration of antibacterial compounds into
lipophilic bacterial cell membranes (Pelczar, 1998).
ICEO 2019 - 2nd International Conference of Essential Oil Indonesia
116

Figure 7: Inhibitory diameter of Staphylococcus aureus on
transparent solid soap. The notation followed by the same
letter shows no difference (LSD
0.05
= 0.69 and coefficient
of variation = 8.37%).
4 CONCLUSIONS
Palm oil and VCO can be formulated into transparent
solid soap by adding different concentrations of
cananga oil. Physical and chemical analyses showed
that the soaps have meet the SNI. Unfortunately, the
value of free alkali in soap exceeds the maximum SNI
limit and in bacterial inhibition tests, only VCO is
significant in Staphylococcus aureus. Further
research is needed to reduce the levels of free alkali
in soap and increase the concentration of cananga oil
so that it can inhibit the growth of Escherichia coli.
REFERENCES
Anggria, F.T., Yuharmen., Balatif, N. 2014. Perbandingan
Isolasi Minyak Atsiri dari Bunga Kenanga (Cananga
odorata (Lam.) Hook.f dan Thoms) Cara Konvensional
dan Microwave serta Uji Aktivitas Antibakteri dan
Antioksidan. Fakultas Matematika dan Ilmu
Pengetahuan Alam, Universitas Riau, Riau.
Amin, H. 2006. Kajian Penggunaan Kitosan Sebagai
Pengisi dalam Pembuatan SabunTransparan. Skripsi,
Fakultas Perikanan dan Ilmu Kelautan, Institut
Pertanian Bogor, Bogor.
Badan Standarisasi Nasional. 1996. Standar Sabun Mandi
Cair, SNI 06-4085-1996, Dewan Standarisasi Nasional,
Jakarta.
Babbar, N. 2015. An Introduction to Alkaloids and Their
Applications in Pharmaceutical Chemistry. The
Pharma Innovation Journal, 4 (10), 74-75.
Edoga, M, O. 2009. Comparison of various fatty acid
sources for making soft soap (Part 1). Qualitative
analysis. Department of Chemical Engineering, Federal
University of Technology, Minna, Nigeria.
Erindyah, R,W., Maryati. 2002. Aktivitas Antibakteri
Minyak Atsiri Pinus Terhadap S. aureus dan E.coli.
Jurnal Farmasi Indonesia Pharmacon 4 (1) : 20-24.
Fachmi, C. 2008. Pengaruh Penambahan Gliserin dan
Sukrosa Terhadap Mutu Sabun Transparan. Skripsi.
Fakultas Tekhnik Pertanian, Institut Pertanian Bogor,
Bogor.
Febriyenti., Sari, L. I., Nofita, R. 2014. Formulasi Sabun
Transparan Minyak Ylang-Ylang dan Uji Efektivitas
terhadap Bakteri Penyebab Jerawat, Fakultas Farmasi,
Universitas Andalas, Padang.
Giang, P. M., Phan, T. S. 2016. GC and GC-MS Analysis
of The Fresh Flower Essential Oil of Cananga odorata
(Lam.) Hook. f. et Th. var. fruticosa (Craib) J. Sincl.
American Journal of Essential Oils and Natural
Products, 4 (4) : 09-11.
Gromophone, M, A. 1983. Lather Stability of Soap
Solutions. JAOCS. 60 (5) : 1022-1024.
Gusviputri, A., Meliana, N. P. S., Aylianawati., Indraswati,
N. 2013. Pembuatan Sabun dengan Lidah Buaya (Aloe
vera) Sebagai Antiseptik Alami. Fakultas Teknik.
Universitas Katolik Widya Mandala. Surabaya.
Hambali, E., Bunasor, T. K., Suryani, A., Kusumah, G. A.
2005. Aplikasi Dietanolamida dari Asam Laurat
Minyak Inti Sawit pada Pembuatan Sabun Transparan.
Fakultas Teknologi Industri Pertanian, Bogor.
Karo, A. Y. 2011. Pengaruh Penggunaan Kombinasi Jenis
Minyak terhadap Mutu Sabun Transparan. Skripsi.
Institut Pertanian Bogor. Bogor.
Lenny, S. 2006. Senyawa Flavonoida, Fenilpropanoida
dan Alkaloida. Karya Ilmiah. Universitas Sumatera
Utara, Medan.
Maulidya, R., Aisyah, Y., Haryani, S. 2016. Pengaruh Jenis
Bunga dan Waktu Pemetikan Terhadap Sifat
Fisikokimia dan Aktivitas Antibakteri Minyak Atsiri
Bunga Kenanga (Cananga odorata). Jurnal Teknologi
dan Industri Pertanian Indonesia, 8(2), 53-60.
Momuat, L, I., Wuntu, A, D. 2017. Produksi Sabun Mandi
Transparan Berbahan Baku Vco Mengandung
Karotenoid Tomat.
Program Studi Kimia FMIPA,
Universitas Sam Ratulangi, Manado.
Nurhadi, S, C. 2012. Pembuatan Sabun Mandi Gel Alami
dengan Bahan Aktif Mikroalga Chlorrela pyrenoidosa
Beyerinck dan Minyak Atsiri Lavandula lativolia
Chaix. Fakultas sains dan Teknologi, Universitas Ma
Chug, Malang.
Oktapiyani, S. 2004. Respon Penyimpanan Bunga Ylang-
ylang (Cananga odorata forma genuina) terhadap
Rendemen dan Kualitas Minyak Atsiri. Institut
Pertanian Bogor, Bogor.
Pelczar, M, J. 1988. Dasar-Dasar Mikrobiologi. UI Press,
Jakarta.
Priani, S, E., Lukmayani, Y. 2010. Pembuatan Sabun
Transparan Berbahan Dasar Minyak Jelantah Serta
Hasil Uji Iritasinya Pada Kelinci. Jurusan Farmasi,
Universitas Islam Bandung, Bandung.
Prihandana, R., Erliza, H., Siti, M., Roy, H. 2007. Meraup
Untung dengan Jarak Pagar. Agromedia Pustaka,
Jakarta.
Quality Characteristics and Antibacterial Activity of Transparent Solid Soap with Addition of Cananga Oil (Cananga odorata)
117

Rahmanto, A., 2011. Pemanfaatan Minyak Jarak Pagar
(Jatropha curcas, Linn) Sebagai Komponen Sediaan
dalam Formulasi Produk Hand and Body Cream.
Program Studi Teknologi Industri Pertanian
Pascasarjana, Institut Pertanian Bogor, Bogor.
Roslan, A, N., Sunariani, J., Irmawati, A. 2009. Penurunan
Sensitivitas Rasa Manis Akibat Pemakaian Pasta Gigi
yang Mengandung Sodium Lauryl Sulphate 5%. Jurnal
Persatuan Dokter Gigi Indonesia Vol 58 (2) : 10-13.
Rozi, M., Sulaiman, T, N, S., Indrayudha, P. 2013.
Formulasi Sediaan Sabun Mandi Transparan Minyak
Atsiri Jeruk Nipis (Citrus Aurantifolia) Dengan
Cocamid Dea Sebagai Surfaktan. Fakultas Farmasi,
Universitas Muhammadiyah Surakarta.
Setyoningrum., Maharani, E, N. 2010. Optimasi Formula
Sabun Transparan dengan Fase Minyak Virgin
Coconut Oil dan Surfaktan Cocoamidopropil Betaine.
Fakultas Farmasi, Universitas Sanata Dharma,
Yogyakarta.
Sotyati. 2016. Kenanga, Flora Identitas Aceh.
http://www.satuharapan.com/read detail/read/kenanga-
flora-identitas-aceh-kaya-manfaat.16 Desember 2019.
Steve., 2008. Saponification Table Plus The
Characteristics of Oils in Soap. USA. http://www.soap-
making-resource.com/saponification-table.html. 19
Desember 2019.
Tan, L. T. H., Learn, H. L., Wai, F. Y., Chim, K. C.,
Habsah, A. K., Kok, G. C., Bey, H. G. 2015. Traditional
Uses, Phytochemistry, and Bioactivities of Cananga
odprata (Ylang-Ylang). Evidence-Based
Complementary and Alternative Medicine : 1-30.
Wasitaatmadja, S. M. 1997. Penuntun Ilmu Kosmetik
Medik. Universitas Indonesia, Jakarta.
Widyasanti, A., Farddani, C, L., Rohdiana, D. 2016.
Pembuatan Sabun Padat Transparan Menggunakan
Minyak Kelapa Sawit (Palm Oil) dengan Penambahan
Bahan Aktif Ekstrak Teh Putih (Camellia sinensis).
Fakultas Teknologi Industri Pertanian Universitas
Padjadjaran, Bandung.
Wijana, S., Tika, P., Nur, L. R. 2019. Optimization of
Solubilizers Combinations on The Transparent Liquid
Soap with The Addition of Peppermint (Mentha
piperita L.) and Lavender (Lavandula L.) Oil. AIP
Conference Proceedings 2120, 050020 (2019).
Williams, D, F., Schmitt, W, H. 2002. Kimia dan Teknologi
Industri Kosmetika dan Produk-produk Perawatan
Diri. Fakultas Teknologi Pertanian,IPB. Bogor.
ICEO 2019 - 2nd International Conference of Essential Oil Indonesia
118
